1. Introduction
Microcystins are produced by certain species of cyanobacteria and are hazardous to humans and animals. Considered to be the most toxic group of cyanobacterial toxins, microcystins are inhibitors of specific protein phosphatases and can cause skin irritations, allergic reactions and gastroenteritis [
1]. Humans are primarily exposed to microcystins via drinking water consumption and accidental ingestion of recreational water [
2,
3]. Recreational exposure by skin contact or inhalation to microcystins is now a recognised cause of a wide range of acute illnesses that can be life-threatening [
4]. While microcystins have been reported across the world [
5], the frequency of their occurrence in aquatic systems is expected to rise as a result of climate change [
6,
7,
8,
9,
10]. To control the associated health risks, it is therefore essential to understand the fate of microcystins in aquatic systems.
In aquatic systems, microcystins can be present not only in the water but also in sediments. They have been reported in a variety of sediments, with and without the occurrence of benthic cyanobacteria [
11,
12,
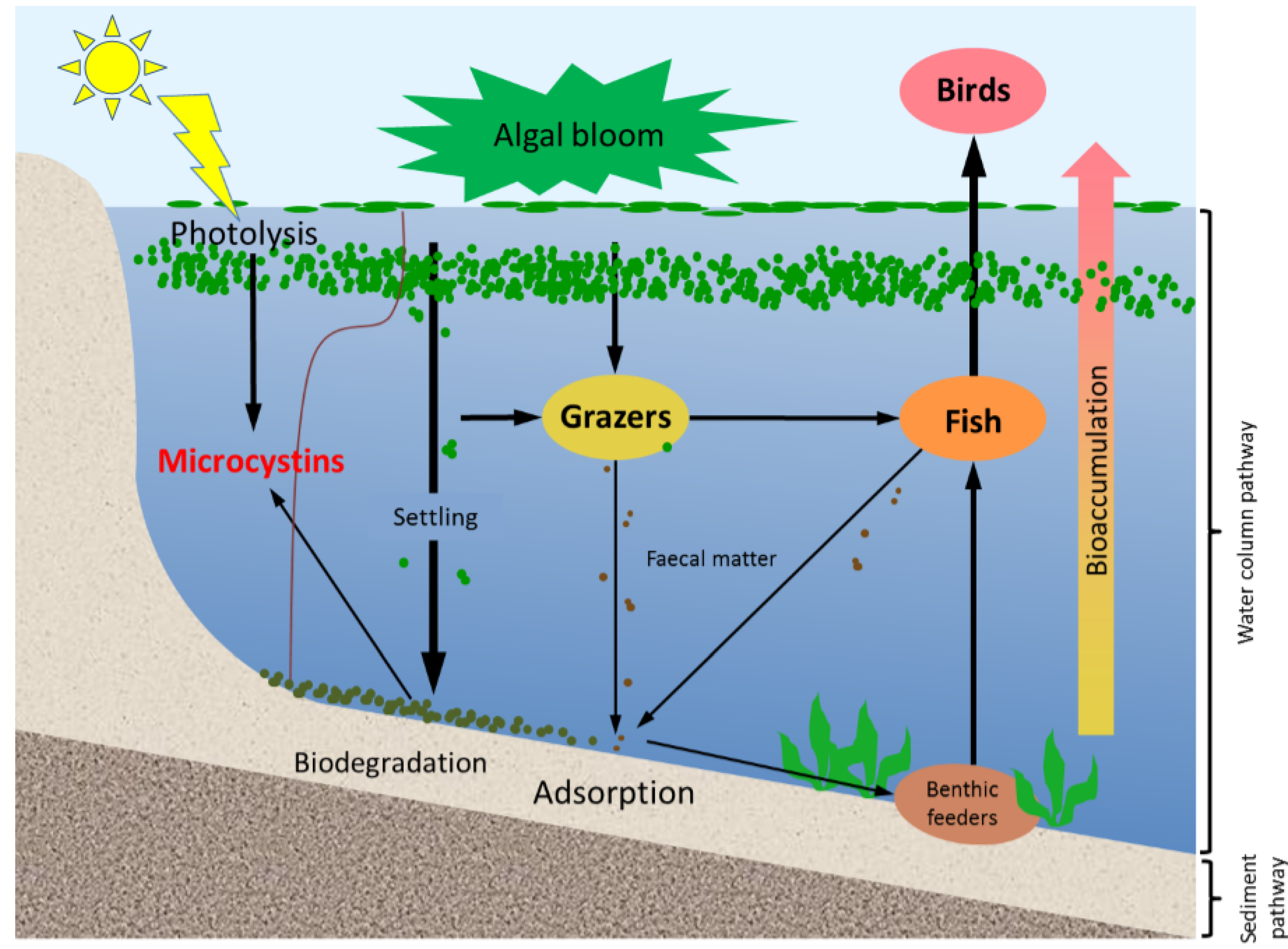
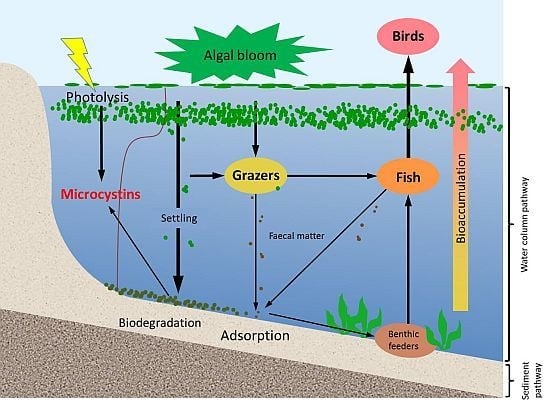
13]. Microcystins in lake sediments have their origin in at least three main processes (
Figure 1); including the lysis of cyanobacterial cells in sediments, transfer from the dissolved form to sediments by adsorption, and grazing by aquatic animals followed by the sinking of microcystin-containing faecal pellets [
14]. Their occurrence in lake sediments is a potential hazard to aquatic animals and benthic organisms, for example, by altering the organisms’ metabolism [
15,
16]. In addition, the release of toxins that are loosely bound to the sediment might re-dissolve into the water column long after a bloom, and in absence of any visual indication of the presence of cyanobacterial blooms [
17].
Studies on the distribution of total microcystins in sediments of aquatic systems are limited, likely due to technical difficulties in extracting total toxins from sediments [
11]. An earlier study indicates that sediments contain two microcystin fractions: a loosely adsorbed and therefore “readily extractable” fraction, and a bound fraction [
17]. The MMPB (2-methyl-3-methoxy-4-phenylbutyric acid) method is considered to be an effective analytical procedure for the quantification of total microcystins (free and bound) in lake sediments. However, this method requires a very low reaction temperature of −78 °C [
11], which might be a limiting factor. Conversely, the loosely adsorbed microcystin fraction, which is possibly the more hazardous fraction for organisms due to its potential to easily re-dissolve back into the water column, is easier to extract, and some studies have focused on this fraction [
14,
18,
19]. With conventional solvents, the extraction efficiency of loosely adsorbed microcystins strongly depends on the solvent type, the sediment characteristics, and the chemical structure of microcystins [
12].
Figure 1.
A schematic diagram showing the various pathways of microcystin dynamics during and following a bloom event. The sediment compartment plays a key role in release, adsorption and degradation of microcystins. Please note that stratification does not occur in all systems; especially shallow systems often have mixed water columns.
Figure 1.
A schematic diagram showing the various pathways of microcystin dynamics during and following a bloom event. The sediment compartment plays a key role in release, adsorption and degradation of microcystins. Please note that stratification does not occur in all systems; especially shallow systems often have mixed water columns.
Microcystin concentrations in sediments have been shown to be highly variable on a temporal and spatial scale. High microcystin concentrations have been found in sediments during spring, followed by a decrease in the summer, when cyanobacterial biomass in the water was at a maximum [
19]. Moreover, Chen
et al. [
14] found that the highest microcystin concentrations in sediments occurred during summer, when cyanobacterial biomass and microcystin production were at their maximum. Microcystins have been reported at different depths of sediments [
11,
20], with concentrations decreasing with increased depth of sediment in Lake Taihu, China [
14].
Many factors can contribute to the variability of microcystins in lake sediments. Ihle
et al. [
19] reported that microcystin concentrations in the sediments of a shallow lake correlated to
Microcystis biomass in the sediments. Similarly, microcystin concentrations in the sediments of the Nile River and irrigation canal sediments correlated to the total count of cyanobacteria, particularly
Microcystis spp. and intracellular microcystins in the water [
18]. The concentration of microcystins in sediments can also be influenced by the sedimentation of suspended particles with absorbed microcystins [
21] and the adsorption of dissolved microcystins from the water [
11,
12,
14,
18,
22,
23]. Furthermore, organic matter content and particle size fraction (sand, silt and clay) [
14,
16,
22,
24], as well as the physicochemical parameters of lake water, such as temperature, salinity, and pH [
24,
25,
26,
27], can influence the sediment’s capacity to adsorb and degrade microcystins in aquatic systems. In shallow systems, wind-induced mixing of the water column and the associated redistribution of toxin-containing sediment potentially plays an important role in explaining the spatial distribution of toxins in the sediment.
Shallow lakes have a complex interplay between being stratified during times of high solar irradiation without wind and being completely mixed during periods of wind. Our study lake, Lake Yangebup, which is representative of a shallow water body, is a typical example of such a system with stratification only occurring during periods of wind speed <6 m/s [
28]. In such well mixed water bodies, the horizontal distribution of allochthonous contaminants in sediments can be expected to differ very little, due to the highly dynamic nature of the sediment, which is being resuspended and redeposited during mixing events [
28,
29]. With autochthonous substances, such as cyanotoxins, the spatial distribution of toxins in the sediment depends on the location where it is produced and on the redistribution of sediments. Thus, in the absence of wind mixing, we could expect higher concentrations of toxins in the sediment at locations that have cyanobacterial blooms (
Figure 1), while these horizontal differences should be reduced during wind-induced mixing events. The spatial distribution of toxins in the sediments of shallow lakes should therefore be the result of a complex interaction of biological and physicochemical processes, including wind-driven mixing.
To date, systematic studies on the relationship between the variability of microcystins in lake sediments and the environmental factors are lacking. While a number of studies have looked at the temporal variability of microcystins in sediments, the spatial variability of microcystins within aquatic systems, especially in shallow systems has not been investigated. This study aims to identify the spatial and temporal variability of microcystins in the upper layer of sediments in a shallow, eutrophic lake, and to determine the biological and physical parameters contributing to the variability. We hypothesised that the variability of microcystin concentration in lake sediments can be predicted by a combination of biological and physical parameters such as cyanobacterial biomass in the sediments, cyanobacterial biomass and microcystins in the water, and physicochemical parameters of lake water and the sediment characteristics.
3. Discussion
Microcystins were detected in all sediment samples in this study, including at site 4 where only a low level of cyanobacterial biomass occurred, indicating the wide presence of microcystins in the lake’s sediments. Similar to previous studies [
30,
31,
32], we found that cyanobacterial blooms, dominated by toxic
Microcystis spp., and microcystins in the water occurred most of the year in Lake Yangebup. Microcystins can therefore accumulate in the sediments as a result of its long-term exposure to cyanobacterial biomass at the bottom of the lake and microcystins in the water (
Figure 1).
The occurrence of microcystins in lake sediments associated with cyanobacterial blooms has been reported in earlier studies [
11,
12,
14,
18,
19]. Microcystin concentrations in the sediment depend on a number of factors, including toxin production within the cyanobacterial community, and the biomass of toxic cyanobacteria in the sediment and in the water column [
18,
19]. Once the toxins that are produced within the water column reach the sediment, physical, chemical, and biological factors that influence the sediment’s capacity to adsorb and degrade microcystins play an important role. The spatial variability of toxins in the sediment of a lake will therefore be the result of a complex interaction between the toxin producing processes in the water column, the physico-bio-chemical processes in the sediment, and a redistribution of toxin containing sediment. The latter is of extreme importance in shallow lakes, where wind is responsible for de-stratification of the water column and re-distribution of sediments [
28,
29]. An earlier study in Lake Yangebup showed that there is complete mixing of the water column at wind speeds >6 m/s with intermittent stratification at lower wind speeds, which can overturn within a few days [
28]. Although wind speed was partially <6 m/s during our study (
Figure 4B), these earlier results from Arnold and Oldham [
28], in addition to the fact that we did not find a difference between the surface water layer and the water layer directly above the sediment (overlying) in cyanobacterial biomass, temperature, salinity and pH, indicates that Lake Yangebup is a typical representative of a shallow lake, that can be considered a mixed system due to wind effects and diurnal convectional cooling of the water.
Figure 4.
Study site and wind conditions. (A) Map of Lake Yangebup with sampling sites; (B) Wind speed and direction for the sampling days and the two antecedent days for each month. Wind measurements were taken at 9 am and 3 pm of each day, resulting in 6 measurements for each month. Please note different axes for wind speeds.
Figure 4.
Study site and wind conditions. (A) Map of Lake Yangebup with sampling sites; (B) Wind speed and direction for the sampling days and the two antecedent days for each month. Wind measurements were taken at 9 am and 3 pm of each day, resulting in 6 measurements for each month. Please note different axes for wind speeds.
Interestingly, microcystin concentration and cyanobacterial biomass in the sediment was different between sites in three out of five months. This indicates that site specific processes are important for these variables, and that a re-distribution of toxin and cell containing sediment by wind-mixing might not have been complete. At the same time, we did not find a difference in the sediment toxin concentration between sites in September, when site 3 had a significantly higher cyanobacterial biomass in the water than all other sites, and differences in the sediment toxin concentration in November also did not coincide with different cyanobacterial biomass in the water column. This shows that some level of mixing is important in explaining the distribution of microcystins in the sediment. We can therefore conclude that local and wind-mixing processes are both important factors affecting the concentration of microcystin in the sediment.
We could explain the variability of microcystin concentration in the sediments by a combination of environmental variables, namely total microcystin concentration in the water, cyanobacterial biomass in the water, cyanobacterial biomass in the sediment and pH. These factors have also been shown to be of importance in previous studies, where significant and close relationships between the concentration of microcystins in sediments and intracellular microcystins or cyanobacterial biomass in the water column and the sediment have been reported [
18,
19]. The microcystins produced in the cyanobacteria in the water column can reach the sediment via a number of pathways, including settlement of cyanobacterial cells with subsequent cell lysis, and the adsorption of dissolved toxins to the sediment (
Figure 1). The percentage of explained variability could be increased significantly by forcing variables indicative of smaller scale processes, such as cyanobacterial biomass in the overlying water, cyanobacterial biomass in the surface water, and intracellular and dissolved microcystin concentrations into the model. This clearly highlights again that, although Lake Yangebup is a mixed system, local processes are important to explain the local microcystin concentration in the sediment. Furthermore, it emphasises the importance of the link between processes in the water column in explaining the microcystin concentration in sediments [
14,
21].
The multiple linear regression analysis shows that microcystin concentration in sediments decreased with increasing pH and temperature. This could be due to the fact that higher temperatures increased the sediments capability to biodegrade microcystins, aiding the removal of these toxins from aquatic systems [
25,
33], and due to higher temperatures leading to an increased metabolic activity of the microcystin-degrading bacteria. In addition, previous studies have also shown that lower pH increases the capacity of sediments to adsorb microcystins [
24,
26,
27], by influencing the surface charge heterogeneity of microcystins and sediment [
17].
The temporal variability of microcystins in the lake’s sediments was site specific, and the spatial variability in their concentration was different in each sampling month. Site 3 experienced a massive development of cyanobacterial biomass in November and December, and this possibly resulted in the increase of toxin concentration in the sediment at this site. On average, cyanobacterial biomass in the water was lowest at site 4 and highest at site 3. Wind is known to be a main driver of horizontal distribution of cyanobacterial biomass in lakes [
34]. Wind speed and direction was variable in our study, however site 3 was the most downwind site in all months except September (
Figure 4B). Therefore, it is likely that wind is the reason why we detected the highest biomass at this site.
The physical and chemical properties of sediments, for example, organic matter content and particle size fraction (sand, silt, and clay), will affect the sediment’s capacity to adsorb and degrade microcystins [
14,
16,
22,
23,
24]. Mohamed
et al. [
18] observed that in the Nile River, the capacity of sediments to adsorb microcystins was significantly correlated to their clay and organic matter content. Wu
et al. [
27] found that the adsorption process for microcystins to sediments mainly depended on the sediment’s organic matter content. The significance of organic matter and clay in the binding and degradation of microcystins to soils and sediments has been reported in many other studies [
24,
35,
36,
37,
38,
39,
40]. In our study, no clay was present in the sediments and no significant correlation between the concentration of microcystins and organic matter content in sediments was observed, probably due to only minor differences in the organic matter content of the samples.
4. Experimental Section
4.1. Study Site
This study was conducted at Lake Yangebup in Western Australia (32°6'40"S, 115°50'00"E). The field permit was provided by the City of Cockburn, Western Australia. The field study did not involve endangered or protected species. Lake Yangebup is a shallow, eutrophic, permanent lake with approximately 68.4 ha of open water and a mean water depth of 2.5 m [
28]. Total phosphorus and total nitrogen in the water column of Lake Yangebup was previously reported to be 0.49–6.98 µM and 0.14–0.37 mM, respectively [
32,
41]. Toxic cyanobacterial blooms, dominated by
Microcystis spp., frequently occur in this lake, and microcystins are present in the water for most of the year [
30,
31,
32]. In a previous study in 2008–2009 [
32,
42], the range of concentration of microcystins in the water column was 1–80 µg equivalent microcystin-LR/L. Lake Yangebup is a groundwater through-flow lake that receives groundwater from its eastern side and discharges it towards the west. An earlier study, over 20 months using a weather station and a thermistor chain in the water column with sensors every 40 cm, showed that Lake Yangebup is mixed without stratification when wind speed is >6 m/s [
28]. Sediment resuspension and redistribution during periods of wind-mixing has been hypothesised to be an important factor responsible for the horizontal distribution of sediments and contaminants in this and a neighbouring lake [
28,
29]. Lake Yangebup is surrounded by patches of fringing vegetation and an earlier investigation has reported the presence of a flocculate sediment layer, consisting mainly of dead and decaying organic matter covering a more consolidated sediment layer [
28].
4.2. Sampling Design
Samples were taken monthly between August and December 2010, from four shore sites of Lake Yangebup with coordinates for sites 1–4 being 32°07'21"S, E115°49'45"; 32°07'07"S, 115°49'31"E; 32°6'50"S, 115°49'43"E; 32°07'11"S, 115°50'07"E, respectively (
Figure 4A). Sampling sites were chosen based on their accessibility and geographic locations with the aim to distribute our sampling sites around the whole lake. All samples were taken between 7:30 am and 1:30 pm from water with a depth of 0.6–0.7 m and were stored on ice, in the dark, for transport to the laboratory. Temperature, pH, salinity were taken
in situ from 10 cm below the water surface (referred to as “surface water”) in all months, and additionally directly above the sediments (referred to as “overlying water”) in October to December with probes (TPS WP-81); the average values of these parameters are given in
Table 3. Dissolved oxygen was only measured at a depth of 10 cm (TPS-DO2). At each site, measurements for both surface water and overlying water were taken above the points where sediment samples were collected. There was no significant difference in temperature and salinity between the surface and the overlying water layer during the sampling period (student
t-test;
Figure 5), indicating that the water column was well mixed. Dissolved and intracellular toxins in surface water samples and cyanobacterial biomass in surface water and overlying water samples were quantified in the laboratory. For each sampling site, three sediment samples (0–4 cm) were collected using a transparent, polycarbonate sediment corer with a stainless-steel cutter (50 mm in diameter). Sediments in each sample were thoroughly mixed for homogenous quantification of cyanobacterial biomass, microcystin concentrations, and physicochemical properties. Wind direction and speed data came from the Australian Bureau of Meteorology’s monitoring station at Jandakot Airport, which is 3 km from Lake Yangebup (
Figure 4B).
Table 3.
Physicochemical parameter means in each sampling month in Lake Yangebup. - indicates no measurement due to failure of the probe. DO = dissolved oxygen.
Table 3.
Physicochemical parameter means in each sampling month in Lake Yangebup. - indicates no measurement due to failure of the probe. DO = dissolved oxygen.
| Month | Temperature (°C) | Salinity (mg/L) | DO (%) | DO (mg/L) | pH |
|---|
| August | 16.4 | 946 | 44.9 | 4.9 | 8.4 |
| September | 18.9 | 947 | 54.9 | 5.0 | 8.6 |
| October | 21.4 | 1115 | 63.3 | 5.6 | 8.4 |
| November | 23.1 | 1202 | - | - | 8.6 |
| December | 22.2 | 1254 | 123.9 | 11.1 | 9.4 |
Figure 5.
Mean (±1 SD) temperature (°C) and salinity (mg/L) in the surface and overlying water layers measured at the four sites in October to December in Lake Yangebup during this study. * = missing data; horizontal line indicates that no significant difference between data were detected (student t-test).
Figure 5.
Mean (±1 SD) temperature (°C) and salinity (mg/L) in the surface and overlying water layers measured at the four sites in October to December in Lake Yangebup during this study. * = missing data; horizontal line indicates that no significant difference between data were detected (student t-test).
The mean daily air temperature, recorded at the nearest weather station to Lake Yangebup, taken over the 14 days prior to the sampling day, was used to represent surface water temperature in the data analysis. This was chosen instead of direct water temperature measurements, as water temperature strongly depends on the time of day sampling takes place. Earlier studies reported a good correlation between water temperature in shallow lakes and air temperature, and found that microcystin concentration in the water could be best predicted by mean daily air temperature of the preceding 14 days [
43].
4.3. Sediment Analysis
The particle size of sediment samples was measured using a method adapted from Bowmanand Hutka [
44]. The adaptations included heating the samples for a quicker digestion of the organic matter and removing the hydrogen peroxide by sequential dilution instead of evaporation. The particle size distribution analyser used was the Malvern Mastersizer 2000 with a Malvern Hydro 2000G automated wet dispersion accessory. Organic matter, measured by loss-on-ignition, was analysed according to Heiri
et al. [
45] but with samples dried in an oven at 110 °C overnight instead of freeze drying. The organic matter content of the sediment samples in Lake Yangebup ranged from 0.55% to 6.17%. With respect to particle size distribution, all sediment samples were sand, consisting of coarse and fine sand particles with a coarse sand content of 88% ± 10.12%. The particle size distribution for sediment samples at each sampling site is given in
Table 4.
Table 4.
Particle size fractions of sediment samples at each sampling site.
Table 4.
Particle size fractions of sediment samples at each sampling site.
| Sampling Sites | Clay % (<2 µm) | Silt % (2–20 µm) | Fine Sand % (20–200 µm) | Coarse Sand % (200–2000 µm) | Total Sand % |
|---|
| Site 1 | 0 | 0 | 13.12 ± 6.45 | 86.88 ± 6.45 | 100 |
| Site 2 | 0 | 0 | 21.05 ± 2.71 | 78.95 ± 2.72 | 100 |
| Site 3 | 0 | 0 | 10.66 ± 2.88 | 89.40 ± 2.88 | 100 |
| Site 4 | 0 | 0 | 3.22 ± 0.87 | 96.78 ± 0.87 | 100 |
4.4. Analysis of Dissolved Microcystins in the Water
Surface water samples, 900–1000 mL for each water sample, were first filtered through pre-combusted GF/C filters (Whatman). Each filtrate was then applied to a solid phase extraction (SPE) cartridge (Oasis HLB 6 cc/500 mg, Waters, Rydalmere, NSW, Australia) with a flow rate <10 mL per minute for cleaning and concentration. The cartridge was then washed with 5 mL of 10% and 20% (
v/v) methanol, in sequence, before blowing a constant air flow through the cartridge for drying. Microcystins were eluted from the cartridge with 5 mL of 100% methanol +0.1% trifluoroacetic acid (
v/v). The elutes were dried with nitrogen gas at 45 °C before being re-dissolved in 1 mL of 30% acetonitrile (
v/v) and analysed by high-performance liquid chromatography (HPLC) with a photodiode detector (1.2 nm resolution; Alliance 2695, Waters Corporation, Rydalmere, NSW, Australia) and an Atlantis
® T3 separation column (4.6 × 150mm i.d, 3 µm, 100 Å; Waters Corporation). The HPLC gradient was identical to Lawton
et al. [
46] but with a maximum of 100% acetonitrile and, therefore, a longer run time of 37 min. Column temperature was 37.5 ± 2.5 °C and the limit of detection was 1.12 ng. Our limit of quantification was 3× the limit of detection. Peaks that showed a typical microcystin absorption spectrum with a maximum at 238.8 nm were quantified by comparing the peak area with the area of a known standard (microcystin-LR; Sapphire, Sydney, Australia).
4.5. Analysis of Intracellular Microcystins in the Water
Water samples were filtered on pre-combusted and pre-weighed GF/C filters (Whatman). The filters were dried at 60 °C, for 24 h, before being re-weighed to quantify biomass and then stored at −21 °C until microcystin extraction. Before the extraction of microcystins, the dry filters were thawed and re-frozen three times. The extraction was achieved with 6 mL of 75% methanol (v/v) per filter. Filters were sonicated on ice for 25 min, followed by gentle shaking for another 25 min. The extracts were then centrifuged at 3273× g (Allegra X-12 Series; Beckman and Coulter Inc., Lane Cove, NSW, Australia) for 10 min at room temperature. Extracts were collected and filters were extracted two more times. After the three extractions, the supernatants were combined and diluted with Milli-Q water from 75% to 20% methanol (v/v) before they were applied to SPE cartridges (Oasis HLB 6 cc/500 mg, Waters, Australia). The subsequent analysis procedure was identical to that used for the dissolved microcystin analysis.
4.6. Analysis of Microcystins in the Sediments
The method used for quantification of microcystins in the sediments was adapted from Babica
et al. [
12]. In short, each freeze-dried sediment sample, 2.0–2.2 g (dry mass), was extracted twice with 20 mL of 5% acetic acid in methanol containing 0.2%
v/v trifluoroacetic acid (TFA), using an ultrasonic bath for 30 min. After each extraction, the sample was centrifuged for 10 min at 3273×
g (Allegra X-12, Beckman Coulter) and the supernatants from both extractions were combined, diluted with Milli-Q water from 95% to 20% methanol (
v/v) and then applied to SPE cartridges (Oasis HLB 6 cc/500 mg, Waters, Australia). The subsequent analysis procedures, such as the cleaning and concentration of microcystins using SPE cartridges and the quantification of microcystins with HPLC, were identical to those used for the dissolved microcystin analysis. In a preliminary laboratory test, we determined the recovery rate for easily extractable microcystin-LR in sediment samples using this method was above 87% (data not shown).
Throughout this study, we refer to the total concentration of microcystin variants per sample as microcystin concentration. Total microcystin concentration (intracellular + dissolved) was expressed as micrograms (microcystin-LR mass equivalents) per litre water, intracellular microcystin concentration was expressed as micrograms (microcystin-LR mass equivalents) per gram cyanobacterial dry mass, and the total concentration of microcystin variants per sediment (easily extractable microcystins) was micrograms (microcystin-LR mass equivalents) per gram dry sediments.
4.7. Cyanobacterial Biomass in the Sediments and Water
The cyanobacterial biomass of each water sample was measured in the laboratory with a bench top version of the FluoroProbe (BBE Moldaenke, Schwentinental, Germany) as µg chl-
a/L [
47,
48], for which chorophyll-
a is a proxy for cyanobacterial biomass. The validation of the measurement of total chlorophyll-
a with the Fluoroprobe against the values obtained from samples extracted according to standard methods [
49] was described in Sinang
et al. [
32]. A strong linear correlation was found between the chlorophyll-
a concentration measured with FluoroProbe and the chlorophyll-
a concentration measured with the standard method (
R2 = 0.94,
N = 32,
p < 0.05). Sediment samples were washed with tap water until no cyanobacteria colonies in the sediments could be observed microscopically. The tap water was filtered through gauze (63 µm) to capture these colonies, which were then transferred with 30 mL of deionized water into a 100 mL glass bottle. This washing procedure was repeated twice and the cyanobacterial colonies were combined in a total of 100 mL deionized water. The concentration of cyanobacterial biomass in this bottle was quantified using the bench top version of the FluoroProbe as µg chl-
a/L.
4.8. Statistical Analyses
Differences between sites were analysed using a one-way analysis of variance (ANOVA) when the data was normally distributed, and a Kruskal-Wallis ANOVA on ranks with a Bonferroni t-test when the data failed the normality test. Differences between months were analysed with a repeated-measures ANOVA with a Bonferroni t-test when the data was normally distributed, and a Friedman repeated-measures ANOVA on ranks with a Bonferroni t-test when the data failed the normality test. Pearson’s correlations were calculated to identify the correlation between the concentration of microcystins in lake sediments and biological and physical parameters.
Two multiple linear regression analysis (backward) were carried out on the log transformed data to identify models that best explained the variability in the concentration of microcystins in the sediments. The first analysis included the following explanatory variables, which are representative for systems that are vertically well-mixed: cyanobacterial biomass in the sediment (c(CBsedim); µg chl-a/g d.m.); total microcystin concentration in the water (c(tMCwater)); µg/L); average cyanobacterial biomass in water (c(CBaverage); µg/L); and pH and temperature. The second analysis included explanatory variables, which present processes on a smaller local scales: cyanobacterial biomass in the sediment (c(CBsedim); µg chl-a/g d.m.); intracellular microcystin concentration in the water (c(MCintra)); µg/L); dissolved microcystin concentration in the water (c(MCdiss)); µg/L); cyanobacterial biomass in overlying (c(CBoverl); µg/L) and surface water (c(CBsurf); µg/L); and pH and temperature. We used 0.05 as the probability of F-to-remove. Prior to the statistical analyses, the linearity of the microcystin concentration in sediments and the biological and physical parameters hypothesised to control microcystin variability were tested using scatterplots. Both dependent and independent variables were log-transformed and checked for normality using a Kologorov-Smirnov test. Statistical analyses were conducted in SigmaPlot 12.0 (Systat Software Int., San Jose, CA, USA) and IBM SPSS 21.0 (SPSS Inc., Chicago, IL, USA). Significance levels were set as p < 0.05 unless stated otherwise.