Novel Treatment of Chronic Bladder Pain Syndrome and Other Pelvic Pain Disorders by OnabotulinumtoxinA Injection
Abstract
:1. Introduction
2. History of BoNT-A in LUTDs
3. Mechanism Action of BoNT-A on CPP
3.1. Effectiveness of BoNT-A in the Treatment of Pelvic Pain
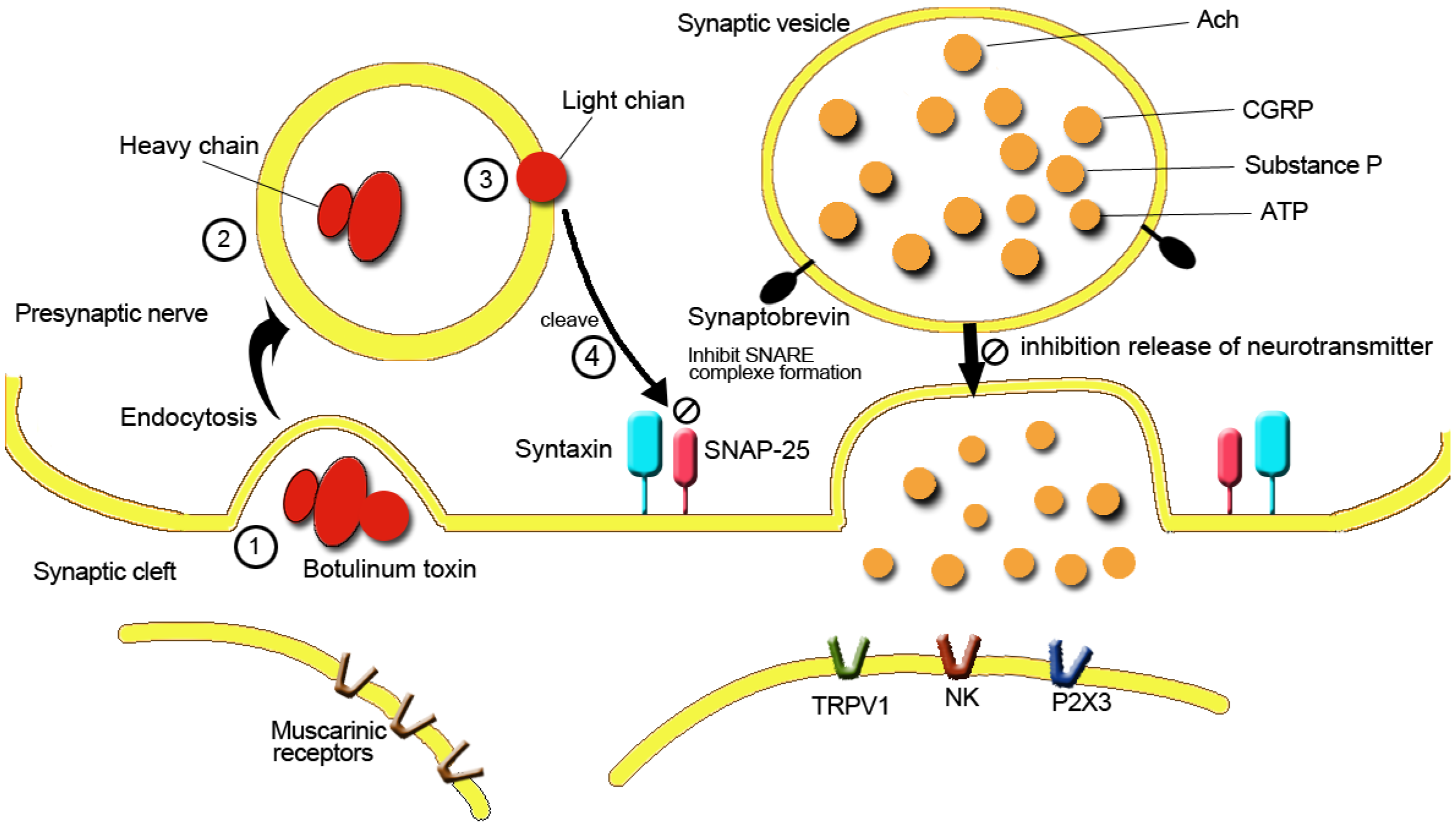
3.2. Reducing Pelvic Muscle Spasm and Pain
3.3. Reduction of Central and Peripheral Nerve Sensitization and Decrease of Noxious Neurotransmitter Release
3.4. Reducing Neurogenic Inflammation
4. Clinical Application of BoNT-A in IC/BPS
| Authors, year | N | Follow-up | BoNT-A preparation, dose | Injection sites and volume | Frequency, Δ% | VAS, Δ% | ICPI, ICSI, Δ% | LoE | Others |
|---|---|---|---|---|---|---|---|---|---|
| Smith et al. 2004 [21] | 13 | 3 mo | 100 U to 200 U, 10 to 20 mL | 20 to 30 sites, trigone and bladder floor | −44% * | −79% * | −69,−71% * | 3 | - |
| Giannantoni et al. 2006 [63] | 14 | 3 mo | 200 U, 20 mL, | 20 sites, bladder floor and trigone | −35% * | −34% * | - | 3 | - |
| Giannantoni et al. 2008 [67] | 15 | 3 mo | 200 U, 20 mL, | 20 sites, bladder floor and trigone | −43% * | −28% * | - | 3 | - |
| Kuo and Chancellor 2009 [64] | 15 | 3 mo | 200 U 20 mL + HD 100 U 20 mL + HD HDonly | 40 sites, bladder floor except trigone | −34% * | −55% * | −42, −36% * | 2 | - |
| 29 | −25% | −39% * | −38, −35% * | ||||||
| 23 | −14% | −18% | −23, −23% * | ||||||
| Chung et al. 2012 [65] | 67 | 6 mo | 100 U, 20 mL | 40 sites, bladder floor except trigone | −31% * | −37% * | −38, −34% * | 3 | - |
| Kuo, 2013 [69] | 81 | 12 mo | 100 U, 20 mL, 1 injection | 40 sites, bladder floor except trigone | −23% * | −30% * | −28,−27% * | 3 | Repeat injection better than single injection |
| 30 | 100 U, 20 mL, 4 injections | −21% * | −37% * | ||||||
| Lee et al. 2013 [70] | 10 ulcer | 6 mo | 100 U, 20 mL, 4 injection | 40 sites, bladder floor except trigone | 0% | −10% | 0%, −8% | 2 | BoNT-A injection is not effective in ulcer IC/BPS |
| 30 non ulcer | 100 U, 20 mL, 4 injection | −68 * | −62% * | −65% *, −54% * | |||||
| Pinto et al. 2014 [71] | 10 ulcer | 1 mo | 100 U, 10 mL | 10 sites, trigone only | −29% * | −54% * | −46% *, −40% * | 2 | BoNT-A injection is effective in ulcer IC/BPS |
| 14 non ulcer | 100 U, 10 mL | −23% * | −57% * | −47% *, −45% * | |||||
| Kuo et al. 2015 [66] | 40 | 2 mo | 100 U, 10 mL, +HD | 20 sites, bladder floor except trigone | −27% * | −49% *† | −40% *, −34% * | 1 | Randomized study |
| 20 | Normal saline 10 mL +HD | −9% | −24% | −30% *, 21% * |
5. Clinical Application of BoNT-A in CP/CPPS
6. Clinical Application of BoNT-A in Pelvic Floor Muscle and Fascial Pain
| Authors, year | N | Follow-up | BoNT-A dose | Injection sites | Results |
|---|---|---|---|---|---|
| Zermann et al. 2000 [85] | 11 | 2–4 weeks | 200 U | transurethral perisphincteric injection | 1. relief of prostatic pain and urethral hypersensitivity/hyperalgesia |
| 2. decrease of the urethral sphincter closure pressure and increase maxima flow rate | |||||
| Gottsch et al. 2011 [86] | 29 | 1 mo | 100 U or normal saline | perineal body and bulbospongiosus muscle. | 1. 30% response rate for BoNT-A treatment compared with 13% for placebo (p = 0.0002). |
| 2. Pain score significantly better in BoNT-A group | |||||
| Falahatkar et al. 2014 [87] | 30 | 1, 3, 6 mo | 100 or 200 U Normal saline | transurethral intraprostatic injection into 3 different points of each lobe | 1. NIH-CPSI total and subscale scores and urinary frequency had significantly improved in BoNT-A injection, no significant improvement in placebo group |
| 30 | 2. Pain score decreased by 64.76%, 75.63%, and 79.97% |
| Authors, year | N | Follow-up | BoNT-A dose | Injection sites | Dyspareunia, Δ% | Non-menstrual pelvic pain, Δ% | Pelvic floor pressure, Δ% |
|---|---|---|---|---|---|---|---|
| Jarvis et al. 2004 [94] | 12 | 4 weeks | 40 U | bilaterally puborectalis and pubococcygeus muscles | −65% * | −42% | −37% * |
| Abbott et al. 2006 [22] | 30 | 6 mo | 80 U | pelvic floor muscles | −81% * | −57% * | −35% *† |
| 30 | Normal saline | −58% * | −18% | −11% * | |||
| Nesbitt-Hawes et al. 2013 [95] | 26 | 26 weeks | 100 U single | puborectalis and pubococcygeous muscles | −44% * | −32% * | −17.5% * |
| 11 | 100 U repeat | −55% * |
7. Other Applications of BoNT-A in CPP
8. Adverse Events of BoNT-A injection in CPP
9. Conclusions
Author Contributions
Conflicts of Interest
References
- Fall, M.; Baranowski, A.P.; Elneil, S.; Engeler, D.; Hughes, J.; Messelink, E.J.; Oberpenning, F.; de C Williams, A.C. European Association of Urology. EAU guidelines on chronic pelvic pain. Eur. Urol. 2010, 57, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Dressler, D.; Adib Saberi, F. Botulinum toxin: Mechanisms of action. Eur. Neurol. 2005, 53, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.B. Botulinum toxin injection of eye muscles to correct strabismus. Trans. Am. Ophthalmol. Soc. 1981, 79, 734–770. [Google Scholar] [PubMed]
- Charles, P.D. Botulinum neurotoxin serotype A: A clinical update on non-cosmetic uses. Am. J. Health Syst. Pharm. 2004, 15, S11–S23. [Google Scholar]
- Brown, E.A.; Schütz, S.G.; Simpson, D.M. Botulinum toxin for neuropathic pain and spasticity: An overview. Pain Manag. 2014, 4, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Kharkar, S.; Ambady, P.; Venkatesh, Y.; Schwartzman, R.J. Intramuscular botulinum toxin in complex regional pain syndrome: Case series and literature review. Pain Phys. 2011, 14, 419–424. [Google Scholar]
- Karsenty, G.; Denys, P.; Amarenco, G.; de Seze, M.; Gamé, X.; Haab, F.; Kerdraon, J.; Perrouin-Verbe, B.; Ruffion, A.; Saussine, C.; et al. Botulinum toxin A (Botox) intradetrusor injections in adults with neurogenic detrusor overactivity/neurogenic overactive bladder: A systematic literature review. Eur. Urol. 2008, 53, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Sacco, E.; Bientinesi, R.; Marangi, F.; Totaro, A.; D’Addessi, A.; Racioppi, M.; Pinto, F.; Vittori, M.; Bassi, P. Patient-reported outcomes in men with lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH) treated with intraprostatic OnabotulinumtoxinA: 3-month results of a prospective single-armed cohort study. BJU Int. 2012, 110, E837–E844. [Google Scholar] [CrossRef] [PubMed]
- Adelowo, A.; Hacker, M.R.; Shapiro, A.; Modest, A.M.; Elkadry, E. Botulinum toxin type A (BOTOX) for refractory myofascial pelvic pain. Female Pelvic Med. Reconstr. Surg. 2013, 19, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Erbguth, F.J. Historical notes on botulism, Clostridium botulinum, botulinum toxin, and the idea of the therapeutic use of the toxin. Mov. Disord. 2004, 19, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Tessmer, S.P.; Sommer, H. Studies on Botulinus Toxin: 3. Acid Precip. Botulinus Toxin J. Infect. Dis. 1928, 43, 152–160. [Google Scholar]
- Dykstra, D.D.; Sidi, A.A.; Scott, A.B.; Pagel, J.M.; Goldish, G.D. Effects of botulinum A toxin on detrusor-sphincter dyssynergia in spinal cord injury patients. J. Urol. 1988, 139, 919–922. [Google Scholar] [PubMed]
- Schurch, B.; Stohrer, M.; Kramer, G.; Schmid, D.M.; Gaul, G.; Hauri, D. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: A new alternative to anticholinergic drugs? Preliminary results. J. Urol. 2000, 164, 692–697. [Google Scholar] [CrossRef]
- Giannantoni, A.; Mearini, E.; Del Zingaro, M; Porena, M. Six-year follow-up of botulinum toxin A intradetrusorial injections in patients with refractory neurogenic detrusor overactivity: Clinical and urodynamic results. Eur Urol. 2009, 55, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Visco, A.G.; Brubaker, L.; Richter, H.E.; Nygaard, I.; Paraiso, M.F.; Menefee, S.A.; Schaffer, J.; Lowder, J.; Khandwala, S.; Sirls, L.; et al. Anticholinergic therapy vs. onabotulinumtoxinA for urgency urinary incontinence. N. Engl. J. Med. 2012, 367, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. FDA approves Botox to treat specific form of urinary incontinence. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm269509.htm (accessed on 25 August 2011).
- U.S. Food and Drug Administration. FDA approves Botox to treat overactive bladder. Available online: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm336101.htm (accessed on 18 January 2013).
- Duthie, J.B.; Vincent, M.; Herbison, G.P.; Wilson, D.I.; Wilson, D. Botulinum toxin injections for adults with overactive bladder syndrome. Cochrane Database Syst. Rev. 2011, 7, CD005493. [Google Scholar]
- Giannantoni, A.; Conte, A.; Farfariello, V.; Proietti, S.; Vianello, A.; Nardicchi, V.; Santoni, G.; Amantini, C. Onabotulinumtoxin-A intradetrusorial injections modulate bladder expression of NGF, TrkA, p75 and TRPV1 in patients with detrusor overactivity. Pharmacol Res. 2013, 68, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Conte, A.; Giannantoni, A.; Proietti, S.; Giovannozzi, S.; Fabbrini, G.; Rossi, A.; Porena, M.; Berardelli, A. Botulinum toxin A modulates afferent fibers in neurogenic detrusor overactivity. Eur. J. Neurol. 2012, 19, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.P.; Radziszewski, P.; Borkowski, A.; Somogyi, G.T.; Boone, T.B.; Chancellor, M.B. Botulinum toxin A has antinociceptive effects in treating interstitial cystitis. Urology 2004, 64, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Abbott, J.A.; Jarvis, S.K.; Lyons, S.D.; Thomson, A.; Vancaille, T.G. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: A randomized controlled trial. Obstet Gynecol. 2006, 108, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Strauss, A.C.; Dimitrakov, J.D. New treatments for chronic prostatitis/chronic pelvic pain syndrome. Nat. Rev. Urol. 2010, 7, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Kim, D.K.; Chiang, P.H.; Chancellor, M.B. Bladder botulinum toxin A injection can benefit patients with radiation and chemical cystitis. BJU Int. 2008, 102, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.L.; Yang, F.; Zhan, H.L.; Feng, Z.Y.; Zhang, Z.G.; Li, W.B.; Zhou, X.F. Increased severity of inflammation correlates with elevated expression of TRPV1 nerve fibers and nerve growth factor on interstitial cystitis/bladder pain syndrome. Urol. Int. 2014, 92, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Tempest, H.V.; Dixon, A.K.; Turner, W.H.; Elneil, S.; Sellers, L.A.; Ferguson, D.R. P2X and P2X receptor expression in human bladder urothelium and changes in interstitial cystitis. BJU Int. 2004, 93, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, E.; Luongo, L.; de Novellis, V.; Rossi, F.; Marabese, I.; Maione, S. Transient receptor potential vanilloid type 1 and pain development. Curr. Opin. Pharmacol. 2012, 12, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Cheng, J.; Dai, J.; Zhang, D. Botulinum toxin decreases hyperalgesia and inhibits P2X3 receptor over-expression in sensory neurons induced by ventral root transection in rats. Pain Med. 2011, 12, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.B. NGF as a mediator of inflammatory pain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996, 351, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.H.; Peng, C.H.; Liu, H.T.; Kuo, H.C. Increased pro-inflammatory cytokines, C-reactive protein and nerve growth factor expressions in serum of patients with interstitial. PLoS ONE 2013, 17, e76779. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.C.; Zhang, W.; Yan, S.; Liu, Y.L.; Wang, P. Urinary nerve growth factor could be a biomarker for interstitial cystitis/painful bladder syndrome: A meta-analysis. PLoS ONE 2014, 9, e106321. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.J.; Moldwin, R.M.; Cossons, N.; Darekar, A.; Mills, I.W.; Scholfield, D. Proof of concept trial of tanezumab for the treatment of symptoms associated with interstitial cystitis. J. Urol. 2011, 185, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, M.L.; Mateus-Vasconcelos, E.C.; Rosa e Silva, J.C.; Nogueira, A.A.; Dos Reis, F.J.; Poli Neto, O.B. Importance of pelvic muscle tenderness evaluation in women with chronic pelvic pain. Pain 2010, 11, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Tu, F.F.; As-Sanie, S.; Steege, J.F. Prevalence of pelvic musculoskeletal disorders in a female chronic pelvic pain clinic. J. Reprod. Med. 2006, 51, 185–189. [Google Scholar] [PubMed]
- Arezzo, J.C. Possible mechanisms for the effects of botulinum toxin on pain. Clin. J. Pain 2002, 18, S125–S132. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.G.; McMahon, S.B. ATP as a peripheral mediator of pain. J. Auton. Nerv. Syst. 2000, 81, 187–194. [Google Scholar] [CrossRef]
- Kaya, S.; Hermans, L.; Willems, T.; Roussel, N.; Meeus, M. Central sensitization in urogynecological chronic pelvic pain: A systematic literature review. Pain Phys. 2013, 16, 291–308. [Google Scholar]
- Aoki, K.R. Evidence for antinociceptive activity of botulinum toxin type A in pain management. Headache 2003, 43, S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Foran, P.G.; Mohammed, N.; Lisk, G.O.; Nagwaney, S.; Lawrence, G.W.; Johnson, E.; Smith, L.; Aoki, K.R.; Dolly, J.O. Evaluation of the therapeutic usefulness of botulinum neurotoxin B, C1, E, and F compared with the long lasting type A. Basis for distinct durations of inhibition of exocytosis in central neurons. J. Biol. Chem. 2003, 278, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Durham, P.L.; Cady, R. Regulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: Implications for migraine therapy. Headache 2004, 44, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Khera, M.; Somogyi, G.T.; Kiss, S.; Boone, T.B.; Smith, C.P. Botulinum toxin A inhibits ATP release from bladder urothelium after chronic spinal cord injury. Neurochem. Int. 2004, 45, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; van Houdenhove, B.; Oostendorp, R.A. Recognition of central sensitization in patients with musculoskeletal pain: Application of pain neurophysiology in manual therapy practice. Man. Ther. 2010, 15, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, F.; Rossi, C.; Gianfranceschi, L.; Rossetto, O.; Caleo, M. Long-distance retrograde effects of botulinum neurotoxin A. J. Neurosci. 2008, 28, 3689–3696. [Google Scholar] [CrossRef] [PubMed]
- Bach-Rojecky, L.; Lacković, Z. Central origin of the antinociceptive action of botulinum toxin type A. Pharmacol. Biochem. Behav. 2009, 94, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Akaike, N.; Shin, M.C.; Wakita, M.; Torii, Y.; Harakawa, T.; Ginnaga, A.; Kato, K.; Kaji, R.; Kozaki, S. Transsynaptic inhibition of spinal transmission by A2 botulinum toxin. J. Physiol. 2013, 15, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Drinovac, V.; Bach-Rojecky, L.; Matak, I.; Lacković, Z. Involvement of μ-opioid receptors in antinociceptive action of botulinum toxin type A. Neuropharmacology 2013, 70, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Restani, L.; Antonucci, F.; Gianfranceschi, L.; Rossi, C.; Rossetto, O.; Caleo, M. Evidence for anterograde transport and transcytosis of botulinum neurotoxin A (BoNT/A). J. Neurosci. 2011, 31, 15650–15659. [Google Scholar] [CrossRef] [PubMed]
- Papagiannopoulou, D.; Vardouli, L.; Dimitriadis, F.; Apostolidis, A. Retrograde transport of radiolabelled botulinum neurotoxin type a (bont/a) to the central nervous system following intradetrusor injection in rats. BJU Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Kuo, H.C. Increased urine and serum nerve growth factor levels in interstitial cystitis suggest chronic inflammation is involved in the pathogenesis of disease. PLoS ONE 2012, 7, e44687. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Chen, H.; Yang, J.; Wang, L.; Cui, Y.; Guan, X.; Wang, Z.; Niu, J.; Zu, X.; Qi, L.; et al. Development and validation of an animal model of prostate inflammation-induced chronic pelvic pain: Evaluating from inflammation of the prostate to pain behavioral modifications. PLoS ONE 2014, 13, e96824. [Google Scholar] [CrossRef] [PubMed]
- Graziottin, A.; Skaper, S.D.; Fusco, M. Mast cells in chronic inflammation, pelvic pain and depression in women. Gynecol. Endocrinol. 2014, 30, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Logadottir, Y.; Delbro, D.; Fall, M.; Gjertsson, I.; Jirholt, P.; Lindholm, C.; Peeker, R. Cytokine expression in patients with bladder pain syndrome/interstitial cystitis ESSIC type 3C. J. Urol. 2014, 192, 1564–1568. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.S.; Xie, A.; La, J.H.; Gebhart, G.F. Nociceptive and Inflammatory Mediator Upregulation in a Mouse Model of Chronic Prostatitis. Pain 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Ameda, K.; Furuno, T.; Okada, H.; Date, I.; Kakizaki, H. Evaluation of Prostaglandin E2 and E-Series Prostaglandin Receptor in Patients with Interstitial Cystitis. J. Urol. 2015, 193, 1987–1993. [Google Scholar] [CrossRef] [PubMed]
- Shie, J.H.; Kuo, H.C. Higher levels of cell apoptosis and abnormal E-cadherin expression in the urothelium are associated with inflammation in patients with interstitial cystitis/painful bladder syndrome. BJU Int. 2011, 108, E136–E141. [Google Scholar] [CrossRef] [PubMed]
- Hegele, A.; Knippschild, S.; Frohme, C.; Hänze, J.; Olbert, P.; Hofmann, R. Changes in prostaglandin E2 in patients with idiopathic overactive bladder syndrome after botulinum toxin type A treatment: Is there a clinical benefit? BMC Urol. 2014, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.P.; Gangitano, D.A.; Munoz, A.; Salas, N.A.; Boone, T.B.; Aoki, K.R.; Francis, J.; Somogyi, G.T. Botulinum toxin type A normalizes alterations in urothelial ATP and NO release induced by chronic spinal cord injury. Neurochem. Int. 2008, 52, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Benemei, S.; Nicoletti, P.; Capone, J.G.; Geppetti, P. CGRP receptors in the control of pain and inflammation. Curr. Opin. Pharmacol. 2009, 9, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Van de Merwe, J.P.; Nordling, J.; Bouchelouche, P.; Bouchelouche, K.; Cervigni, M.; Daha, L.K.; Elneil, S.; Fall, M.; Hohlbrugger, G.; Irwin, P.; et al. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: An ESSIC proposal. Eur. Urol. 2008, 53, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Shie, J.H.; Liu, H.T.; Kuo, H.C. Increased Cell Apoptosis of Urothelium Mediated by Inflammation in Interstitial Cystitis/Painful Bladder Syndrome. Urology 2012, 79, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Keay, S.K.; Birder, L.A.; Chai, T.C. Evidence for bladder urothelial pathophysiology in functional bladder disorders. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Hanno, P.M.; Burks, D.A.; Clemens, J.Q.; Dmochowski, R.R.; Erickson, D.; Fitzgerald, M.P.; Forrest, J.B.; Gordon, B.; Gray, M.; Mayer, R.D.; et al. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J. Urol. 2011, 185, 2162–2170. [Google Scholar] [CrossRef] [PubMed]
- Giannantoni, A.; Costantini, E.; di Stasi, S.M.; Tascini, M.C.; Bini, V.; Porena, M. Botulinum A toxin intravesical injections in the treatment of painful bladder syndrome: A pilot study. Eur. Urol. 2006, 49, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C.; Chancellor, M.B. Comparison of intravesical botulinum toxin type A injections plus hydrodistention with hydrodistention alone for the treatment of refractory interstitial cystitis/painful bladder syndrome. BJU Int. 2009, 104, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.D.; Kuo, Y.C.; Kuo, H.C. Intravesical onabotulinumtoxinA injections for refractory painful bladder syndrome. Pain Phys. 2012, 15, 197–202. [Google Scholar]
- Kuo, H.C.; Jiang, Y.H.; Tsai, Y.C.; Kuo, Y.C. Intravesical botulinum toxin-A injection reduce bladder pain of interstital cystitis/bladder pain syndrome refractory to conventional treatment—A prospective, multicenter, randomized, double blind, placebo-controlled clinical trial. Neuroruol. Urodyn. 2015. [Google Scholar] [CrossRef]
- Giannantoni, A.; Porena, M.; Costantini, E.; Zucchi, A.; Mearini, L.; Mearini, E. Botulinum A toxin intravesical injection in patients with painful bladder syndrome: 1-year followup. J. Urol. 2008, 179, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.; Lopes, T.; Silva, J.; Silva, C.; Dinis, P.; Cruz, F. Persistent therapeutic effect of repeated injections of onabotulinum toxin a in refractory bladder pain syndrome/interstitial cystitis. J. Urol. 2013, 189, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C. Repeated onabotulinumtoxin-a injections provide better results than single injection in treatment of painful bladder syndrome. Pain Phys. 2013, 16, E15–E23. [Google Scholar]
- Lee, C.L.; Kuo, H.C. Intravesical botulinum toxin a injections do not benefit patients with ulcer type interstitial cystitis. Pain Phys. 2013, 16, 109–116. [Google Scholar]
- Pinto, R.; Lopes, T.; Costa, D.; Barros, S.; Silva, J.; Silva, C.; Cruz, C.; Dinis, P.; Cruz, F. Ulcerative and nonulcerative forms of bladder pain syndrome/interstitial cystitis do not differ in symptom intensity or response to onabotulinum toxin A. Urology 2014, 83, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Kuo, H.C. Intravesical botulinum toxin A injections plus hydrodistension can reduce nerve growth factor production and control bladder pain in interstitial cystitis. Urology 2007, 70, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.; Lopes, T.; Silva, J. Clinical Response to Intra-Trigonal Onabotulinum Toxin A Injections is not Related to the Presence of Ulcers in Bladder Pain Syndrome/Interstitial Cystitis Patient; AUA abstract: Linthicum, MD, USA, 2013. [Google Scholar]
- Shie, J.H.; Liu, H.T.; Wang, Y.S.; Kuo, H.C. Immunohistochemical evidence suggests repeated intravesical application of botulinum toxin A injections may improve treatment efficacy of interstitial cystitis/bladder pain syndrome. BJU Int. 2013, 111, 638–446. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.H.; Jhang, J.F.; Shie, J.H.; Kuo, H.C. Down regulation of vascular endothelial growth factor is associated with decreased inflammation after intravesical OnabotulinumtoxinA injections combined with hydrodistention for patients with interstitial cystitis--clinical results and immunohistochemistry analysis. Urology 2013, 82, 1452.e1–1452.e6. [Google Scholar] [PubMed]
- Scaldaferri, F.; Vetrano, S.; Sans, M.; Arena, V.; Straface, G.; Stigliano, E.; Repici, A.; Sturm, A.; Malesci, A.; Panes, J.; et al. VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology 2009, 136, 585–595. [Google Scholar] [CrossRef] [PubMed]
- De la Rosette, J.J.; Hubregtse, M.R.; Meuleman, E.J.; Stolk-Engelaar, M.V.; Debruyne, F.M. Diagnosis and treatment of 409 patients with prostatitis syndromes. Urology 1993, 41, 301–307. [Google Scholar] [CrossRef]
- Krieger, J.N.; Nyberg, L.; Nickel, J.C. NIH consensus definition and classification of prostatitis. JAMA 1999, 282, 236–237. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.M.; Stafford, R.S.; O’Leary, M.P.; Barry, M.J. How common is prostatitis? A national survey of physician visits. J. Urol. 1998, 159, 1224–1228. [Google Scholar] [CrossRef]
- Anothaisintawee, T.; Attia, J.; Nickel, J.C.; Thammakraisorn, S.; Numthavaj, P.; McEvoy, M.; Thakkinstian, A. Management of chronic prostatitis/chronic pelvic pain syndrome: A systematic review and network meta-analysis. JAMA 2011, 305, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Pavone-Macaluso, M. Chronic prostatitis syndrome: A common but poorly understood condition. part II. EAU-EBU Update Ser. 2007, 5, 16–25. [Google Scholar] [CrossRef]
- Rees, J.; Abrahams, M.; Doble, A.; Cooper, A.; The Prostatitis Expert Reference Group (PERG). Diagnosis and treatment of chronic bacterial prostatitis and chronic prostatitis/chronic pelvic pain syndrome: a consensus guideline. BJU Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Maria, G.; Destito, A.; Lacquaniti, S.; Bentivoglio, A.R.; Brisinda, G.; Albanese, A. Relief by botulinum toxin of voiding dysfunction due to prostatitis. Lancet 1998, 22, 625. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Yoshimura, N.; Huang, C.C.; Wu, M.; Chiang, P.H.; Chancellor, M.B. Intraprostatic botulinum toxin A injection inhibits cyclooxygenase-2 expression and suppresses prostatic pain on capsaicin induced prostatitis model in rat. J. Urol. 2008, 180, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Zermann, D.H.; Ishigooka, M.; Schubert, J.; Schmidt, R.A. Perisphincteric injection of botulinum toxin type A. A treatment option for patients with chronic prostatic pain? Eur. Urol. 2000, 38, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Gottsch, H.P.; Yang, C.C.; Berger, R.E. A pilot study of botulinum toxin A for male chronic pelvic pain syndrome. Scand. J. Urol. Nephrol. 2011, 45, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Falahatkar, S.; Shahab, E.; Gholamjani Moghaddam, K.; Kazemnezhad, E. Transurethral Intraprostatic Injection of Botulinum Toxin Type A for the Treatment of Chronic Prostatitis/Chronic Pelvic Pain Syndrome: Results of a Prospective Pilot Double-Blind and Randomized Placebo-Controlled study. BJU Int. 2014. [Google Scholar] [CrossRef]
- Hetric, D.C.; Ciol, M.A.; Rothman, I.; Turner, J.A.; Frest, M.; Berger, R.E. Musculoskeletal dysfunction in men with chronic pelvic pain syndrome type III: A case-control study. J. Urol. 2003, 170, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Clemens, J.Q.; Nadler, R.B.; Schaeffer, A.J.; Belani, J.; Albaugh, J.; Bushman, W. Biofeedback, pelvic floor re-education, and bladder training for male chronic pelvic pain syndrome. Urology 2000, 56, 951–955. [Google Scholar] [CrossRef]
- Anderson, R.U.; Sawyer, T.; Wise, D.; Morey, A.; Nathanson, B.H. Painful myofascial trigger points and pain sites in men with chronic prostatitis/chronic pelvic pain syndrome. J. Urol. 2009, 182, 2753–2758. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, M.P.; Anderson, R.U.; Potts, J.; Payne, C.K.; Peters, K.M.; Clemens, J.Q.; Kotarinos, R.; Fraser, L.; Cosby, A.; Fortman, C.; et al. Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J. Urol. 2009, 182, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Cornel, E.B.; van Haarst, E.P.; Schaarserg, R.W.; Geels, J. The effect of biofeedback physical therapy in men with chronic pelvic pain syndrome type III. Eur. Urol. 2005, 47, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.E.; Young, C.J.; Young, J.M.; Solomon, M.J. Systematic review of randomized controlled trials of the effectiveness of biofeedback for pelvic floor dysfunction. Br. J. Surg. 2008, 95, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, S.K.; Abbott, J.A.; Lenart, M.B.; Steensma, A.; Vancaillie, T.G. Pilot study of botulinum toxin type A in the treatment of chronic pelvic pain associated with spasm of the levator ani muscles. Aust. N. Z. J. Obstet. Gynaecol. 2004, 44, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt-Hawes, E.M.; Won, H.; Jarvis, S.K.; Lyons, S.D.; Vancaillie, T.G.; Abbott, J.A. Improvement in pelvic pain with botulinum toxin type A-Single vs. repeat injections. Toxicon 2013, 63, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Bhide, A.A.; Puccini, F.; Khullar, V.; Elneil, S.; Digesu, G.A. Botulinum neurotoxin type A injection of the pelvic floor muscle in pain due to spasticity: A review of the current literature. Int. Urogynecol. J. 2013, 24, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.Y.; Tan, K.H. Botulinum toxin A for myofascial trigger point injection: A qualitative systematic review. Eur. J. Pain 2007, 11, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Cayan, S.; Coşkun, B.; Bozlu, M.; Acar, D.; Akbay, E.; Ulusoy, E. Botulinum toxin type A may improve bladder function in a rat chemical cystitis model. Urol. Res. 2003, 30, 399–404. [Google Scholar] [PubMed]
- Chu, P.S.; Ma, W.K.; Wong, S.C.; Chu, R.W.; Cheng, C.H.; Wong, S.; Tse, J.M.; Lau, F.L.; Yiu, M.K.; Man, C.W. The destruction of the lower urinary tract by ketamine abuse: A new syndrome? BJU Int. 2008, 102, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xie, K.; Cai, Y.; et al. Treatment ketamine-related bladder dysfunction by intravesical injection of botulinum toxin A. J. Third Mil. Med. Univ. 2012, 34, 1120–1122. [Google Scholar]
- Lieb, M.; Bader, M.; Palm, U.; Stief, C.G.; Baghai, T.C. Ketamine-induced vesicopathy. Psychiatr Prax. 2012, 39, 43–45. [Google Scholar] [PubMed]
- Soljanik, I. Efficacy and safety of botulinum toxin A intradetrusor injections in adults with neurogenic detrusor overactivity/neurogenic overactive bladder: A systematic review. Drugs 2013, 73, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, D.; Gousse, A.; Keppenne, V.; Sievert, K.D.; Thompson, C.; Lam, W.; Brin, M.F.; Jenkins, B.; Haag-Molkenteller, C. Phase 3 efficacy and tolerability study of onabotulinumtoxinA for urinary incontinence from neurogenic detrusor overactivity. J. Urol. 2012, 187, 2131–2139. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jhang, J.-F.; Kuo, H.-C. Novel Treatment of Chronic Bladder Pain Syndrome and Other Pelvic Pain Disorders by OnabotulinumtoxinA Injection. Toxins 2015, 7, 2232-2250. https://doi.org/10.3390/toxins7062232
Jhang J-F, Kuo H-C. Novel Treatment of Chronic Bladder Pain Syndrome and Other Pelvic Pain Disorders by OnabotulinumtoxinA Injection. Toxins. 2015; 7(6):2232-2250. https://doi.org/10.3390/toxins7062232
Chicago/Turabian StyleJhang, Jia-Fong, and Hann-Chorng Kuo. 2015. "Novel Treatment of Chronic Bladder Pain Syndrome and Other Pelvic Pain Disorders by OnabotulinumtoxinA Injection" Toxins 7, no. 6: 2232-2250. https://doi.org/10.3390/toxins7062232
APA StyleJhang, J.-F., & Kuo, H.-C. (2015). Novel Treatment of Chronic Bladder Pain Syndrome and Other Pelvic Pain Disorders by OnabotulinumtoxinA Injection. Toxins, 7(6), 2232-2250. https://doi.org/10.3390/toxins7062232








