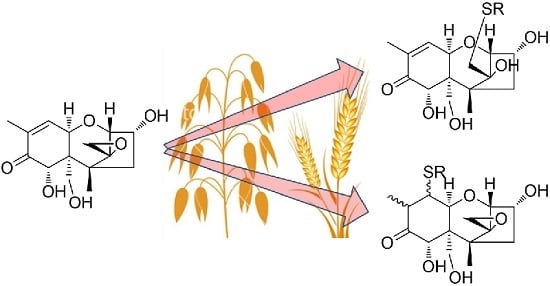
Glutathione-Conjugates of Deoxynivalenol in Naturally Contaminated Grain Are Primarily Linked via the Epoxide Group
Abstract
:1. Introduction
2. Results and Discussion
2.1. DON–GSH and Related Adducts in DON–Treated Wheat and Naturally Contaminated Grain
2.2. Mercapturates of DON
2.3. Separation of DON–Thiol Adducts
2.4. DON–Acetates
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Reference Standard Mixtures
4.3. Samples and Extraction
4.4. LC-HRMS(/MS) Analyses
4.5. Oxidation of DON–GSH to DON–GS(O)H with Hydrogen Peroxide
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Cys | l-cysteine |
| DON | 4-deoxynivalenol |
| γ-GluCys | γ-l-glutamyl-l-cysteine |
| CysGly | l-cysteinyl-l-glycine |
| GSH | l-glutathione |
| HRMS | high-resolution mass spectrometry |
| LC | liquid chromatography |
| MS/MS | tandem mass spectrometry |
| NAC | N-acetylcysteine |
| PFPP | pentafluorophenylpropyl |
| tR | retention time |
References
- Nesic, K.; Ivanovic, S.; Nesic, V. Fusarial toxins: Secondary metabolites of Fusarium fungi. Rev. Environ. Contam. Toxicol. 2014, 228, 101–120. [Google Scholar] [PubMed]
- Bonnet, M.S.; Roux, J.; Mounien, L.; Dallaporta, M.; Troadec, J.-D. Advances in deoxynivalenol toxicity mechanisms: The brain as a target. Toxins 2012, 4, 1120–1138. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Oswald, I.P. Effect of deoxynivalenol and other type B trichothecenes on the intestine: A review. Toxins 2014, 6, 1615–1643. [Google Scholar] [CrossRef] [PubMed]
- Kluger, B.; Bueschl, C.; Lemmens, M.; Michlmayr, H.; Malachova, A.; Koutnik, A.; Maloku, I.; Berthiller, F.; Adam, G.; Krska, R.; et al. Biotransformation of the mycotoxin deoxynivalenol in Fusarium resistant and susceptible near isogenic wheat lines. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Kluger, B.; Bueschl, C.; Lemmens, M.; Berthiller, F.; Häubl, G.; Jaunecker, G.; Adam, G.; Krska, R.; Schuhmacher, R. Stable isotopic labelling-assisted untargeted metabolic profiling reveals novel conjugates of the mycotoxin deoxynivalenol in wheat. Anal. Bioanal. Chem. 2013, 405, 5031–5036. [Google Scholar] [CrossRef] [PubMed]
- Stanic, A.; Uhlig, S.; Solhaug, A.; Rise, F.; Wilkins, A.L.; Miles, C.O. Preparation and characterization of cysteine adducts of deoxynivalenol. J. Agric. Food Chem. 2016, 64, 4777–4785. [Google Scholar] [CrossRef] [PubMed]
- Stanic, A.; Uhlig, S.; Sandvik, M.; Rise, F.; Wilkins, A.L.; Miles, C.O. Characterization of deoxynivalenol–glutathione conjugates using nuclear magnetic resonance spectroscopy and liquid chromatography–high-resolution mass spectrometry. J. Agric. Food Chem. 2016, 64, 6903–6910. [Google Scholar] [CrossRef] [PubMed]
- Stanic, A.; Uhlig, S.; Solhaug, A.; Rise, F.; Wilkins, A.L.; Miles, C.O. Nucleophilic addition of thiols to deoxynivalenol. J. Agric. Food Chem. 2015, 63, 7556–7566. [Google Scholar] [CrossRef] [PubMed]
- Fruhmann, P.; Weigl-Pollack, T.; Mikula, H.; Wiesenberger, G.; Adam, G.; Varga, E.; Berthiller, F.; Krska, R.; Hametner, C.; Frohlich, J. Methylthiodeoxynivalenol (MTD): Insight into the chemistry, structure and toxicity of thia-Michael adducts of trichothecenes. Org. Biomol. Chem. 2014, 12, 5144–5150. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.-M. Sulfones and Sulfoxides. In Ullmann's Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Sato, K.; Hyodo, M.; Aoki, M.; Zheng, X.-Q.; Noyori, R. Oxidation of sulfides to sulfoxides and sulfones with 30% hydrogen peroxide under organic solvent- and halogen-free conditions. Tetrahedron 2001, 57, 2469–2476. [Google Scholar] [CrossRef]
- Miles, C.O.; Melanson, J.E.; Ballot, A. Sulfide oxidations for LC-MS analysis of methionine-containing microcystins in Dolichospermum flos-aquae NIVA-CYA 656. Environ. Sci. Technol. 2014, 48, 13307–13315. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.; Blake-Kalff, M.; Davies, E. Detoxification of xenobiotics by plants: Chemical modification and vacuolar compartmentation. Trends Plant Sci. 1997, 2, 144–151. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef] [PubMed]
- Brazier-Hicks, M.; Evans, K.M.; Cunningham, O.D.; Hodgson, D.R.W.; Steel, P.G.; Edwards, R. Catabolism of glutathione conjugates in Arabidopsis thaliana: Role in metabolic reactivation of the herbicide safener fenclorim. J. Biol. Chem. 2008, 283, 21102–21112. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.J.L.; Krasnikov, B.F.; Niatsetskaya, Z.V.; Pinto, J.T.; Callery, P.S.; Villar, M.T.; Artigues, A.; Bruschi, S.A. Cysteine S-conjugate β-lyases: Important roles in the metabolism of naturally occurring sulfur and selenium-containing compounds, xenobiotics and anticancer agents. Amino Acids 2011, 41, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Havlíková, L.; Matysová, L.; Hájková, R.; Šatínský, D.; Solich, P. Advantages of pentafluorophenylpropyl stationary phase over conventional C18 stationary phase—Application to analysis of triamcinolone acetonide. Talanta 2008, 76, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Mochizuki, N.; Nagatomi, Y.; Harayama, K.; Toriba, A.; Hayakawa, K. A method for simultaneous determination of 20 Fusarium toxins in cereals by high-resolution liquid chromatography-orbitrap mass spectrometry with a pentafluorophenyl column. Toxins 2015, 7, 1664–1682. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, S.; Eriksen, G.S.; Hofgaard, I.S.; Krska, R.; Beltrán, E.; Sulyok, M. Faces of a changing climate: Semi-quantitative multi-mycotoxin analysis of grain grown in exceptional climatic conditions in Norway. Toxins 2013, 5, 1682–1697. [Google Scholar] [CrossRef] [PubMed]
- Aamot, H.U.; Ward, T.J.; Brodal, G.; Vrålstad, T.; Larsen, G.B.; Klemsdal, S.S.; Elameen, A.; Uhlig, S.; Hofgaard, I.S. Genetic and phenotypic diversity within the Fusarium graminearum species complex in Norway. Eur. J. Plant Pathol. 2015, 142, 501–519. [Google Scholar] [CrossRef]
- Hofgaard, I.S.; Aamot, H.U.; Torp, T.; Jestoi, M.; Lattanzio, V.M.T.; Klemsdal, S.S.; Waalwijk, C.; Van der Lee, T.; Brodal, G. Associations between Fusarium species and mycotoxins in oats and spring wheat from farmers’ fields in Norway over a six-year period. World Mycotoxin J. 2016, 9, 365–378. [Google Scholar] [CrossRef]
- Miles, C.O. Fusarium Toxin Mass Calculator (Version 9 in Excel). 2016. Available online: https://www.researchgate.net/publication/304249256_Fusarium_toxin_mass_calculator_version_9_in_Excel (accessed on 23 June 2016).








| Compound | Ion Species ESI+ | Calculated Mass (m/z) | Ion Species ESI− | Calculated Mass (m/z) |
|---|---|---|---|---|
| Deoxynivalenol (DON) | - | - | [M + formate]− | 341.1242 |
| 3/15-O-acetyl-DON | [M + H]+ | 339.1438 | - | - |
| [M + NH4]+ | 356.1704 | |||
| [M + Na]+ | 361.1258 | |||
| DON–GSH | [M + H]+ | 604.2171 | [M − H]− | 602.2025 |
| DON–GS(O)H | [M + H]+ | 620.2120 | [M − H]− | 618.1974 |
| DON–CysGly | [M + H]+ | 475.1745 | [M − H]− | 473.1599 |
| DON–γ-GluCys | [M + H]+ | 547.1956 | [M − H]− | 545.1811 |
| DON–Cys | [M + H]+ | 418.1530 | [M − H]− | 416.1385 |
| DON–NAC | [M + H]+ | 460.1636 | [M − H]− | 458.1490 |
| DON-3-β-d-glucoside | [M + NH4]+ | 476.2126 | [M + formate]− | 503.1770 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uhlig, S.; Stanic, A.; Hofgaard, I.S.; Kluger, B.; Schuhmacher, R.; Miles, C.O. Glutathione-Conjugates of Deoxynivalenol in Naturally Contaminated Grain Are Primarily Linked via the Epoxide Group. Toxins 2016, 8, 329. https://doi.org/10.3390/toxins8110329
Uhlig S, Stanic A, Hofgaard IS, Kluger B, Schuhmacher R, Miles CO. Glutathione-Conjugates of Deoxynivalenol in Naturally Contaminated Grain Are Primarily Linked via the Epoxide Group. Toxins. 2016; 8(11):329. https://doi.org/10.3390/toxins8110329
Chicago/Turabian StyleUhlig, Silvio, Ana Stanic, Ingerd S. Hofgaard, Bernhard Kluger, Rainer Schuhmacher, and Christopher O. Miles. 2016. "Glutathione-Conjugates of Deoxynivalenol in Naturally Contaminated Grain Are Primarily Linked via the Epoxide Group" Toxins 8, no. 11: 329. https://doi.org/10.3390/toxins8110329
APA StyleUhlig, S., Stanic, A., Hofgaard, I. S., Kluger, B., Schuhmacher, R., & Miles, C. O. (2016). Glutathione-Conjugates of Deoxynivalenol in Naturally Contaminated Grain Are Primarily Linked via the Epoxide Group. Toxins, 8(11), 329. https://doi.org/10.3390/toxins8110329