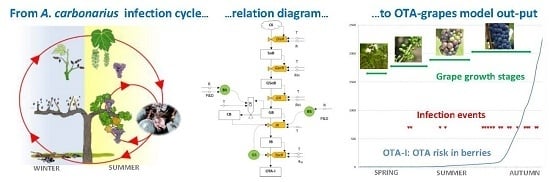
OTA-Grapes: A Mechanistic Model to Predict Ochratoxin A Risk in Grapes, a Step beyond the Systems Approach
Abstract
:1. Introduction
2. Results
2.1. Prototype Predictive Model OTA-Grapes

2.2. Overwintering Inoculum (Oi)
2.3. Spore Dispersal (DisR)
| Rate | Variable | Function | Ref. text | Parameters * | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | x | β | R2 | Tmin | Tmax | ||||
| GeR † | T | Bete | 1 | 4.879 | 0.023 | 9.921 | - # | - | 0.98 | 5 | 45 |
| aw | Polynomial | −0.458 | 89.842 | −4323.4 | - | - | 1 | - | - | ||
| GR | T | Bete | 1 | 4.692 | 1.277 | 1.899 | - | - | 0.84 | 5 | 45 |
| aw | Linear | - | - | - | 7.275 | −6.257 | 0.83 | - | - | ||
| ToxR | T | Bete | 1 | 2.778 | 0.650 | 2.760 | - | - | 0.54 | 10 | 37 |
| T1 | Bete | 1 | 2.770 | 0.562 | 6.559 | - | - | 0.87 | 10 | 37 | |
| T2 | Bete | 1 | 9.915 | 3.201 | 0.588 | - | - | 0.76 | 10 | 37 | |
| aw | Linear | - | - | - | 8.153 | −7.135 | 1 | - | - | ||
2.4. Germination Rate (GeR)
2.5. Growth Rate (GR)
| Symbols | Selected paper | GR | ToxR |
|---|---|---|---|
| ∆ | Astoreca et al. [49] | * | |
| ■ | Battilani, Giorni and Pietri [8] | * | |
| ▲ | Belli et al. [50] | * | |
| + | Belli et al. [54] | * | |
| □ | Esteban et al. [55] | * | |
| ● | Lasram, Oueslati, Valero, Marin, Ghorbel and Sanchis [21] | * | |
| ◊ | Leong et al. [51] | * | * |
| ♦ | Romero, Pinto, Patriarca and Vaamonde [22] | * | |
 | Selouane, Bouya, Lebrihi, Decock and Bouseta [23] | * | |
| ○ | Spadaro et al. [52] | * |

2.6. Infection Rate (IR)
2.7. Toxin Production Rate (ToxR)

2.8. Effect of Weather Data on ToxRT
| % ToxRT1 | % ToxRT2 | Mean ToxRT * |
|---|---|---|
| 0 | 100 | 603.24 a |
| 10 | 90 | 611.84 ab |
| 20 | 80 | 620.44 ab |
| 30 | 70 | 629.05 ab |
| 40 | 60 | 637.65 ab |
| 50 | 50 | 646.25 b |
| 60 | 40 | 654.86 bc |
| 70 | 30 | 663.46 bc |
| 80 | 20 | 672.06 bc |
| 90 | 10 | 680.66 bc |
| 100 | 0 | 689.27 c |
2.9. Model Output (OTA-I)
3. Discussion
4. Material and Methods
4.1. Literature Search
4.2. Model Development
4.3. Grape Growth Stage Model
4.4. OTA-Grapes Data Input and Output
4.5. Meteorological Data
4.6. Effect of Weather Data on ToxRT
4.7. Preliminary OTA-Grapes Validation
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pappalardo, G.; Scienza, A.; Vindigni, G.; D’Amico, M. Profitability of wine grape growing in the EU member states. J. Wine Res. 2013, 24, 59–76. [Google Scholar] [CrossRef]
- Battilani, P.; Pietri, A. Ochratoxin A in grapes and wine. Eur. J. Plant Pathol. 2002, 108, 639–643. [Google Scholar] [CrossRef]
- Battilani, P.; Silva, A. Controlling ochratoxin A in the vineyard and winery. In Managing Wine Quality. Volume 1: Viticulture and Wine Quality; Reynold, A., Ed.; Woodhead Publishing in Food Science: Cambridge, UK, 2010; pp. 515–546. [Google Scholar]
- Barberis, M.G.; Merlera, G.G.; Reynoso, M.M.; Chulze, S.N.; Torres, A.M. Factors affecting distribution and abundance of Aspergillus section Nigri in vineyard soils from grapevine growing regions of Argentina. J. Sci. Food Agric. 2014, 94, 3001–3007. [Google Scholar] [CrossRef] [PubMed]
- Cabanes, F.J.; Bragulat, M.R.; Castella, G. Characterization of nonochratoxigenic strains of Aspergillus carbonarius from grapes. Food Microbiol. 2013, 36, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Heenan, C.N.; Shaw, K.J.; Pitt, J.I. Ochratoxin A production by Aspergillus carbonarius and A. niger isolates and detection using coconut cream agar. J. Food Mycol. 1998, 1, 67–72. [Google Scholar]
- Bau, M.; Castella, G.; Bragulat, M.R.; Cabanes, F.J. Rflp characterization of Aspergillus niger aggregate species from grapes from Europe and Israel. Int. J. Food Microbiol. 2006, 111, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P.; Giorni, P.; Pietri, A. Epidemiology of toxin-producing fungi and ochratoxin A occurrence in grape. Eur. J. Plant Pathol. 2003, 109, 715–722. [Google Scholar] [CrossRef]
- Kuiper-Goodman, T. Risk assessment of ochratoxin A: An update. Food Addit. Contam. 1996, 13, 53–57. [Google Scholar] [PubMed]
- Petzinger, E.; Ziegler, K. Ochratoxin A from a toxicological perspective. J. Vet. Pharmacol. Ther. 2000, 23, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Walker, R. Risk Assessment of Ochratoxin: Current Views of the European Scientific Committee on Food, the Jecfa and the Codex Committee on Food Additives and Contaminants; Advances in experimental medicine and biology volume 504; Mycotoxins and food safety Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 249–255. [Google Scholar]
- Gareis, M.; Rosner, H.; Ehrhardt, S. Blood serum levels of ochratoxin A and nutrition habits. Archiv. Fur. Lebensm. 2000, 51, 108–110. [Google Scholar]
- Gareis, M.; Wolff, J.; Bresch, H.; Engel, G.; Rosner, H.; Majerus, P.; Scheuer, R.; Cholmakow-Bodechtel, C. The german ochratoxin A project: Contamination of foods and consumer exposure. Mycotoxins 2001, 51, 31–35. [Google Scholar] [CrossRef]
- Marquardt, R.R.; Frohlich, A.A. A review of recent advances in understanding ochratoxicosis. J. Anim. Sci. 1992, 70, 3968–3988. [Google Scholar] [PubMed]
- Clark, H.A.; Snedeker, S.M. Ochratoxin A: Its cancer risk and potential for exposure. J. Toxicol. Environ. Health. 2006, 9, 265–296. [Google Scholar] [CrossRef] [PubMed]
- Interantional Agency for Research on Cancer (IARC). Monographs on the Evaluation of Carcinogenic Risks to Humans. Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; IARC: Lyon, France, 1993. [Google Scholar]
- Zimmerli, B.; Dick, R. Ochratoxin A in table wine and grape-juice: Occurrence and risk assessment. Food Addit. Contam. 1996, 13, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P. Prevention of Ochratoxin A in Grapes and Wine; Leslie, J.F., Bandyopadhyay, R., Visconti, A., Eds.; Mycotoxins CABI: Wallingford, CT, USA, 2008; pp. 245–256. [Google Scholar]
- Belli, N.; Mitchell, D.; Marin, S.; Alegre, I.; Ramos, A.J.; Naresh, M.; Sanchis, V. Ochratoxin A-producing fungi in spanish wine grapes and their relationship with meteorological conditions. Eur. J. Plant Pathol. 2005, 113, 233–239. [Google Scholar] [CrossRef]
- Esteban, A.; Abarca, M.L.; Bragulat, M.R.; Cabanes, F.J. Effect of water activity on ochratoxin A production by Aspergillus niger aggregate species. Int. J. Food Microbiol. 2006, 108, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Lasram, S.; Oueslati, S.; Valero, A.; Marin, S.; Ghorbel, A.; Sanchis, V. Water activity and temperature effects on fungal growth and ochratoxin A production by ochratoxigenic Aspergillus carbonarius isolated from tunisian grapes. J. Food Sci. 2010, 75, M89–M97. [Google Scholar] [CrossRef] [PubMed]
- Romero, S.M.; Pinto, V.F.; Patriarca, A.; Vaamonde, G. Ochratoxin A production by a mixed inoculum of Aspergillus carbonarius at different conditions of water activity and temperature. Int. J. Food Microbiol. 2010, 140, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Selouane, A.; Bouya, D.; Lebrihi, A.; Decock, C.; Bouseta, A. Impact of some environmental factors on growth and production of ochratoxin a of/by Aspergillus tubingensis, A. niger, and A. carbonarius isolated from moroccan grapes. J. Microbiol. 2009, 47, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Rosseau, J.; Blateyron, L. Ochratoxin A in wines: No curative solution in wine, priority in the vineyard sanitary management. Rev. Oenol. Fr. 2002, 29, 14–16. [Google Scholar]
- Sage, L.; Garon, D.; Seigle-Murandi, F. Fungal microflora and ochratoxin A risk in French vineyards. J. Agric. Food Chem. 2004, 52, 5764–5768. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P.; Barbano, C.; Marin, S.; Sanchis, V.; Kozakiewicz, Z.; Magan, N. Mapping of Aspergillus section nigri in Southern Europe and Israel based on geostatistical analysis. Int. J. Food Microbiol. 2006, 111, S72–S82. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, G.; Haidukowski, M.; Perrone, G.; Visconti, A.; Logrieco, A.F. Influence of Lobesia botrana field control on black aspergilli rot and ochratoxin A contamination in grapes. J. Food Prot. 2009, 72, 894–897. [Google Scholar] [PubMed]
- Cozzi, G.; Pascale, M.; Perrone, G.; Visconti, A.; Logrieco, A. Effect of Lobesia botrana damages on black Aspergilli rot and ochratoxin a content in grapes. Int. J. Food Microbiol. 2006, 111, S88–S92. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, G.; Somma, S.; Haidukowski, M.; Logrieco, A.F. Ochratoxin A management in vineyards by Lobesia botrana biocontrol. Toxins 2013, 5, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Chiotta, M.L.; Ponsone, M.L.; Torres, A.M.; Combina, M.; Chulze, S.N. Influence of Planococcus ficus on Aspergillus section nigri and ochratoxin A incidence in vineyards from Argentina. Lett. Appl. Microbiol. 2010, 51, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Minguez, S.; Cantus, J.M.; Pons, A.; Margot, P.; Cabanes, F.X.; Masque, C.; Accensi, F.; Elorduy, X.; Giralt, L.L.; Vilavella, M.; et al. Influence of the fungus control strategy in the vineyard on the presence of ochratoxin A in the wine. Bull. l'OIV 2004, 77, 821–831. [Google Scholar]
- Hocking, A.D.; Leong, S.L.L.; Kazi, B.A.; Emmett, R.W.; Scott, E.S. Fungi and mycotoxins in vineyards and grape products. Int. J. Food Microbiol. 2007, 119, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, G.; Paciolla, C.; Haidukowski, M.; de Leonardis, S.; Mule, G.; Logrieco, A. Increase of fumonisin B2 and ochratoxin A production by black Aspergillus species and oxidative stress in grape berries damaged by powdery mildew. J. Food Prot. 2013, 76, 2031–2036. [Google Scholar] [CrossRef] [PubMed]
- Tjamos, S.E.; Antoniou, P.P.; Kazantzidou, A.; Antonopoulos, D.F.; Papageorgiou, I.; Tjamos, E.C. Aspergillus niger and Aspergillus carbonarius in corinth raisin and wine-producing vineyards in Greece: Population composition, ochratoxin a production and chemical control. J. Phytopathol. 2004, 152, 250–255. [Google Scholar] [CrossRef]
- Bleve, G.; Grieco, F.; Cozzi, G.; Logrieco, A.; Visconti, A. Isolation of epiphytic yeasts with potential for biocontrol of Aspergillus carbonarius and A. niger on grape. Int. J. Food Microbiol. 2006, 108, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P.; Pietri, A.; Silva, A.; Giorni, P. Critical control points for ochratoxin A control in the grape-wine chain. J. Plant Pathol. 2003, 85, 285. [Google Scholar]
- Battilani, P.; Logrieco, A.; Giorni, P.; Cozzi, G.; Bertuzzi, T.; Pietri, A. Ochratoxin A production by Aspergillus carbonarius on some grape varieties grown in italy. J. Sci. Food Agric. 2004, 84, 1736–1740. [Google Scholar] [CrossRef]
- Covarelli, L.; Beccari, G.; Marini, A.; Tosi, L. A review on the occurrence and control of ochratoxigenic fungal species and ochratoxin A in dehydrated grapes, non-fortified dessert wines and dried vine fruit in the mediterranean area. Food Control 2012, 26, 347–356. [Google Scholar] [CrossRef]
- Kazi, B.A.; Emmett, R.W.; Nancarow, N.; Clarke, K. Incidence of Aspergillus carbonarius in australian vineyards. In Proceeding of the 3rd Australian Soil-born Symposium, Adelaide, Australia, 18–19 March 2004; pp. 75–76.
- Battilani, P.; Giorni, P.; Bertuzzi, T.; Formenti, S.; Pietri, A. Black Aspergilli and ochratoxin A in grapes in Italy. Int. J. Food Microbiol. 2006, 111, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.; Hocking, A.D.; Pitt, J.I. Occurrence of fruit rot fungi (Aspergillus section nigri) on some drying varieties of irrigated grapes. Aust. J. Grape Wine Res. 2004, 10, 83–88. [Google Scholar] [CrossRef]
- Leong, S.L.; Hocking, A.D.; Pitt, J.I.; Kazi, B.A.; Emmett, R.W.; Scott, E.S. Australian research on ochratoxigenic fungi and ochratoxin A. Int. J. Food Microbiol. 2006, 111, S10–S17. [Google Scholar] [CrossRef] [PubMed]
- El-Nagerabi, S.A.F.; Ahmed, A.H.M. Storability of onion bulbs contaminated by Aspergillus niger mold. Phytoparasitica 2003, 31, 515–523. [Google Scholar] [CrossRef]
- Ko, S.-S.; Huang, J.-W.; Wang, J.-F.; Shanmugasundaram, S.; Chang, W.-N. Evaluation of onion cultivars for resistance to Aspergillus niger, the causal agent of black mold. Am. Soc. Hortic. Sci. 2002, 127, 697–702. [Google Scholar]
- Battilani, P.; Camardo Leggieri, M.; Rossi, V.; Giorni, P. Afla-maize, a mechanistic model for Aspergillus flavus infection and aflatoxin B1 contamination in maize. Comput. Electron. Agric. 2013, 94, 38–46. [Google Scholar] [CrossRef]
- Camardo Leggieri, M.; Mitchell, D.; Aldred, D.; Battilani, P.; Magan, N. Hydro- and thermotimes for conidial germination kinetics of the ochratoxigenic species Aspergillus carbonarius in vitro, on grape skin and grape flesh. Fungal Biol. 2014, 118, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Dantigny, P.; Marin, S.; Beyer, M.; Magan, N. Mould germination: Data treatment and modelling. Int. J. Food Microbiol. 2007, 114, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Nanguy, S.P.M.; Perrier-Cornet, J.M.; Bensoussan, M.; Dantigny, P. Impact of water activity of diverse media on spore germination of Aspergillus and Penicillium species. Int. J. Food Microbiol. 2010, 142, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Astoreca, A.; Magnoli, C.; Ramirez, M.L.; Combina, M.; Dalcero, A. Water activity and temperature effects on growth of Aspergillus niger, A. awamori and A. carbonarius isolated from different substrates in Argentina. Int. J. Food Microbiol. 2007, 119, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Belli, N.; Marin, S.; Sanchis, V.; Ramos, A.J. Influence of water activity and temperature on growth of isolates of Aspergillus section nigri obtained from grapes. Int. J. Food Microbiol. 2004, 96, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.L.L.; Hocking, A.D.; Scott, E.S. Effect of temperature and water activity on growth and ochratoxin a production by australian Aspergillus carbonarius and A. niger isolates on a simulated grape juice medium. Int. J. Food Microbiol. 2006, 110, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, D.; Subban, P.; Lore, A.; Gullino, M.L.; Garibaldi, A. Effect of pH, water activity and temperature on the growth and accumulation of ochratoxin A produced by three strains of Aspergillus carbonarius isolated from italian vineyards. Phytopathol. Mediterr. 2010, 49, 65–73. [Google Scholar]
- Analytis, S. Über die relation zwischen biologischer entwicklung und temperatur bei phytopathogenen pilzen. J. Phytopathol. 1977, 90, 64–76. [Google Scholar] [CrossRef]
- Belli, N.; Ramos, A.J.; Sanchis, V.; Marin, S. Incubation time and water activity effects on ochratoxin a production by Aspergillus section nigri strains isolated from grapes. Lett. Appl. Microbiol. 2004, 38, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Esteban, A.; Abarca, M.L.; Bragulat, M.R.; Cabanes, F.J. Effects of temperature and incubation time on production of ochratoxin A by black aspergilli. Res. Microbiol. 2004, 155, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Belli, N.; Marin, S.; Coronas, I.; Sanchis, V.; Ramos, A.J. Skin damage, high temperature and relative humidity as detrimental factors for Aspergillus carbonarius infection and ochratoxin a production in grapes. Food Control 2007, 18, 1343–1349. [Google Scholar] [CrossRef]
- Paola, B.; (Università Cattolica del Sacro Cuore, Piacenza, Italy). Personal communication, 2015.
- Bau, M.; Bragulat, M.R.; Abarca, M.L.; Minguez, S.; Cabanes, F.J. Ochratoxigenic species from spanish wine grapes. Int. J. Food Microbiol. 2005, 98, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Guzev, L.; Danshin, A.; Ziv, S.; Lichter, A. Occurrence of ochratoxin A producing fungi in wine and table grapes in Israel. Int. J. Food Microbiol. 2006, 111, 67–71. [Google Scholar]
- Serra, R.; Mendonca, C.; Venancio, A. Fungi and ochratoxin A detected in healthy grapes for wine production. Lett. Appl. Microbiol. 2006, 42, 42–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otteneder, H.; Majerus, P. Occurence of ochratoxin A (OTA) in wine: Influence of the type of wines and its geographical origin. Food Addit. Contam. 2000, 17, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Pietri, A.; Bertuzzi, T.; Pallaroni, L.; Piva, G. Occurrence of ochratoxin A in Italian wines. Food Addit. Contam. 2001, 18, 647–654. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) no 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs; Official Journal of European Union: Bruxelles, Belgium, 2006; pp. 5–24. [Google Scholar]
- Comuzzo, P.; Rauhut, D.; Werner, M.; Lagazio, C.; Zironi, R. A survey on wines from organic viticulture from different European countries. Food Control 2013, 34, 274–282. [Google Scholar] [CrossRef]
- Dimakopoulou, M.; Tjamos, S.E.; Antoniou, P.P.; Pietri, A.; Battilani, P.; Avramidis, N.; Markakis, E.A.; Tjamos, E.C. Phyllosphere grapevine yeast Aureobasidium pullulans reduces Aspergillus carbonarius (sour rot) incidence in wine-producing vineyards in Greece. Biol. Control. 2008, 46, 158–165. [Google Scholar] [CrossRef]
- Trnka, M.; Rötter, R.P.; Ruiz-Ramos, M.; Kersebaum, K.C.; Olesen, J.E.; Žalud, Z.; Semenov, M.A. Adverse weather conditions for european wheat production will become more frequent with climate change. Nat. Clim. Chang. 2014, 4, 637–643. [Google Scholar] [CrossRef]
- Battilani, P.; Camardo Leggieri, M. Predictive modelling of aflatoxin contamination to support maize chain management. World Mycotoxin J. 2015, 8, 161–170. [Google Scholar] [CrossRef]
- Cabanes, F.J.; Accensi, F.; Bragulat, M.R.; Abarca, M.L.; Castella, G.; Minguez, S.; Pons, A. What is the source of ochratoxin a in wine? Int. J. Food Microbiol. 2002, 79, 213–215. [Google Scholar] [CrossRef]
- Varga, J.; Kozakiewicz, Z. Ochratoxin a in grapes and grape-derived products. Trends Food Sci. Technol. 2006, 17, 72–81. [Google Scholar] [CrossRef]
- Van Maanen, A.; Xu, X.M. Modelling plant disease epidemics. Eur. J. Plant Pathol. 2003, 109, 669–682. [Google Scholar] [CrossRef]
- McCatney, H.A. The influence of environment on the development and control disease. In Environmentally Safe Approaches to Crop Disease Control; Rechcigl, J., Ed.; CRC Press: Bocan Ratan, FL, USA, 1997. [Google Scholar]
- Kapetanakou, A.E.; Panagou, E.Z.; Gialitaki, M.; Drosinos, E.H.; Skandamis, P.N. Evaluating the combined effect of water activity, pH and temperature on ochratoxin A production by Aspergillus ochraceus and Aspergillus carbonarius on culture medium and corinth raisins. Food Control 2009, 20, 725–732. [Google Scholar] [CrossRef]
- Tassou, C.C.; Natskoulis, P.I.; Magan, N.; Panagou, E.Z. Effect of temperature and water activity on growth and ochratoxin A production boundaries of two Aspergillus carbonarius isolates on a simulated grape juice medium. J. Appl. Microbiol. 2009, 107, 257–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marin, S.; Hodzic, I.; Ramos, A.J.; Sanchis, V. Predicting the growth/no-growth boundary and ochratoxin A production by Aspergillus carbonarius in pistachio nuts. Food Microbiol. 2008, 25, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.; Belli, N.; Lasram, S.; Chebil, S.; Ramos, A.J.; Ghorbel, A.; Sanchis, V. Kinetics of ochratoxin a production and accumulation by Aspergillus carbonarius on synthetic grape medium at different temperature levels. J. Food Sci. 2006, 71, M196–M200. [Google Scholar] [CrossRef]
- Mateo, E.M.; Valle-Algarra, F.M.; Mateo-Castro, R.; Jimenez, M. Impact of non-selective fungicides on the growth and production of ochratoxin a by Aspergillus ochraceus and A. carbonarius in barley-based medium. Food Addit. Contam. A 2011, 28, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Dachoupakan, C.; Ratomahenina, R.; Martinez, V.; Guiraud, J.P.; Baccou, J.C.; Schorr-Galindo, S. Study of the phenotypic and genotypic biodiversity of potentially ochratoxigenic black Aspergilli isolated from grapes. Int. J. Food Microbiol. 2009, 132, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Lasram, S.; Oueslati, S.; Ben Jouira, H.; Chebil, S.; Mliki, A.; Ghorbel, A. Identification of ochratoxigenic Aspergillus section nigri isolated from grapes by its-5.8s rDNA sequencing analysis and in silico rflp. J. Phytopathol. 2013, 161, 280–283. [Google Scholar] [CrossRef]
- Belli, N.; Bau, M.; Marin, S.; Abarca, M.L.; Ramos, A.J.; Bragulat, M.R. Mycobiota and ochratoxin a producing fungi from spanish wine grapes. Int. J. Food Microbiol. 2006, 111, 40–45. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Directive 2009/128/EC Estabilishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides; Official Journal of European Union: Bruxelles, Belgium, 2009; pp. 71–86. [Google Scholar]
- Campbell, C.L.; Madden, M.V. Introduction to Plant Disease Epidemiology; John Wiley & Sons: New York, NY, USA, 1990. [Google Scholar]
- Dantigny, P.; Guilmart, A.; Bensoussan, M. Basis of predictive mycology. Int. J. Food Microbiol. 2005, 100, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Kapetanakou, A.E.; Ampavi, A.; Yanniotis, S.; Drosinos, E.H.; Skandamis, P.N. Development of a model describing the effect of temperature, water activity and (gel) structure on growth and ochratoxin A production by Aspergillus carbonarius in vitro and evaluation in food matrices of different viscosity. Food Microbiol. 2011, 28, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Jargot, D.; Melin, S. Characterization and validation of sampling and analytical methods for mycotoxins in workplace air. Environ. Sci. 2013, 5, 633–644. [Google Scholar] [CrossRef]
- Okoli, C.; Schabram, K. A guide to conducting a systematic literature review of information systems research. Sprotus: Working Papers on Information Systems 2010, 10, 26. Available online: http://sprouts.aisnet.org/10–26 (accessed on 5 May 2010). [Google Scholar] [CrossRef]
- Lefferlaar, P.A. Basic elements of dynamic simulation. In On System Analysis and Simulation of Ecological Processes; Leffelaar, P.A., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993; pp. 11–28. [Google Scholar]
- Mariani, L.; Alilla, R.; Cola, G.; Monte, G.D.; Epifani, C.; Puppi, G.; Failla, O. Iphen-a real-time network for phenological monitoring and modelling in Italy. Int. J. Biometeorol. 2013, 57, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Cola, G.; Mariani, L.; Salinari, F.; Civardi, S.; Bernizzoni, F.; Gatti, M.; Poni, S. Description and testing of a weather-based model for predicting phenology, canopy development and source-sink balance in Vitis vinifera L. cv. Barbera. Agric. For. Meteorol. 2014, 184, 117–136. [Google Scholar] [CrossRef]
- Lorenz, D.H.; Eichhorn, K.W.; Bleiholder, H.; Klose, R.; Meier, U.; Weber, E. Phenological growth stages of the grapevine (vitis vinifera l. Spp. Vinifera)—codes and descriptions according to the extended bbch scale. Aust. J. Grape Wine Res. 1995, 1, 100–103. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paola, B.; Marco, C.L. OTA-Grapes: A Mechanistic Model to Predict Ochratoxin A Risk in Grapes, a Step beyond the Systems Approach. Toxins 2015, 7, 3012-3029. https://doi.org/10.3390/toxins7083012
Paola B, Marco CL. OTA-Grapes: A Mechanistic Model to Predict Ochratoxin A Risk in Grapes, a Step beyond the Systems Approach. Toxins. 2015; 7(8):3012-3029. https://doi.org/10.3390/toxins7083012
Chicago/Turabian StylePaola, Battilani, and Camardo Leggieri Marco. 2015. "OTA-Grapes: A Mechanistic Model to Predict Ochratoxin A Risk in Grapes, a Step beyond the Systems Approach" Toxins 7, no. 8: 3012-3029. https://doi.org/10.3390/toxins7083012
APA StylePaola, B., & Marco, C. L. (2015). OTA-Grapes: A Mechanistic Model to Predict Ochratoxin A Risk in Grapes, a Step beyond the Systems Approach. Toxins, 7(8), 3012-3029. https://doi.org/10.3390/toxins7083012