N-methyl-2-pyridone-5-carboxamide (2PY)—Major Metabolite of Nicotinamide: An Update on an Old Uremic Toxin
Abstract
:1. Introduction
2. Metabolism of NAM and Production of 2PY
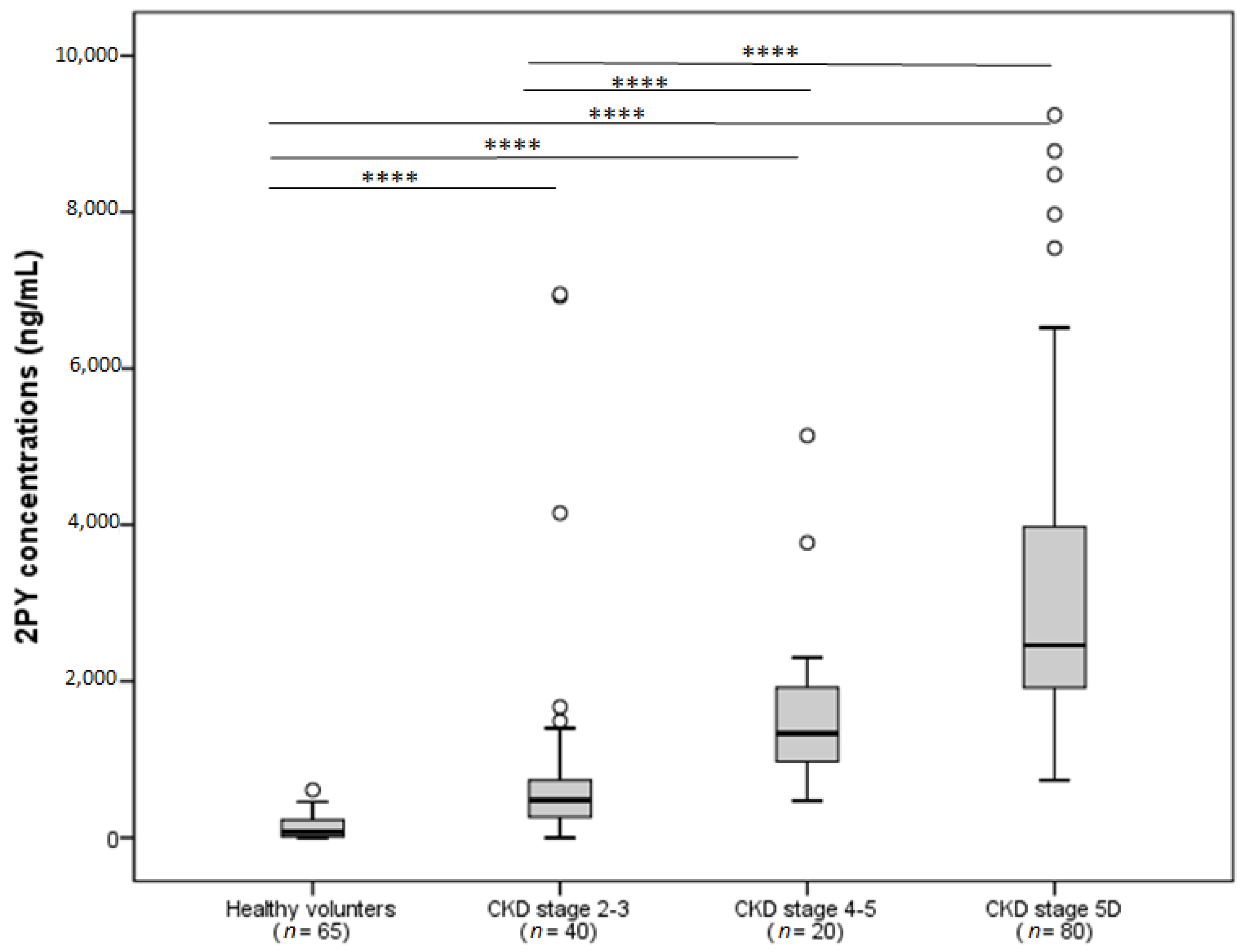
3. Elevated 2PY Concentrations in Patients with Chronic Kidney Disease (CKD)
4. Toxicological Profile
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Katai, K.; Tanaka, H.; Tatsumi, S.; Fukunaga, Y.; Genjida, K.; Morita, K.; Kuboyama, N.; Suzuki, T.; Akiba, T.; Miyamoto, K.; et al. Nicotinamide inhibits sodium-dependent phosphate cotransport activity in rat small intestine. Nephrol. Dial. Transplant. 1999, 14, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Eto, N.; Miyata, Y.; Ohno, H.; Yamashita, T. Nicotinamide prevents the development of hyperphosphataemia by suppressing intestinal sodium-dependent phosphate transporter in rats with adenine-induced renal failure. Nephrol. Dial. Transplant. 2005, 20, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Kempson, S.A.; Colon-Otero, G.; Ou, S.Y.; Turner, S.T.; Dousa, T.P. Possible role of nicotinamide adenine dinucleotide as an intracellular regulator of renal transport of phosphate in the rat. J. Clin. Investig. 1981, 67, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Lenglet, A.; Liabeuf, S.; Guffroy, P.; Fournier, A.; Brazier, M.; Massy, Z.A. Use of nicotinamide to treat hyperphosphatemia in dialysis patients. Drugs R D 2013, 13, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. European Uremic Toxin Work Group (EUTox) Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Van Laecke, S.; Glorieux, G. What is new in uremic toxicity? Pediatr. Nephrol. 2008, 23, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Lenglet, A.; Liabeuf, S.; El Esper, N.; Brisset, S.; Mansour, J.; Lemaire-Hurtel, A.-S.; Mary, A.; Brazier, M.; Kamel, S.; Mentaverri, R.; et al. Efficacy and safety of nicotinamide in haemodialysis patients: The NICOREN study. Nephrol. Dial. Transplant. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.B. Niacin status, NAD distribution and ADP-ribose metabolism. Curr. Pharm. Des. 2009, 15, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Mattiussi, A.J.; Blais, D. Niacin versus niacinamide. CMAJ 1992, 147, 990–991. [Google Scholar] [PubMed]
- Inamadugu, J.K.; Damaramadugu, R.; Mullangi, R.; Ponneri, V. Simultaneous determination of niacin and its metabolites—Nicotinamide, nicotinuric acid and N-methyl-2-pyridone-5-carboxamide—In human plasma by LC-MS/MS and its application to a human pharmacokinetic study. Biomed. Chromatogr. 2010, 24, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Szafarz, M.; Lomnicka, M.; Sternak, M.; Chlopicki, S.; Szymura-Oleksiak, J. Simultaneous determination of nicotinic acid and its four metabolites in rat plasma using high performance liquid chromatography with tandem mass spectrometric detection (LC/MS/MS). J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Terry, R.C.; Simon, M. Determination of niacin metabolites 1-methyl-5-carboxylamide-2-pyridone and N-1-methylnicotinamide in urine by high-performance liquid chromatography. J. Chromatogr. 1982, 232, 261–274. [Google Scholar] [CrossRef]
- Shibata, K.; Kawada, T.; Iwai, K. Simultaneous micro-determination of nicotinamide and its major metabolites, N1-methyl-2-pyridone-5-carboxamide and N1-methyl-4-pyridone-3-carboxamide, by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1988, 424, 23–28. [Google Scholar] [CrossRef]
- Stratford, M.R.; Dennis, M.F. High-performance liquid chromatographic determination of nicotinamide and its metabolites in human and murine plasma and urine. J. Chromatogr. 1992, 582, 145–151. [Google Scholar] [CrossRef]
- Mullangi, R.; Srinivas, N.R. Niacin and its metabolites: Role of LC-MS/MS bioanalytical methods and update on clinical pharmacology. An overview. Biomed. Chromatogr. 2011, 25, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.M.; Adams, M.H.; González, M.A.; Tolbert, D.S.; Leu, J.H.; Cefali, E.A. Plasma and urine pharmacokinetics of niacin and its metabolites from an extended-release niacin formulation. Int. J. Clin. Pharmacol. Ther. 2007, 45, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Beyer, K.H.; Russo, H.F. Renal tubular elimination of N1-methylnicotinamide. Am. J. Physiol. 1950, 160, 311–320. [Google Scholar] [PubMed]
- Ito, S.; Kusuhara, H.; Kumagai, Y.; Moriyama, Y.; Inoue, K.; Kondo, T.; Nakayama, H.; Horita, S.; Tanabe, K.; Yuasa, H.; et al. N-methylnicotinamide is an endogenous probe for evaluation of drug-drug interactions involving multidrug and toxin extrusions (MATE1 and MATE2-K). Clin. Pharmacol. Ther. 2012, 92, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Abelson, D.; Boyle, A.; Depatie, C.; Seligson, H. N′-methyl-2-pyridone-5-carboxamide in human plasma. Clin. Chim. Acta 1963, 8, 603–606. [Google Scholar] [CrossRef]
- Slominska, E.M.; Rutkowski, P.; Smolenski, R.T.; Szutowicz, A.; Rutkowski, B.; Swierczynski, J. The age-related increase in N-methyl-2-pyridone-5-carboxamide (NAD catabolite) in human plasma. Mol. Cell. Biochem. 2004, 267, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Fukuwatari, T.; Suzuki, C. Pharmacological doses of nicotinic acid and nicotinamide are independently metabolized in rats. J. Nutr. Sci. Vitaminol. 2014, 60, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Kramer, B.; Seligson, H.; Seligson, D.; Baltrush, H. Isolation of n-methyl-2-pyridone-5-carboxamide from hemodialysis fluid obtained from uremic patients. Clin. Chim. Acta 1964, 10, 447–452. [Google Scholar] [CrossRef]
- Rutkowski, P.; Slominska, E.M.; Wołyniec, W.; Smoleński, R.T.; Szolkiewicz, M.; Swierczyński, J.; Rutkowski, B. Nicotinamide metabolites accumulate in the tissues of uremic rats. J. Ren. Nutr. 2008, 18, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Carrey, E.A.; Smolenski, R.T.; Edbury, S.M.; Laurence, A.; Marinaki, A.M.; Duley, J.A.; Zhu, L.; Goldsmith, D.J.A.; Simmonds, H.A. Origin and characteristics of an unusual pyridine nucleotide accumulating in erythrocytes: Positive correlation with degree of renal failure. Clin. Chim. Acta 2003, 335, 117–129. [Google Scholar] [CrossRef]
- Slominska, E.M.; Smolenski, R.T.; Szolkiewicz, M.; Leaver, N.; Rutkowski, B.; Simmonds, H.A.; Swierczynski, J. Accumulation of plasma N-methyl-2-pyridone-5-carboxamide in patients with chronic renal failure. Mol. Cell. Biochem. 2002, 231, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A. European Uremic Toxin Work Group Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Isakova, T.; Ix, J.H.; Sprague, S.M.; Raphael, K.L.; Fried, L.; Gassman, J.J.; Raj, D.; Cheung, A.K.; Kusek, J.W.; Flessner, M.F.; et al. Rationale and Approaches to Phosphate and Fibroblast Growth Factor 23 Reduction in CKD. J. Am. Soc. Nephrol. 2015, 26, 2328–2339. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, B.; Slominska, E.; Szolkiewicz, M.; Smolenski, R.T.; Striley, C.; Rutkowski, P.; Swierczynski, J. N-methyl-2-pyridone-5-carboxamide: A novel uremic toxin? Kidney Int. Suppl. 2003, S19–S21. [Google Scholar] [CrossRef] [PubMed]
- Slominska, E.M.; Kowalik, K.; Smolenski, R.T.; Szolkiewicz, M.; Rutkowski, P.; Rutkowski, B.; Swierczynski, J. Accumulation of poly(ADP-ribose) polymerase inhibitors in children with chronic renal failure. Pediatr. Nephrol. 2006, 21, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, B.; Rutkowski, P.; Słomińska, E.; Smolenski, R.T.; Swierczyński, J. Cellular toxicity of nicotinamide metabolites. J. Ren. Nutr. 2012, 22, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Synesiou, E.; Fairbanks, L.D.; Simmonds, H.A.; Slominska, E.M.; Smolenski, R.T.; Carrey, E.A. 4-Pyridone-3-carboxamide-1-β-D-ribonucleoside triphosphate (4PyTP), a novel NAD metabolite accumulating in erythrocytes of uremic children: A biomarker for a toxic NAD analogue in other tissues? Toxins (Basel) 2011, 3, 520–537. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Martin, A.J.; Choy, B.; Fernández-Peñas, P.; Dalziell, R.A.; McKenzie, C.A.; Scolyer, R.A.; Dhillon, H.M.; Vardy, J.L.; Kricker, A.; et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N. Engl. J. Med. 2015, 373, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.; Silverberg, J.D.; Nguyen, T.T. Nicotinic acid-induced toxicity associated with cytopenia and decreased levels of thyroxine-binding globulin. Mayo Clin. Proc. 1992, 67, 465–468. [Google Scholar] [CrossRef]
- Rottembourg, J.B.; Launay-Vacher, V.; Massard, J. Thrombocytopenia induced by nicotinamide in hemodialysis patients. Kidney Int. 2005, 68, 2911–2912. [Google Scholar] [CrossRef] [PubMed]
- Fong, P.C.; Boss, D.S.; Yap, T.A.; Tutt, A.; Wu, P.; Mergui-Roelvink, M.; Mortimer, P.; Swaisland, H.; Lau, A.; O’Connor, M.J.; et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 2009, 361, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Audeh, M.W.; Carmichael, J.; Penson, R.T.; Friedlander, M.; Powell, B.; Bell-McGuinn, K.M.; Scott, C.; Weitzel, J.N.; Oaknin, A.; Loman, N.; et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: A proof-of-concept trial. Lancet 2010, 376, 245–251. [Google Scholar] [CrossRef]
- Tutt, A.; Robson, M.; Garber, J.E.; Domchek, S.M.; Audeh, M.W.; Weitzel, J.N.; Friedlander, M.; Arun, B.; Loman, N.; Schmutzler, R.K.; et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: A proof-of-concept trial. Lancet 2010, 376, 235–244. [Google Scholar] [CrossRef]
- Lin, C.-J.; Wu, V.; Wu, P.-C.; Wu, C.-J. Meta-Analysis of the Associations of p-Cresyl Sulfate (PCS) and Indoxyl Sulfate (IS) with Cardiovascular Events and All-Cause Mortality in Patients with Chronic Renal Failure. PLoS ONE 2015, 10, e0132589. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Massy, Z.; Argiles, A.; Spasovski, G.; Verbeke, F.; Lameire, N. European Uremic Toxin Work Group Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol. Dial. Transplant. 2005, 20, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Liabeuf, S.; Villain, C.; Massy, Z.A. Protein-bound toxins: Has the Cinderella of uraemic toxins turned into a princess? Clin. Sci. 2016, 130, 2209–2216. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Miyamoto, Y.; Honda, D.; Tanaka, H.; Wu, Q.; Endo, M.; Noguchi, T.; Kadowaki, D.; Ishima, Y.; Kotani, S.; et al. p-Cresyl sulfate causes renal tubular cell damage by inducing oxidative stress by activation of NADPH oxidase. Kidney Int. 2013, 83, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-Y.; Chang, S.-C.; Wu, M.-S. Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLoS ONE 2012, 7, e34026. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-Y.; Hsu, H.-H.; Wu, M.-S. p-Cresol sulfate and indoxyl sulfate induce similar cellular inflammatory gene expressions in cultured proximal renal tubular cells. Nephrol. Dial. Transplant. 2013, 28, 70–78. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenglet, A.; Liabeuf, S.; Bodeau, S.; Louvet, L.; Mary, A.; Boullier, A.; Lemaire-Hurtel, A.S.; Jonet, A.; Sonnet, P.; Kamel, S.; et al. N-methyl-2-pyridone-5-carboxamide (2PY)—Major Metabolite of Nicotinamide: An Update on an Old Uremic Toxin. Toxins 2016, 8, 339. https://doi.org/10.3390/toxins8110339
Lenglet A, Liabeuf S, Bodeau S, Louvet L, Mary A, Boullier A, Lemaire-Hurtel AS, Jonet A, Sonnet P, Kamel S, et al. N-methyl-2-pyridone-5-carboxamide (2PY)—Major Metabolite of Nicotinamide: An Update on an Old Uremic Toxin. Toxins. 2016; 8(11):339. https://doi.org/10.3390/toxins8110339
Chicago/Turabian StyleLenglet, Aurélie, Sophie Liabeuf, Sandra Bodeau, Loïc Louvet, Aurélien Mary, Agnès Boullier, Anne Sophie Lemaire-Hurtel, Alexia Jonet, Pascal Sonnet, Said Kamel, and et al. 2016. "N-methyl-2-pyridone-5-carboxamide (2PY)—Major Metabolite of Nicotinamide: An Update on an Old Uremic Toxin" Toxins 8, no. 11: 339. https://doi.org/10.3390/toxins8110339
APA StyleLenglet, A., Liabeuf, S., Bodeau, S., Louvet, L., Mary, A., Boullier, A., Lemaire-Hurtel, A. S., Jonet, A., Sonnet, P., Kamel, S., & Massy, Z. A. (2016). N-methyl-2-pyridone-5-carboxamide (2PY)—Major Metabolite of Nicotinamide: An Update on an Old Uremic Toxin. Toxins, 8(11), 339. https://doi.org/10.3390/toxins8110339








