Venoms of Heteropteran Insects: A Treasure Trove of Diverse Pharmacological Toolkits
Abstract
:1. Evolution of Venom Systems in Heteroptera
1.1. Introduction: Are Heteropterans Venomous Animals?
1.2. Evolution of the Heteropteran Venom Apparatus
1.3. Diversification of Trophic Strategies in the Heteropteran Radiation
2. Diversification of Venom Pharmacology in the Evolution of Heteroptera
2.1. Aquatic and Semi-Aquatic Hunters: Nepomorpha, Gerromorpha and Leptopodomorpha
2.1.1. Habitat and Prey Range
2.1.2. Activity and Composition of Nepomorphan Venoms
2.2. Assassin’s Creed: Terrestrial Predators in Cimicomorpha and Pentatomomorpha
2.2.1. Efficient Predation through Envenomation, Prey-Capture Organs and Dietary Specialisation
2.2.2. Physiological Effect of Venoms of Terrestrial Predaceous Heteropterans
Effects of venom on invertebrates
Effects of venom on vertebrates
2.2.3. Composition of Venoms from Terrestrial Predators.
2.3. The Blood Feeders
2.3.1. Convergent Evolution of Blood-Feeding in Heteroptera
2.3.2. Venoms of Haematophagous Heteroptera
3. Future Directions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Henry, T.J. Biodiversity of Heteroptera. In Insect Biodiversity: Science and Society; Foottit, R.G., Adler, P.H., Eds.; Blackwell Publishing: Oxford, UK, 2009; pp. 223–264. [Google Scholar]
- Weirauch, C.; Schuh, R.T. Systematics and evolution of Heteroptera: 25 years of progress. Ann. Rev. Entomol. 2011, 56, 487–510. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Investing to Overcome the Global Impact of Neglected Tropical Diseases; World Health Organisation: Geneva, Switzerland, 2015. [Google Scholar]
- Walter, J.; Fletcher, E.; Moussaoui, R.; Gandhi, K.; Weirauch, C. Do bites of kissing bugs cause unexplained allergies? Results from a survey in triatomine-exposed and unexposed areas in southern California. PLoS ONE 2012, 7, e44016. [Google Scholar]
- Caras, R.A. Dangerous to Man; Barrie & Jenkins: London, UK, 1976. [Google Scholar]
- Edwards, J.S. The action and compostion of the saliva of an assassin bug Platymeris rhadamanthus Gaerst. (Hemiptera, Reduviidae). J. Exp. Biol. 1961, 38, 61–77. [Google Scholar]
- Haddad, V.; Schwartz, E.F.; Schwartz, C.A.; Carvalho, L.N. Bites caused by giant water bugs belonging to Belostomatidae family (Hemiptera, Heteroptera) in humans: A report of seven cases. Wilderness Environ. Med. 2010, 21, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Readio, P.A. Studies on the biology of the Reduviidae of America North of Mexico. Kans. Univ. Sci. Bull. 1927, 17, 5–249. [Google Scholar]
- Zerachia, T.; Bergmann, F.; Shulov, A. Pharmacological activities of the venom of the predaceous bug Holotrichius innesi H. (Heteroptera, Reduviidae). In Animal and Plant Toxins; Kaiser, E., Ed.; Goldman: Munich, Germany, 1973; Volume 143–146. [Google Scholar]
- Schaefer, C.W.; Panizzi, A.R. Heteroptera of Economic Importance; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Annu. Rev. Genom. Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef] [PubMed]
- Baptist, B.A. The morphology and physiology of the salivary glands of Hemiptera-Heteroptera. Q. J. Microsc. Sci. 1941, S2–S83, 91–139. [Google Scholar]
- Azevedo, D.O.; Zanuncio, J.C.; Zanuncio, J.S.J.; Martins, G.F.; Marques-Silva, S.; Sossai, M.F.; Serrão, J.E. Biochemical and morphological aspects of salivary glands of the predator Brontocoris tabidus (Heteroptera: Pentatomidae). Braz. Arch. Biol. Technol. 2007, 50, 469–477. [Google Scholar] [CrossRef]
- Escoubas, P.; Diochot, S.; Corzo, G. Structure and pharmacology of spider venom neurotoxins. Biochimie 2000, 82, 893–907. [Google Scholar] [CrossRef]
- Fry, B.G. Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Lewis, R.J.; Dutertre, S.; Vetter, I.; Christie, M.J. Conus venom peptide pharmacology. Pharmacol. Rev. 2012, 64, 259–298. [Google Scholar] [CrossRef] [PubMed]
- Undheim, E.A.B.; Fry, B.G.; King, G.F. Centipede venom: Recent discoveries and current state of knowledge. Toxins 2015, 7, 679–704. [Google Scholar] [CrossRef] [PubMed]
- Zhijian, C.; Feng, L.; Yingliang, W.; Xin, M.; Wenxin, L. Genetic mechanisms of scorpion venom peptide diversification. Toxicon 2006, 47, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Jouiaei, M.; Yanagihara, A.A.; Madio, B.; Nevalainen, T.J.; Alewood, P.F.; Fry, B.G. Ancient venom systems: A review on cnidaria toxins. Toxins 2015, 7, 2251–2271. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.C. Solid-to-liquid feeding: The inside(s) out story of extra-oral digestion in predaceous arthropods. Am. Entomol. 1998, 44, 103–116. [Google Scholar] [CrossRef]
- Cohen, A.C. Extra-oral digestion in predaceous terrestrial arthropoda. Annu. Rev. Entomol. 1995, 40, 85–103. [Google Scholar] [CrossRef]
- Schmidt, J.O. Biochemistry of insect venoms. Annu. Rev. Entomol. 1982, 27, 339–368. [Google Scholar] [CrossRef] [PubMed]
- Zlotkin, E. Toxins derived from arthropod venoms specifically affecting insects. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon: Oxford, UK, 1984; Volume 10, pp. 499–546. [Google Scholar]
- Picado, C.T. Estudo experimental sobre o veneno de Lethocerus delpontei (de Carlo) (Hemiptero. Belostomatidae). Mem. Inst. Butantan 1936, 10, 303–311. [Google Scholar]
- Zhu, S.; Darbon, H.; Dyason, K.; Verdonck, F.; Tytgat, J. Evolutionary origin of inhibitor cystine knot peptides. FASEB J. 2003, 17, 1765–1767. [Google Scholar] [CrossRef] [PubMed]
- Undheim, E.A.; Mobli, M.; King, G.F. Toxin structures as evolutionary tools: Using conserved 3D folds to study the evolution of rapidly evolving peptides. Submitted to Bioessays. 2016. [Google Scholar]
- Bernard, C.; Corzo, G.; Mosbah, A.; Nakajima, T.; Darbon, H. Solution structure of Ptu1, a toxin from the assassin bug Peirates turpis that blocks the voltage-sensitive calcium channel N-type. Biochemistry 2001, 40, 12795–12800. [Google Scholar] [CrossRef] [PubMed]
- Corzo, G.; Adachi-Akahane, S.; Nagao, T.; Kusui, Y.; Nakajima, T. Novel peptides from assassin bugs (Hemiptera: Reduviidae): Isolation, chemical and biological characterization. FEBS Lett. 2001, 499, 256–261. [Google Scholar] [CrossRef]
- Ribeiro, J.M.C.; Assumpção, T.C.; Francischetti, I.M.B. An insight into the sialomes of bloodsucking Heteroptera. Psyche (Stuttg.) 2012, 2012, 1–16. [Google Scholar] [CrossRef]
- Cobben, R.H. Evolutionary trends in Heteroptera, part II: Mouthpart structures and feeding strategies. Meded Landbouwhogesch. Wagening. 1978, 78, 1–407. [Google Scholar]
- Cohen, A.C. Feeding adaptations in some predaceous Hemiptera. Ann. Entomol. Soc. Am. 1990, 83, 1215–1223. [Google Scholar] [CrossRef]
- Smith, J.J.B. Feeding mechanisms. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; Volume 4, pp. 34–85. [Google Scholar]
- Sahayaraj, K.; Kanna, A.V.; Kumar, S.M. Gross morphology of feeding canal, salivary apparatus and digestive enzymes of salivary gland of Catamirus brevipennis (Servile) (Hemiptera: Reduviidae). J. Entomol. Res. Soc. 2010, 12, 37–50. [Google Scholar]
- Swart, C.C.; Felgenhauer, B.E. Structure and function of the mouthparts and salivary gland complex of the giant waterbug, Belostoma lutarium (Stål) (Hemiptera: Belostomatidae). Ann. Entomol. Soc. Am. 2003, 95, 870–882. [Google Scholar] [CrossRef]
- Haridass, E.T.; Ananthakrishnan, T.N. Functional morphology of the salivary system in some Reduviidae (Insecta-Heteroptera). Proc. Indian Acad. Sci. 1981, 90, 145–160. [Google Scholar] [CrossRef]
- Louis, D.; Kumar, R. Morphology of the alimentary and reproductive organs in Reduviidae (Hemiptera: Heteroptera) with comments on interrelationships within the family. Ann. Entomol. Soc. Am. 1973, 66, 635–639. [Google Scholar] [CrossRef]
- Barth, R. Estudos anatômicos e histológicos sôbre a subfamília Triatominae (Heteroptera, Reduviidae): IV. parte: O complexo das glândulas salivares de Triatoma infestans. Mem. Inst. Oswaldo Cruz 1954, 52, 517–583. [Google Scholar] [CrossRef]
- Miles, P.W.; Slowiak, D. The accessory salivary gland as the source of water in the saliva of Hemiptera: Heteroptera. Experientia 1976, 32, 1011–1012. [Google Scholar] [CrossRef] [PubMed]
- Swart, C.C.; Deaton, L.E.; Felgenhauer, B.E. The salivary gland and salivary enzymes of the giant waterbugs (Heteroptera; Belostomatidae). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 145, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.R. Histological evidence on the secretory activity of the accessory salivary gland of Lethocerus indicus Lep. & Serv. (Heteroptera—Belostomatidae). Proc. Natl. Acad. Sci. India Sect. B 1992, 62B, 284–287. [Google Scholar]
- Miles, P.W.; Sloviak, D. Transport of whole protein molecules from blood to saliva of a plant-bug. Experientia 1970, 26, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Miles, P.W. The saliva of Hemiptera. In Advances in Insect Physiology; Treherne, J.E., Berridge, M.J., Wigglesworth, V.B., Eds.; Academic Press: Cambridge, UK, 1972; Volume 9, pp. 183–255. [Google Scholar]
- Friend, W.G.; Smith, J.J.B. Feeding in Rhodnius prolixus: Mouthpart activity and salivation, and their correlation with changes of electrical resistance. J. Insect Physiol. 1971, 17, 233–243. [Google Scholar] [CrossRef]
- Miles, P.W. Studies on the salivary physiology of plant bugs: Oxidase activity in the salivary apparatus and saliva. J. Insect Physiol. 1964, 10, 121–129. [Google Scholar] [CrossRef]
- Miles, P.W. The physiological division of labour in the salivary glands of Oncopeltus fasciatus (Dall.) (Heteroptera: Lygaeidae). Aust. J. Biol. Sci. 1967, 20, 785–798. [Google Scholar] [PubMed]
- Maran, S.P.M.; Selvamuthu, K.; Rajan, K.; Kiruba, D.A.; Ambrose, D.P. The salivary protein profile and paralytic potential of three species of Rhynocoris (Hemiptera: Reduviidae) to three insect pests. In Insect Pest Management, a Current Scenario; Ambrose, D.P., Ed.; Entomology Research Unit: Palayamkottai, India, 2011; pp. 346–361. [Google Scholar]
- Morrison, M.N. Gel electrophoretic studies with reference to functional morphology of the salivary glands of Acanthaspis pedestris Stål. (Insecta: Heteroptera : Reduviidae). Proc. Indian Acad. Sci. 1989, 98, 167–173. [Google Scholar] [CrossRef]
- Dutertre, S.; Jin, A.H.; Vetter, I.; Hamilton, B.; Sunagar, K.; Lavergne, V.; Dutertre, V.; Fry, B.G.; Antunes, A.; Venter, D.J.; et al. Evolution of separate predation- and defence-evoked venoms in carnivorous cone snails. Nat. Commun. 2014, 5, 3521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Undheim, E.A.B.; Hamilton, B.R.; Kurniawan, N.D.; Bowlay, G.; Cribb, B.W.; Merritt, D.J.; Fry, B.G.; King, G.F.; Venter, D.J. Production and packaging of a biological arsenal: Evolution of centipede venoms under morphological constraint. Proc. Natl. Acad. Sci. USA 2015, 112, 4026–4031. [Google Scholar] [CrossRef] [PubMed]
- Stern, D.L.; Foster, W.A. The evolution of soldiers in aphids. Biol. Rev. 1996, 71, 27–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Cui, Y.; Rédei, D.; Baňař, P.; Xie, Q.; Štys, P.; Damgaard, J.; Chen, P.P.; Yi, W.B.; Wang, Y.; et al. Phylogenetic divergences of the true bugs (Insecta: Hemiptera: Heteroptera), with emphasis on the aquatic lineages: The last piece of the aquatic insect jigsaw originated in the Late Permian/Early Triassic. Cladistics 2015. [Google Scholar] [CrossRef]
- Schuh, R.T.; Weirauch, C.; Wheeler, W.C. Phylogenetic relationships within the Cimicomorpha (Hemiptera: Heteroptera): A total-evidence analysis. Syst. Entomol. 2009, 34, 15–48. [Google Scholar] [CrossRef]
- Hua, J.; Li, M.; Dong, P.; Cui, Y.; Xie, Q.; Bu, W. Comparative and phylogenomic studies on the mitochondrial genomes of Pentatomomorpha (Insecta: Hemiptera: Heteroptera). BMC Genom. 2008, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tian, Y.; Zhao, Y.; Bu, W. Higher level phylogeny and the first divergence time estimation of Heteroptera (Insecta: Hemiptera) based on multiple genes. PLoS ONE 2012, 7, e32152. [Google Scholar] [CrossRef] [PubMed]
- Cobben, R.H. On the original feeding habits of the Hemiptera (Insecta): A reply to Merrill Sweet. Ann. Entomol. Soc. Am. 1979, 72, 711–715. [Google Scholar] [CrossRef]
- Schuh, R.T.; Slater, J.A. True Bugs of the World (Hemiptera: Heteroptera): Classification and Natural History; Cornell University Press: New York, NY, USA, 1995. [Google Scholar]
- Conticello, S.G.; GIlad, Y.; Avidan, N.; Ben-Asher, E.; Fainzilber, M. Mechanisms for evolving hypervariability: The case of conopeptides. Mol. Biol. Evol. 2001, 18, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Olivera, B.M.; Walker, C.; Cartier, G.E.; Hooper, D.; Santos, A.D.; Schoenfeld, R.; Shetty, R.; Watkins, M.; Bandyopadhyay, P.K.; Hillyard, D.R. Speciation of cone snails and interspecific hyperdivergence of their venom peptides. Ann. N. Y. Acad. Sci. 1999, 870, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Olivera, B.M.; Watkins, M.; Bandyopadhyay, P.; Imperial, J.S.; de la Cotera, E.P.H.; Aguilar, M.B.; Vera, E.L.; Concepcion, G.P.; Lluisma, A. Adaptive radiation of venomous marine snail lineages and the accelerated evolution of venom peptide genes. Ann. N. Y. Acad. Sci. 2012, 1267, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Ohba, S.Y.; Huynh, T.T.T.; Kawada, H.; Le, L.L.; Ngoc, H.T.; Hoang, S.L.; Higa, Y.; Takagi, M. Heteropteran insects as mosquito predators in water jars in southern Vietnam. J. Vector Ecol. 2011, 36, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Ohba, S.Y.; Nakasuji, F. Dietary items of predacious aquatic bugs (Nepoidea: Heteroptera) in Japanese wetlands. Limnology 2006, 7, 41–43. [Google Scholar] [CrossRef]
- Shaalan, E.A.; Canyon, D.V. Aquatic insect predators and mosquito control. Trop. Biomed. 2009, 26, 223–261. [Google Scholar] [PubMed]
- Zuharah, W.F.; Fadzly, N.; Lester, P.J. Lethal and sublethal impacts of predaceous backswimmer Anisops wakefieldi (Hemiptera: Notonectidae) on the life-history traits of the New Zealand mosquito Culex pervigilans (Diptera: Culicidae). J. Med. Entomol. 2013, 50, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Hidaka, K. Anuran-dependent predation by the giant water bug, Lethocerus deyrollei (Hemiptera: Belostomatidae), in rice fields of Japan. Ecol. Res. 2002, 17, 655–661. [Google Scholar] [CrossRef]
- Matheson, R. Bird vs. Insect. Entomol. News 1907, 18, 452. [Google Scholar]
- Mori, A.; Ohba, S. Field observations of predation on snakes by the giant water bug. Bull. Herpetol. Soc. Jpn. 2004, 2004, 78–81. [Google Scholar]
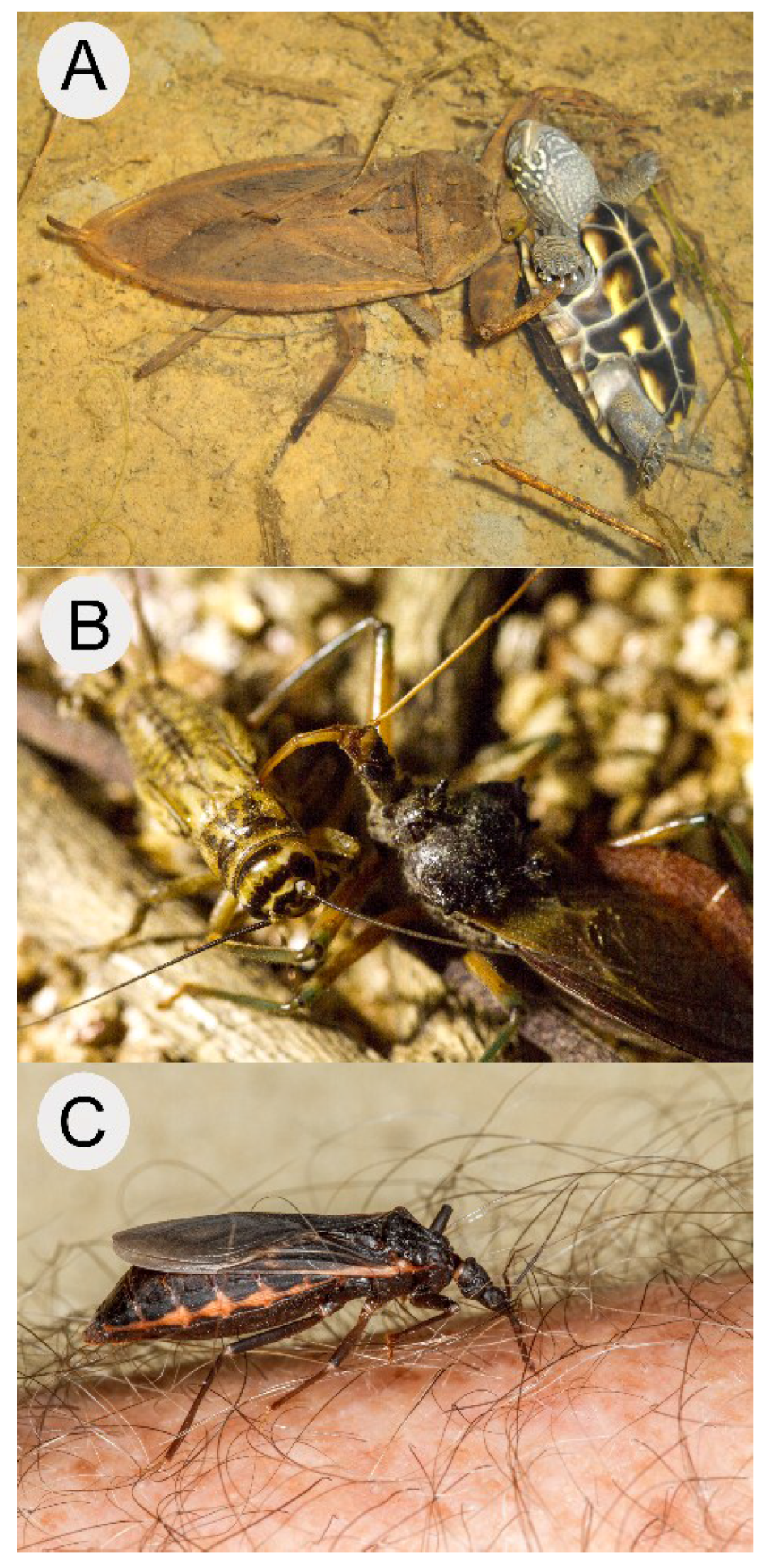
- Ohba, S.Y. Field observation of predation on a turtle by a giant water bug. Entomol. Sci. 2011, 14, 364–365. [Google Scholar] [CrossRef]
- Silva-Cardoso, L.; Caccin, P.; Magnabosco, A.; Patron, M.; Targino, M.; Fuly, A.; Oliveira, G.A.; Pereira, M.H.; do Carmo, M.; Souza, A.S.; et al. Paralytic activity of lysophosphatidylcholine from saliva of the waterbug Belostoma anurum. J. Exp. Biol. 2010, 213, 3305–3310. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.L. Estudo Comparativo da ação da Saliva de Triatomíneos (Heteroptera: Reduviidae) e do Predador Belostoma anurum (Heteroptera: Belostomatidae) Sobre as Preparações de Nervo Isolado de Rattus novergicus e de Vaso Dorsal de Rhodnius prolixus. Master’s Thesis, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 2007. [Google Scholar]
- Dan, A.; Pereira, M.H.; Melo, A.L.; Azeved, A.D.; Freire-Maia, L. Effects induced by the saliva of the aquatic hemipteran Belostoma anurum on the isolated guinea-pig heart. Comp. Biochem. Physiol. 1993, 106, 221–228. [Google Scholar] [CrossRef]
- Rees, A.R.; Offord, R.E. Studies on the protease and other enzymes from the venom of Lethocerus cordofanus. Nature 1969, 221, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.C. On the salivary enzymes of some phytophagous and predaceous heteropterans. Sci. Cult. 1962, 28, 479–480. [Google Scholar]
- Gutiérrez, J.M.; Lomonte, B. Phospholipases A2: Unveiling the secrets of a functionally versatile group of snake venom toxins. Toxicon 2013, 62, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Šribar, J.; Križaj, I. Secreted phospholipases A2—Not just enzymes. Acta Chim. Slov. 2011, 58, 678–688. [Google Scholar] [PubMed]
- Tu, A.T.; Hendon, R.R. Characterization of lizard venom hyaluronidase and evidence for its action as a spreading factor. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1983, 76, 377–383. [Google Scholar] [CrossRef]
- Baek, J.H.; Kang, J.S.; Lee, S.H. Differential gene expression profiles in the salivary gland of Ranatra chinensis (Hemiptera: Nepidae). In Proceedings of the Korean Society of Applied Entomology Biannual Meeting, Vivaldi Park, Korea, 12–14 May 2011; p. 284.
- Caccin, P.; Rigoni, M.; Bisceglie, A.; Rossetto, O.; Montecucco, C. Reversible skeletal neuromuscular paralysis induced by different lysophospholipids. FEBS Lett. 2006, 580, 6317–6321. [Google Scholar] [CrossRef] [PubMed]
- Rigoni, M.; Caccin, P.; Gschmeissner, S.; Koster, G.; Postle, A.D.; Rossetto, O.; Schiavo, G.; Montecucco, C. Equivalent effects of snake PLA2 neurotoxins and lysophospholipid-fatty acid mixtures. Science 2005, 310, 1678–1680. [Google Scholar] [CrossRef] [PubMed]
- Locy, W.A. Anatomy and physiology of the family Nepidae. Am. Nat. 1884, 18, 353–367. [Google Scholar] [CrossRef]
- Neiswander, C.R. On the anatomy of the head and thorax in Ranatra (Heteroptera). Trans. Am. Entomol. Soc. 1925, 51, 311–320. [Google Scholar]
- Grundy, P.; Maelzer, D. Predation by the assassin bug Pristhesancus plagipennis (Walker) (Hemiptera: Reduviidae) of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) and Nezara viridula (L.) (Hemiptera: Pentatomidae) in the laboratory. Aust. J. Entomol. 2000, 39, 280–282. [Google Scholar] [CrossRef]
- Smith, R.F.; Hagen, K.S. Enemies of spotted alfalfa aphid. Calif. Agric. 1956, 10, 8–10. [Google Scholar]
- De Clercq, P.; Coudron, T.A.; Riddick, E.W. Production of heteropteran predators. In Mass Production of Beneficial Organisms; Morales-Ramos, J., Rojas, G., Shapiro-Ilan, D.I., Eds.; Academic Press: London, UK, 2014. [Google Scholar]
- Cohen, A.C. Organization of digestion and preliminary characterization of salivary trypsin-like enzymes in a predaceous heteropteran, Zelus renardii. J. Insect Physiol. 1993, 39, 823–829. [Google Scholar] [CrossRef]
- Cohen, A.C. Plant feeding by predatory Heteroptera: Evolutionary and adaptational aspects of trophic switching. In Zoophytophagous Heteroptera: Implication for Life History and Integrated Pest Management; Alomar, O., Ed.; Thomas Say Publications in Entomology: Lanham, MD, USA, 1996; pp. 1–7. [Google Scholar]
- Ambrose, D.P.; Maran, S.P.M. Polymorphic diversity in salivary and haemolymph proteins and digestive physiology of assassin bug Rhynocoris marginatus (Fab.) (Het., Reduviidae). J. Appl. Entomol. 2000, 124, 315–317. [Google Scholar] [CrossRef]
- Sahayaraj, K.; Sankaralinkam, K.; Balasubramanian, S. Prey influence on the salivary gland and gut enzymes qualitative profile of Rhynocoris marginatus (Fab.) and Catamirus brevipennis (Serville) (Heteroptera: Reduviidae). J. Entomol. 2007, 4, 331–336. [Google Scholar]
- Sahayaraj, K.; Subramanium, M.; Rivers, D. Biochemical and electrophoretic analyses of saliva from the predatory reduviid species Rhynocoris marginatus (Fab.). Acta Biochim. Pol. 2013, 60, 91–97. [Google Scholar]
- Zeng, F.; Cohen, A.C. Demonstration of amylase from the zoophytophagous anthocorid Orius insidiosus. Arch. Insect Biochem. Physiol. 2000, 44, 136–139. [Google Scholar] [CrossRef]
- Boyd, D.W. Digestive enzymes and stylet morphology of Deraeocoris nigritulus (Uhler) (Hemiptera: Miridae) reflect adaptations for predatory habits. Ann. Entomol. Soc. Am. 2003, 96, 667–671. [Google Scholar] [CrossRef]
- Agustí, N.; Cohen, A.C. Lygus hesperus and L. lineolaris (Hemiptera: Miridae), phytophages, zoophages, or omnivores: Evidence of feeding adaptations suggested by the salivary and midgut digestive enzymes. J. Entomol. Sci. 2000, 35, 176–186. [Google Scholar]
- Zeng, F.; Cohen, A.C. Comparison of α-amylase and protease activities of a zoophytophagous and two phytozoophagous Heteroptera. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2000, 126, 101–106. [Google Scholar] [CrossRef]
- Zeng, F.; Cohen, A.C. Partial characterization of α-amylase in the salivary glands of Lygus hesperus and L. lineolaris. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2000, 126, 9–16. [Google Scholar] [CrossRef]
- Zeng, F.; Zhu, Y.C.; Cohen, A.C. Molecular cloning and partial characterization of a trypsin-like protein in salivary glands of Lygus hesperus (Hemiptera: Miridae). Insect Biochem. Mol. Biol. 2002, 32, 455–464. [Google Scholar] [CrossRef]
- Colebatch, G.; Cooper, P.; East, P. cDNA cloning of a salivary chymotrypsin-like protease and the identification of six additional cDNAs encoding putative digestive proteases from the green mirid, Creontiades dilutus (Hemiptera: Miridae). Insect Biochem. Mol. Biol. 2002, 32, 1065–1075. [Google Scholar] [CrossRef]
- Colebatch, G.; East, P.; Cooper, P. Preliminary characterisation of digestive proteases of the green mirid, Creontiades dilutus (Hemiptera: Miridae). Insect Biochem. Mol. Biol. 2001, 31, 415–423. [Google Scholar] [CrossRef]
- Ghamari, M.; Hosseininaveh, V.; Darvishzadeh, A.; Talebi, K. Biochemical characterisation of the tissue degrading enzyme, collagenase, in the spined soldier bug, Podisus maculiventris (Hemiptera: Pentatomidae). J. Plant Prot. Res. 2014, 54, 164–170. [Google Scholar] [CrossRef]
- Martínez, L.; do Carmo Queiroz Fialho, M.; Zanuncio, J.; Serrão, J. Ultrastructure and cytochemistry of salivary glands of the predator Podisus nigrispinus (Hemiptera: Pentatomidae). Protoplasma 2014, 251, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.A.; Oliveira, M.G.A.; Guedes, R.N.C.; Soares, M.J. Morphology and preliminary enzyme characterization of the salivary glands from the predatory bug Podisus nigrispinus (Heteroptera: Pentatomidae). Bull. Entomol. Res. 2006, 96, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Zibaee, A.; Hoda, H.; Fazeli-Dinan, M. Role of proteases in extra-oral digestion of a predatory bug, Andrallus spinidens. J. Insect Sci. 2012, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Weirauch, C. Hairy attachment structures in Reduviidae (Cimicomorpha, Heteroptera), with observations on the fossula spongiosa in some other Cimicomorpha. Zool. Anz. 2007, 246, 155–175. [Google Scholar] [CrossRef]
- Zhang, J.; Gordon, E.R.L.; Forthman, M.; Hwang, W.S.; Walden, K.; Swanson, D.R.; Johnson, K.P.; Meier, R.; Weirauch, C. Evolution of the assassin’s arms: Insights from a phylogeny of combined transcriptomic and ribosomal DNA data (Heteroptera: Reduvioidea). Sci. Rep. 2015. Accepted pending revision press. [Google Scholar]
- Zhang, G.; Weirauch, C. Sticky predators: A comparative study of sticky glands in harpactorine assassin bugs (Insecta: Hemiptera: Reduviidae). Acta Zool. (Stockh.) 2013, 94, 1–10. [Google Scholar] [CrossRef]
- Zhang, G.; Weirauch, C. Molecular phylogeny of Harpactorini (Insecta: Reduviidae): Correlation of novel predation strategy with accelerated evolution of predatory leg morphology. Cladistics 2014, 30, 339–351. [Google Scholar] [CrossRef]
- Davis, N.T. Contribution to the morphology and phylogeny of the Reduvioidea. Part IV. The harpactoroid complex. Ann. Entomol. Soc. Am. 1969, 62, 74–94. [Google Scholar]
- Zhang, J.; Weirauch, C.; Zhang, G.; Forero, D. Molecular phylogeny of Harpactorinae and Bactrodinae uncovers complex evolution of sticky trap predation in assassin bugs (Heteroptera: Reduviidae). Cladistics 2015. [Google Scholar] [CrossRef]
- Weirauch, C.; Forero, D.; Jacobs, D.H. On the evolution of raptorial legs—An insect example (Hemiptera: Reduviidae: Phymatinae). Cladistics 2011, 27, 138–149. [Google Scholar] [CrossRef]
- Weirauch, C. Cladistic analysis of Reduviidae (Heteroptera: Cimicomorpha) based on morphological characters. Syst. Entomol. 2008, 33, 229–274. [Google Scholar] [CrossRef]
- van der Meijden, A.; Herrel, A.; Summers, A. Comparison of chela size and pincer force in scorpions; getting a first grip. J. Zool. 2010, 280, 319–325. [Google Scholar] [CrossRef]
- Livingstone, D.; Ambrose, D.P. Feeding behaviour and predatory efficiency of some reduviids from the Palghat gap, India. J. Madras Univ. Sect. B 1979, 41, 1–25. [Google Scholar]
- Maran, S.P.M.; Ambrose, D.P. Paralytic potential of Catamiarus brevipennis (Serville), a potential biological control agent (Insecta: Heteroptera: Reduviidae). In Biotechnological Applications for Integrated Pest Management; Ignacimuth, A., Sen, A., Janarthanan, S., Eds.; Oxford Publishing: New Delhi, India, 2000; pp. 125–131. [Google Scholar]
- Hwang, W.S.; Weirauch, C. Evolutionary history of assassin bugs (Insecta: Hemiptera: Reduviidae): Insights from divergence dating and ancestral state reconstruction. PLoS ONE 2012, 7, e45523. [Google Scholar] [CrossRef] [PubMed]
- Kerzhner, I.M. Poluzhestkokrylve Semejstva Nabidae; Nauka: Saint Petersburg, Russia, 1981; Volume 13. [Google Scholar]
- Wheeler, A.G. Biology of the Plant Bugs (Hemiptera: Miridae): Pests, Predators, Opportunists; Cornell University Press: Ithaca, NY, USA, 2001. [Google Scholar]
- Hickman, V.V. The biology of two emesine bugs (Hemiptera: Reduvudae) occurring on the nests or webs of spiders. J. Entomol. Soc. Aust. (N.S.W.) 1969, 6, 3–18. [Google Scholar]
- Wygodzinsky, P.W. A Monograph of the Emesinae (Reduviidae, Hemiptera); Bulletin of the American Museum of Natural History; American Museum of Natural History: New York, NY, USA, 1966; Volume 133. [Google Scholar]
- Jackson, R.R.; Wilcox, R.S. Aggressive mimicry, prey-specific predatory behaviour and predator-recognition in the predator-prey interactions of Portia fimbriata and Euryattus sp., jumping spiders from Queensland. Behav. Ecol. Sociobiol. 1990, 26, 111–119. [Google Scholar] [CrossRef]
- Soley, F.G.; Taylor, P.W. Ploys and counterploys of assassin bugs and their dangerous spider prey. Behaviour 2013, 150, 397–425. [Google Scholar] [CrossRef]
- Wignall, A.E.; Taylor, P.W. Alternative predatory tactics of an araneophagic assassin bug (Stenolemus bituberus). Acta Ethol. 2009, 12, 23–27. [Google Scholar] [CrossRef]
- Wignall, A.E.; Taylor, P.W. Predatory behaviour of an araneophagic assassin bug. J. Ethol. 2010, 28, 437–445. [Google Scholar] [CrossRef]
- Wignall, A.E.; Taylor, P.W. Assassin bug uses aggressive mimicry to lure spider prey. Proc. Biol. Sci. 2011, 278, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, E. Biological notes on the hemipteran Ptilocerus ochraceus. Tijdschr. Entomol. 1911, 54, 175–179. [Google Scholar]
- McKeown, K.C. Australian Insects. An Introductory Handbook; Royal Zoological Society of New South Wales: Sydney, Australia, 1944. [Google Scholar]
- Bulbert, M.W.; Herberstein, M.E.; Cassis, G. Assassin bug requires dangerous ant prey to bite first. Curr. Biol. 2014, 24, R220–R221. [Google Scholar] [CrossRef] [PubMed]
- Weirauch, C.; Bulbert, M.; Cassis, G. Comparative trichome morphology in feather-legged assassin bugs (Insecta: Heteroptera: Reduviidae: Holoptilinae). Zool. Anz. 2010, 248, 237–253. [Google Scholar] [CrossRef]
- Weirauch, C.; Cassis, G. Attracting ants: The trichome and novel glandular areas on the sternum of Ptilocnemus lemur (Heteroptera: Reduviidae: Holoptilinae). J. N. Y. Entomol. Soc. 2006, 114, 28–37. [Google Scholar] [CrossRef]
- Gordon, E.R.L.; Weirauch, C. Efficient capture of natural history data reveals prey conservatism of cryptic termite predators. Mol. Phylogenet. Evol. 2016, 94, 65–73. [Google Scholar] [CrossRef] [PubMed]
- McMahan, E.A. Adventures documenting an assassin bug that “fishes” for termites. Am. Entomol. 2005, 51, 202–207. [Google Scholar] [CrossRef]
- Forthman, M.; Weirauch, C. Toxic associations: A review of the predatory behaviors of millipede assassin bugs (Hemiptera: Reduviidae: Ectrichodiinae). Eur. J. Entomol. 2012, 109, 147–153. [Google Scholar] [CrossRef]
- Haviland, M.D. The Reduviidae of Kartabo, Bartica District, British Guiana. Zoologica 1931, 7, 129–154. [Google Scholar]
- Casimir, M. Tegea atropicta Stål (Hemiptera, Reduviidae), an unusual predator of termites. Proc. Linn. Soc. N.S.W. 1960, 85, 230–232. [Google Scholar]
- Bérenger, J.M.; Pluot-Sigwalt, D. Notes sur Micrauchenus lineola (Fabricius 1787), espèce termitophile et termitophage (Heteroptera: Reduviidae: Harpactorinae, Apiomerini). Ann. Soc. Entomol. Fr. 2009, 45, 129–133. [Google Scholar]
- Haridass, E.T. Feeding and ovipositional behaviour in some reduviids (Insecta-Heteroptera). Proc. Anim. Sci. 1985, 94, 239–247. [Google Scholar] [CrossRef]
- Jackson, R.R.; Salm, K.; Nelson, X.J. Specialized prey selection behavior of two East African assassin bugs, Scipinnia [Scipinia] repax and Nagusta sp. that prey on social jumping spiders. J. Insect Sci. 2010, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- King, G.F.; Hardy, M.G. Spider-venom peptides: Structure, pharmacology, and potential for control of insect pests. Annu. Rev. Entomol. 2013, 58, 475–496. [Google Scholar] [CrossRef] [PubMed]
- Barlow, A.; Pook, C.E.; Harrison, R.A.; Wüster, W. Coevolution of diet and prey-specific venom activity supports the role of selection in snake venom evolution. Proc. Biol. Sci. 2009, 276, 2443–2449. [Google Scholar] [CrossRef] [PubMed]
- Duda, T.F.; Palumbi, S.R. Gene expression and feeding ecology: Evolution of piscivory in the venomous gastropod genus Conus. Proc. Biol. Sci. 2004, 271, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Pekar, S.; Toft, S. Trophic specialisation in a predatory group: The case of prey-specialised spiders (Araneae). Biol. Rev. 2015, 90, 744–761. [Google Scholar] [CrossRef] [PubMed]
- Remigio, E.A.; Duda, T.F., Jr. Evolution of ecological specialization and venom of a predatory marine gastropod. Mol. Ecol. 2008, 17, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Sahayaraj, K.; Muthukumar, S. Zootoxic effects of reduviid Rhynocoris marginatus (Fab.) (Hemiptera: Reduviidae) venomous saliva on Spodoptera litura (Fab.). Toxicon 2011, 58, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, D.; Himuro, C.; Fujisaki, K. Prey size affects the costs and benefits of group predation in nymphs of the predatory stink bug Andrallus spinidens (Heteroptera: Pentatomidae). J. Ethol. 2014, 32, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Slater, J.A.; Carayon, J. Ethiopian Lygaeidae IV: A new predatory lygaeid from Africa with a discussion of its biology and morphology (Hemiptera: Heteroptera). Proc. R. Entomol. Soc. Lond. A 1963, 38, 1–11. [Google Scholar] [CrossRef]
- Sahayaraj, K.; Vinothkanna, A. Insecticidal activity of venomous saliva from Rhynocoris fuscipes (Reduviidae) against Spodoptera litura and Helicoverpa armigera by microinjection and oral administration. J. Venom. Anim. Toxins Incl. Trop. Dis. 2011, 17, 486–490. [Google Scholar] [CrossRef]
- Edwards, J.S. Spitting as a defensive mechanism in a predatory reduviid. In Proc XI International Congress on Entomology; ICE: Vienna, 1960; Volume 3, pp. 259–263. [Google Scholar]
- Fink, L.S. Venom spitting by the green lynx spider, Peucetia viridans (Araneae, Oxyopidae). J. Arachnol. 1984, 12, 372–373. [Google Scholar]
- Schmidt, J.O.; Yamane, T.; Matsuura, M.; Starr, C.K. Hornet venoms: Lethalities and lethal capacities. Toxicon 1986, 24, 950–954. [Google Scholar] [CrossRef]
- Zerachia, T.; Shulov, A.; Bergmann, F. Hemolysis induced by the venom of the predaceous bug Holotrichius innesi H. (Heteroptera, Reduviidae). In Animal and Plant Toxins; Kaiser, E., Ed.; Goldman: Munich, Germany, 1973; pp. 147–150. [Google Scholar]
- King, G.F. Venoms as a platform for human drugs: Translating toxins into therapeutics. Expert Opin. Biol. Ther. 2011, 11, 1469–1484. [Google Scholar] [CrossRef] [PubMed]
- Evangelin, G.; Horne, B.; Muthupandi, M.; William, J.S. Venomous saliva of non-haematophagous reduviid bugs (Heteroptera: Reduviidae): A review. Biolife 2014, 2, 615–626. [Google Scholar]
- Ambrose, D.P.; Maran, S.P.M. Quantification, protein content and paralytic potential of saliva of fed and prey deprived reduviid Acanthaspis pedestris Stål (Heteroptera: Reduviidae: Reduviinae). Indian J. Environ. Sci. 1999, 3, 11–16. [Google Scholar]
- Sahayaraj, K.; Kumar, S.M.; Anandh, G.P. Evaluation of milking and electric shocks for venom collection from hunter reduviids. Entomon 2006, 31, 65–68. [Google Scholar]
- Boevé, J.L.; Kuhn-Nentwig, L.; Keller, S.; Nentwig, W. Quantity and quality of venom released by a spider (Cupiennius salei, Ctenidae). Toxicon 1995, 33, 1347–1357. [Google Scholar] [CrossRef]
- Johnson, B.D.; Stahnke, H.L.; Koonce, R. A method for estimating Crotalus atrox venom concentrations. Toxicon 1967, 5, 35–38. [Google Scholar] [CrossRef]
- Holm, L.; Rosenström, P. Dali server: Conservation mapping in 3D. Nucleic Acids Res. 2010, 38, W545–W549. [Google Scholar] [CrossRef] [PubMed]
- Vinoth Kanna, A. Rhynocoris fuscipes Fab. Venomous Saliva Biological Immunomodulatory Activity against Insect Pest and Mice. Ph.D. Thesis, Manonmaniam Sundaranar University, Palayamkottai, India, 2014. [Google Scholar]
- Liu, Z.; Dai, J.; Dai, L.; Deng, M.; Hu, Z.; Hu, W.; Liang, S. Function and solution structure of Huwentoxin-X, a specific blocker of N-type calcium channels, from the Chinese bird spider Ornithoctonus huwena. J. Biol. Chem. 2006, 281, 8628–8635. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.C.; Fialho, M.D.C.Q.; Almeida Barbosa, L.C.; Oliveira, L.L.; Zanuncio, J.C.; Serrão, J.E. Stink bug predator kills prey with salivary non-proteinaceous compounds. Insect Biochem. Mol. Biol. 2016, 68, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Habibi, J.; Backus, E.A.; Coudron, T.A.; Brandt, S.L. Effect of different host substrates on hemipteran salivary protein profiles. Entomol. Exp. Appl. 2001, 98, 369–375. [Google Scholar] [CrossRef]
- Andersen, J.F.; Ding, X.D.; Balfour, C.A.; Shokhireva, T.K.; Champagne, D.E.; Walker, F.A.; Montfort, W.R. Kinetics and equilibria in ligand binding by nitrophorins 1–4: Evidence for stabilization of a nitric oxide-ferriheme complex through a ligand-induced conformational trap. Biochemistry 2000, 39, 10118–10131. [Google Scholar] [CrossRef] [PubMed]
- Fialho, M.C.Q.; Moreira, N.R.; Zanuncio, J.C.; Ribeiro, A.F.; Terra, W.R.; Serrão, J.E. Prey digestion in the midgut of the predatory bug Podisus nigrispinus (Hemiptera: Pentatomidae). J. Insect Physiol. 2012, 58, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Lee, S.H. Differential gene expression profiles in the salivary gland of Orius laevigatus. J. Asia-Pac. Entomol. 2014, 17, 729–735. [Google Scholar] [CrossRef]
- Schofield, C.J. Biosystematics and evolution of the Triatominae. Cad. Saude Publica 2000, 16, S89–S92. [Google Scholar] [CrossRef]
- Ribeiro, J.M.; Schneider, M.; Isaias, T.; Jurberg, J.; Galvão, C.; Guimarães, J.A. Role of salivary antihemostatic components in blood feeding by triatomine bugs (Heteroptera). J. Med. Entomol. 1998, 35, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Schofield, C.J.; Galvao, C. Classification, evolution, and species groups within the Triatominae. Acta Trop. 2009, 110, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Harrington, B.J. Comments on the blood-feeding tribe Cleradini (Hemiptera: Lygaeidae: Rhyparochrominae) and description of a new genus and new species with the legs modified for grasping. Ann. Entomol. Soc. Am. 1988, 81, 577–581. [Google Scholar] [CrossRef]
- Harrington, B.J. Detecting evidence of hematophagy in dry museum specimens of Clerada apicicornis (Hemiptera: Lygaeidae: Rhyparochrominae). Ann. Entomol. Soc. Am. 1990, 83, 545–548. [Google Scholar] [CrossRef]
- Lent, H. Sobre o hematofagismo da Clerada apicicornis e outros artropodos; sua importancia na transmissão da doença de Chagas. Mem. Inst. Oswaldo Cruz 1939, 34, 583–606. [Google Scholar] [CrossRef]
- Torres, M.; Cárdenas, E.; Pérez, S.; Morales, A. Haematophagy and cleptohaematophagy of Clerada apicicornis (Hemiptera: Lygaeidae), a potential biological control agent of Rhodnius prolixus (Hemiptera: Reduviidae). Mem. Inst. Oswaldo Cruz 2000, 95, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Chagas, C. Nova tripanozomiaze humana: Estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Mem. Inst. Oswaldo Cruz 1909, 1, 159–218. [Google Scholar] [CrossRef]
- Andersen, J.F. Structure and mechanism in salivary proteins from blood-feeding arthropods. Toxicon 2010, 56, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Andrade, B.B.; Teixeira, C.R.; Barral, A.; Barral-Netto, M. Haematophagous arthropod saliva and host defense system: A tale of tear and blood. An. Acad. Bras. Cienc. 2005, 77, 665–693. [Google Scholar] [CrossRef] [PubMed]
- Champagne, D.E. Antihemostatic molecules from saliva of blood-feeding arthropods. Pathophysiol. Haemost. Thromb. 2005, 34, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Mans, B.J.; Francischetti, I.M.B. Sialomic perspectives on the evolution of blood-feeding behavior in arthropods: Future therapeutics by natural design. In Toxins and Hemostasis; Kini, R.M., Clemetson, K.J., Markland, F.S., McLane, M.A., Morita, T., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 21–44. [Google Scholar]
- Ribeiro, J.M.; Francischetti, I.M. Role of arthropod saliva in blood feeding: Sialome and post-sialome perspectives. Annu. Rev. Entomol. 2003, 48, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.C.; Arcà, B. From sialomes to the sialoverse. In Adv. Insect Physiol.; Simpson, S.J., Casas, J., Eds.; Academic Press: Amsterdam, The Netherlands, 2009; Volume 37, pp. 59–118. [Google Scholar]
- Alves, C.L.; Araujo, R.N.; Gontijo, N.F.; Pereira, M.H. Importance and physiological effects of hemolymphagy in triatomines (Hemiptera: Reduviidae). J. Med. Entomol. 2011, 48, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Herzig, V. Ontogenesis, gender, and molting influence the venom yield in the spider Coremiocnemis tropix (Araneae, Theraphosidae). J. Venom Res. 2010, 1, 76–83. [Google Scholar] [PubMed]
- Ribeiro, J.M.; Marinotti, O.; Gonzales, R. A salivary vasodilator in the blood-sucking bug, Rhodnius prolixus. Br. J. Pharmacol. 1990, 101, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.C. The antiserotonin and antihistamine activities of salivary secretion of Rhodnius prolixus. J. Insect Physiol. 1982, 28, 69–75. [Google Scholar] [CrossRef]
- Assumpção, T.C.F.; Alvarenga, P.H.; Ribeiro, J.M.C.; Andersen, J.F.; Francischetti, I.M.B. Dipetalodipin, a novel multifunctional salivary lipocalin that inhibits platelet aggregation, vasoconstriction, and angiogenesis through unique binding specificity for TXA2, PGF2α, and 15(S)-HETE. J. Biol. Chem. 2010, 285, 39001–39012. [Google Scholar] [CrossRef] [PubMed]
- Noeske-Jungblut, C.; Krätzschmar, J.; Haendler, B.; Alagon, A.; Possani, L.; Verhallen, P.; Donner, P.; Schleuning, W.D. An inhibitor of collagen-induced platelet aggregation from the saliva of Triatoma pallidipennis. J. Biol. Chem. 1994, 269, 5050–5053. [Google Scholar] [PubMed]
- Ribeiro, J.M.; Garcia, E.S. Platelet antiaggregating activity in the salivary secretion of the blood sucking bug Rhodnius prolixus. Experientia 1981, 37, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, J.G.; Chuffe, O.M.; Ribeiro, J.M.C. Apyrase and anti-platelet activities from the salivary glands of the bed bug Cimex lectularius. Insect Biochem. Mol. Biol. 1996, 26, 557–562. [Google Scholar] [CrossRef]
- Hellmann, K.; Hawkins, R.I. Prolixin-S and Prolixin-G; Two anticoagulants from Rhodnius prolixus Stål. Nature 1965, 207, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Noeske-Jungblut, C.; Haendler, B.; Donner, P.; Alagon, A.; Possani, L.; Schleuning, W.D. Triabin, a highly potent exosite inhibitor of Thrombin. J. Biol. Chem. 1995, 270, 28629–28634. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.; Schneider, M.; Guimarães, J.A. Purification and characterization of prolixin-S (nitrophorin 2), the salivary anticoagulant of the blood-sucking bug Rhodnius prolixus. Biochem. J. 1995, 308, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, J.G.; Guimaraes, J.A.; Ribeiro, J.M.C. A novel inhibitor of Factor X activation from the salivary glands of the bed bug Cimex lectularius. Exp. Parasitol. 1996, 83, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Dan, A.; Pereira, M.H.; Pesquero, J.L.; Diotaiuti, L.; Beirao, P.S. Action of the saliva of Triatoma infestans (Heteroptera: Reduviidae) on sodium channels. J. Med. Entomol. 1999, 36, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.M.; Wood, J.N.; Cox, J.J. Sodium channels and pain. Handb. Exp. Pharmacol. 2015, 227, 39–56. [Google Scholar] [PubMed]
- King, G.F.; Vetter, I. No gain, no pain: NaV1.7 as an analgesic target. ACS Chem. Neurosci. 2014, 5, 749–751. [Google Scholar] [CrossRef] [PubMed]
- Lavoipierre, M.M.; Dickerson, G.; Gordon, R.M. Studies on the methods of feeding of blood-sucking arthropods. I. The manner in which triatomine bugs obtain their blood-meal, as observed in the tissues of the living rodent, with some remarks on the effects of the bite on human volunteers. Ann. Trop. Med. Parasitol. 1959, 53, 235–250. [Google Scholar] [PubMed]
- Ribeiro, J.M.C.; Garcia, E.S. The role of the salivary glands in feeding in Rhodnius prolixus. J. Exp. Biol. 1981, 94, 219–230. [Google Scholar]
- Champagne, D.E.; Nussenzveig, R.H.; Ribeiro, J.M.C. Purification, partial characterization, and cloning of nitric oxide-carrying heme proteins (nitrophorins) from salivary glands of the blood-sucking insect Rhodnius prolixus. J. Biol. Chem. 1995, 270, 8691–8695. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.; Hazzard, J.M.; Nussenzveig, R.H.; Champagne, D.E.; Walker, F.A. Reversible binding of nitric oxide by a salivary heme protein from a bloodsucking insect. Science 1993, 260, 539–541. [Google Scholar] [CrossRef] [PubMed]
- Golodne, D.M.; Monteiro, R.Q.; Graça-Souza, A.V.; Silva-Neto, M.A.C.; Atella, G.C. Lysophosphatidylcholine acts as an anti-hemostatic molecule in the saliva of the blood-sucking bug Rhodnius prolixus. J. Biol. Chem. 2003, 278, 27766–27771. [Google Scholar] [CrossRef] [PubMed]
- Ambrus, A.; Friedrich, K.; Somogyi, Á. Oligomerization of nitrophorins. Anal. Biochem. 2006, 352, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.F.; Champagne, D.E.; Weichsel, A.; Ribeiro, J.M.C.; Balfour, C.A.; Dress, V.; Montfort, W.R. Nitric oxide binding and crystallization of recombinant nitrophorin I, a nitric oxide transport protein from the blood-sucking bug Rhodnius prolixus. Biochemistry 1997, 36, 4423–4428. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.F.; Weichsel, A.; Balfour, C.A.; Champagne, D.E.; Montfort, W.R. The crystal structure of Nitrophorin 4 at 1.5 Å resolution: Transport of nitric oxide by a lipocalin-based heme protein. Structure 1998, 6, 1315–1327. [Google Scholar] [CrossRef]
- Berry, R.E.; Muthu, D.; Yang, F.; Walker, F.A. NMR studies of the dynamics of high-spin Nitrophorins: Comparative studies of NP4 and NP2 at close to physiological pH. Biochemistry 2015, 54, 221–239. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Nishikawa, K.; Erdem, Ö.F.; Reijerse, E.; Ogata, H.; Lubitz, W.; Knipp, M. Complexes of ferriheme nitrophorin 4 with low-molecular weight thiol(ate)s occurring in blood plasma. J. Inorg. Biochem. 2013, 122, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Weichsel, A.; Andersen, J.F.; Champagne, D.E.; Walker, F.A.; Montfort, W.R. Crystal structures of a nitric oxide transport protein from a blood-sucking insect. Nat. Struct. Biol. 1998, 5, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Weichsel, A.; Andersen, J.F.; Roberts, S.A.; Montfort, W.R. Nitric oxide binding to Nitrophorin 4 induces complete distal pocket burial. Nat. Struct. Biol. 2000, 7, 551–554. [Google Scholar] [PubMed]
- Andersen, J.F.; Montfort, W.R. The crystal structure of Nitrophorin 2: A trifunctional antihemostatic protein from the saliva of Rhodnius prolixus. J. Biol. Chem. 2000, 275, 30496–30503. [Google Scholar] [CrossRef] [PubMed]
- Gudderra, N.P.; Ribeiro, J.M.C.; Andersen, J.F. Structural determinants of Factor IX(a) binding in Nitrophorin 2, a lipocalin inhibitor of the intrinsic coagulation pathway. J. Biol. Chem. 2005, 280, 25022–25028. [Google Scholar] [CrossRef] [PubMed]
- Isawa, H.; Yuda, M.; Yoneda, K.; Chinzei, Y. The insect salivary protein, Prolixin-S, inhibits Factor IXa generation and Xase complex formation in the blood coagulation pathway. J. Biol. Chem. 2000, 275, 6636–6641. [Google Scholar] [CrossRef] [PubMed]
- Yuda, M.; Higuchi, K.; Sun, J.; Kureishi, Y.; Ito, M.; Chinzei, Y. Expression, reconstitution and characterization of Prolixin-S as a vasodilator. Eur. J. Biochem. 1997, 249, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ribeiro, J.M.C.; Guimarães, J.A.; Walsh, P.N. Nitrophorin-2:A novel mixed-type reversible specific inhibitor of the intrinsic Factor-X activating complex. Biochemistry 1998, 37, 10681–10690. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.F.; Gudderra, N.P.; Francischetti, I.M.B.; Valenzuela, J.G.; Ribeiro, J.M.C. Recognition of anionic phospholipid membranes by an antihemostatic protein from a blood-feeding insect. Biochemistry 2004, 43, 6987–6994. [Google Scholar] [CrossRef] [PubMed]
- Knipp, M.; Soares, R.P.; Pereira, M.H. Identification of the native N-terminus of the membrane attaching ferriheme protein nitrophorin 7 from Rhodnius prolixus. Anal. Biochem. 2012, 424, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Knipp, M.; Yang, F.; Berry, R.E.; Zhang, H.; Shokhirev, M.N.; Walker, F.A. Spectroscopic and functional characterization of Nitrophorin 7 from the blood-feeding insect Rhodnius prolixus reveals an important role of its isoform-specific N-Terminus for proper protein function. Biochemistry 2007, 46, 13254–13268. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Allegri, A.; Bidon-Chanal, A.; Knipp, M.; Roitberg, A.E.; Abbruzzetti, S.; Viappiani, C.; Luque, F.J. Kinetics and computational studies of ligand migration in nitrophorin 7 and its Δ1–3 mutant. Biochim. Biophys. Acta 2013, 1834, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.; Yang, F.; Pacheco, V.; Wrede, K.; Medvedev, A.; Ogata, H.; Knipp, M.; Heise, H. Expression, purification, and solid-state NMR characterization of the membrane binding heme protein Nitrophorin 7 in two electronic spin states. Biochemistry 2013, 52, 7031–7040. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.F.; Francischetti, I.M.B.; Valenzuela, J.G.; Schuck, P.; Ribeiro, J.M.C. Inhibition of hemostasis by a high affinity biogenic amine-binding protein from the saliva of a blood-feeding insect. J. Biol. Chem. 2003, 278, 4611–4617. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chang, B.W.; Mans, B.J.; Ribeiro, J.M.; Andersen, J.F. Structure and ligand-binding properties of the biogenic amine-binding protein from the saliva of a blood-feeding insect vector of Trypanosoma cruzi. Acta Crystallogr. D Biol. Crystallogr. 2013, 69, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Prior, P.; Noeske-Jungblut, C.; Donner, P.; Schleuning, W.-D.; Huber, R.; Bode, W. Structure of the thrombin complex with triabin, a lipocalin-like exosite-binding inhibitor derived from a triatomine bug. Proc. Natl. Acad. Sci. USA 1997, 94, 11845–11850. [Google Scholar] [CrossRef] [PubMed]
- Glusa, E.; Bretschneider, E.; Daum, J.; Noeske-Jungblut, C. Inhibition of thrombin-mediated cellular effects by triabin, a highly potent anion-binding exosite thrombin inhibitor. Thromb. Haemost. 1997, 77, 1196–1200. [Google Scholar] [PubMed]
- Haendler, B.; Becker, A.; Noeske-Jungblut, C.; Kratzchmar, J.; Donner, P.; Schleuning, W.D. Expression of active recombinant palidipin, a novel platelet aggregation inhibitor, in the periplasm of Escherichia coli. Biochem. J. 1995, 307. [Google Scholar] [CrossRef]
- Haendler, B.; Becker, A.; Noeske-Jungblut, C.; Krãtzschmar, J.; Donner, P.; Schleuning, W.D. Expression, purification and characterisation of recombinant pallidipin, a novel platelet aggregation inhibitor from the haematophageous triatomine bug Triatoma pallidipennis. Blood Coagul. Fibrinolysis 1996, 7, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Francischetti, I.M.B.; Andersen, J.F.; Ribeiro, J.M.C. Biochemical and functional characterization of recombinant Rhodnius prolixus Platelet Aggregation Inhibitor 1 as a novel lipocalin with high affinity for adenosine diphosphate and other adenine nucleotides. Biochemistry 2002, 41, 3810–3818. [Google Scholar] [CrossRef] [PubMed]
- Francischetti, I.M.B.; Ribeiro, J.M.C.; Champagne, D.; Andersen, J. Purification, cloning, expression, and mechanism of action of a novel platelet aggregation inhibitor from the salivary gland of the blood-sucking bug, Rhodnius prolixus. J. Biol. Chem. 2000, 275, 12639–12650. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Assumpção, T.C.F.; Li, Y.; Andersen, J.F.; Ribeiro, J.M.C.; Francischetti, I.M.B. Triplatin, a platelet aggregation inhibitor from the salivary gland of the triatomine vector of Chagas Disease, binds to TXA2 but does not interact with GPVI. Thromb. Haemost. 2012, 107, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Mizurini, D.M.; Aslan, J.S.; Gomes, T.; Ma, D.; Francischetti, I.M.B.; Monteiro, R.Q. Salivary thromboxane A2-binding proteins from triatomine vectors of Chagas disease inhibit platelet-mediated Neutrophil Extracellular Traps (NETs) formation and arterial thrombosis. PLoS Negl. Trop. Dis. 2015, 9, e0003869. [Google Scholar] [CrossRef] [PubMed]
- Morita, A.; Isawa, H.; Orito, Y.; Iwanaga, S.; Chinzei, Y.; Yuda, M. Identification and characterization of a collagen-induced platelet aggregation inhibitor, triplatin, from salivary glands of the assassin bug, Triatoma infestans. FEBS J. 2006, 273, 2955–2962. [Google Scholar] [CrossRef] [PubMed]
- Isawa, H.; Orito, Y.; Jingushi, N.; Iwanaga, S.; Morita, A.; Chinzei, Y.; Yuda, M. Identification and characterization of plasma kallikrein–kinin system inhibitors from salivary glands of the blood-sucking insect Triatoma infestans. FEBS J. 2007, 274, 4271–4286. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, Y.; Gomez, E.A.; Zhang, F.; Martini-Robles, L.; Iwata, H.; Sakurai, T.; Katakura, K.; Hashiguchi, Y.; Kato, H. Dimiconin, a novel coagulation inhibitor from the kissing bug, Triatoma dimidiata, a vector of Chagas disease. J. Exp. Biol. 2012, 215, 3597–3602. [Google Scholar] [CrossRef] [PubMed]
- Assumpção, T.C.F.; Ma, D.; Schwarz, A.; Reiter, K.; Santana, J.M.; Andersen, J.F.; Ribeiro, J.M.C.; Nardone, G.; Yu, L.L.; Francischetti, I.M.B. Salivary antigen-5/CAP family members are Cu2+-dependent antioxidant enzymes that scavenge O2− and inhibit collagen-induced platelet aggregation and neutrophil oxidative burst. J. Biol. Chem. 2013, 288, 14341–14361. [Google Scholar] [CrossRef] [PubMed]
- Faudry, E.; Lozzi, S.P.; Santana, J.M.; D’Souza-Ault, M.; Kieffer, S.; Felix, C.R.; Ricart, C.A.O.; Sousa, M.V.; Vernet, T.; Teixeira, A.R.L. Triatoma infestans apyrases belong to the 5′-nucleotidase family. J. Biol. Chem. 2004, 279, 19607–19613. [Google Scholar] [CrossRef] [PubMed]
- Faudry, E.; Santana, J.M.; Ebel, C.; Vernet, T.; Teixeira, A.R.L. Salivary apyrases of Triatoma infestans are assembled into homo-oligomers. Biochem. J. 2006, 396, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Amino, R.; Martins, R.M.; Procopio, J.; Hirata, I.Y.; Juliano, M.A.; Schenkman, S. Trialysin, a novel pore-forming protein from saliva of hematophagous insects activated by limited proteolysis. J. Biol. Chem. 2002, 277, 6207–6213. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.M.; Amino, R.; Daghastanli, K.R.; Cuccovia, I.M.; Juliano, M.A.; Schenkman, S. A short proregion of trialysin, a pore-forming protein of Triatoma infestans salivary glands, controls activity by folding the N-terminal lytic motif. FEBS J. 2008, 275, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.M.; Sforça, M.L.; Amino, R.; Juliano, M.A.; Oyama, S.; Juliano, L.; Pertinhez, T.A.; Spisni, A.; Schenkman, S. Lytic activity and structural differences of amphipathic peptides derived from Trialysin. Biochemistry 2006, 45, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Amino, R.; Tanaka, A.S.; Schenkman, S. Triapsin, an unusual activatable serine protease from the saliva of the hematophagous vector of Chagas’ disease Triatoma infestans (Hemiptera: Reduviidae). Insect Biochem. Mol. Biol. 2001, 31, 465–472. [Google Scholar] [CrossRef]
- Meiser, C.K.; Piechura, H.; Meyer, H.E.; Warscheid, B.; Schaub, G.A.; Balczun, C. A salivary serine protease of the haematophagous reduviid Panstrongylus megistus: Sequence characterization, expression pattern and characterization of proteolytic activity. Insect Mol. Biol. 2010, 19, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.F.; Ribeiro, J.M.C. A secreted salivary inositol polyphosphate 5-phosphatase from a blood-feeding insect: Allosteric activation by soluble phosphoinositides and phosphatidylserine. Biochemistry 2006, 45, 5450–5457. [Google Scholar] [CrossRef] [PubMed]
- Paddock, C.D.; McKerrow, J.H.; Hansell, E.; Foreman, K.W.; Hsieh, I.; Marshall, N. Identification, cloning, and recombinant expression of Procalin, a major triatomine allergen. J. Immunol. 2001, 167, 2694–2699. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, J.G.; Ribeiro, J.M.C. Purification and cloning of the salivary nitrophorin from the hemipteran Cimex lectularius. J. Exp. Biol. 1998, 201, 2659–2664. [Google Scholar] [PubMed]
- Valenzuela, J.G.; Walker, F.A.; Ribeiro, J.M. A salivary nitrophorin (nitric-oxide-carrying hemoprotein) in the bedbug Cimex lectularius. J. Exp. Biol. 1995, 198, 1519–1526. [Google Scholar] [PubMed]
- Valenzuela, J.G.; Charlab, R.; Galperin, M.Y.; Ribeiro, J.M.C. Purification, cloning, and expression of an apyrase from the bed bug Cimex lectularius : A new type of nucleotide-binding enzyme. J. Biol. Chem. 1998, 273, 30583–30590. [Google Scholar] [CrossRef] [PubMed]
- Assumpção, T.C.F.; Francischetti, I.M.; Andersen, J.F.; Schwarz, A.; Santana, J.M.; Ribeiro, J.M. An insight into the sialome of the blood-sucking bug Triatoma infestans, a vector of Chagas’ disease. Insect Biochem. Mol. Biol. 2008, 38, 213–232. [Google Scholar] [CrossRef] [PubMed]
- Assumpção, T.C.F.; Charneau, S.; Santiago, P.B.M.; Francischetti, I.M.B.; Meng, Z.; Araújo, C.N.; Pham, V.M.; Queiroz, R.M.L.; de Castro, C.N.; Ricart, C.A.; et al. Insight into the salivary transcriptome and proteome of Dipetalogaster maxima. J. Proteome Res. 2011, 10, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Assumpção, T.C.F.; Eaton, D.P.; Pham, V.M.; Francischetti, I.M.; Aoki, V.; Hans-Filho, G.; Rivitti, E.A.; Valenzuela, J.G.; Diaz, L.A.; Ribeiro, J.M. An insight into the sialotranscriptome of Triatoma matogrossensis, a kissing bug associated with fogo selvagem in South America. Am. J. Trop. Med. Hyg. 2012, 86, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Bussacos, A.C.; Nakayasu, E.S.; Hecht, M.M.; Assumpção, T.C.; Parente, J.A.; Soares, C.M.; Santana, J.M.; Almeida, I.C.; Teixeira, A.R. Redundancy of proteins in the salivary glands of Panstrongylus megistus secures prolonged procurement for blood meals. J. Proteomics 2011, 74, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Bussacos, A.C.; Nakayasu, E.S.; Hecht, M.M.; Parente, J.A.; Soares, C.M.; Teixeira, A.R.; Almeida, I.C. Diversity of anti-haemostatic proteins in the salivary glands of Rhodnius species transmitters of Chagas disease in the greater Amazon. J. Proteomics 2011, 74, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.M.; Sousa, M.V.; Ricart, C.A.; Santana, J.M.; Teixeira, A.R.; Roepstorff, P.; Charneau, S. 2-DE-based proteomic investigation of the saliva of the Amazonian triatomine vectors of Chagas disease: Rhodnius brethesi and Rhodnius robustus. J. Proteomics 2011, 74, 1652–1663. [Google Scholar] [CrossRef] [PubMed]
- Francischetti, I.M.; Calvo, E.; Andersen, J.F.; Pham, V.M.; Favreau, A.J.; Barbian, K.D.; Romero, A.; Valenzuela, J.G.; Ribeiro, J.M. Insight into the sialome of the bed bug, Cimex lectularius. J. Proteome Res. 2010, 9, 3820–3831. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Jochim, R.C.; Gomez, E.A.; Sakoda, R.; Iwata, H.; Valenzuela, J.G.; Hashiguchi, Y. A repertoire of the dominant transcripts from the salivary glands of the blood-sucking bug, Triatoma dimidiata, a vector of Chagas disease. Infect. Genet. Evol. 2010, 10, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.; Assumpção, T.C.; Pham, V.M.; Francischetti, I.M.; Reisenman, C.E. An insight into the sialotranscriptome of Triatoma rubida (Hemiptera: Heteroptera). J. Med. Entomol. 2012, 49, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.; Schwarz, A.; Francischetti, I.M. A deep insight into the sialotranscriptome of the Chagas disease vector, Panstrongylus megistus (Hemiptera: Heteroptera). J. Med. Entomol. 2015, 52, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.C.; Andersen, J.; Silva-Neto, M.A.C.; Pham, V.M.; Garfield, M.K.; Valenzuela, J.G. Exploring the sialome of the blood-sucking bug Rhodnius prolixus. Insect Biochem. Mol. Biol. 2004, 34, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Ribeiro, J.M.; Lehane, M.J.; Gontijo, N.F.; Veloso, A.B.; Sant’Anna, M.R.; Nascimento Araujo, R.; Grisard, E.C.; Pereira, M.H. The sialotranscriptome of the blood-sucking bug Triatoma brasiliensis (Hemiptera, Triatominae). Insect Biochem. Mol. Biol. 2007, 37, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Helling, S.; Collin, N.; Teixeira, C.R.; Medrano-Mercado, N.; Hume, J.C.C.; Assumpção, T.C.; Marcus, K.; Stephan, C.; Meyer, H.E.; et al. Immunogenic salivary proteins of Triatoma infestans: Development of a recombinant antigen for the detection of low-level Infestation of Triatomines. PLoS Negl. Trop. Dis. 2009, 3, e532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sant’Anna, M.R.V.; Araújo, J.G.V.C.; Pereira, M.H.; Pesquero, J.L.; Diotaiuti, L.; Lehane, S.M.; Lehane, M.J. Molecular cloning and sequencing of salivary gland-specific cDNAs of the blood-sucking bug Triatoma brasiliensis (Hemiptera: Reduviidae). Insect Mol. Biol. 2002, 11, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.L. Evolution of the salivary apyrases of blood-feeding arthropods. Gene 2013, 527, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Champagne, D.E.; Smartt, C.T.; Ribeiro, J.M.; James, A.A. The salivary gland-specific apyrase of the mosquito Aedes aegypti is a member of the 5′-nucleotidase family. Proc. Natl. Acad. Sci. USA 1995, 92, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, J.G.; Belkaid, Y.; Rowton, E.; Ribeiro, J.M. The salivary apyrase of the blood-sucking sand fly Phlebotomus papatasi belongs to the novel Cimex family of apyrases. J. Exp. Biol. 2001, 204, 229–237. [Google Scholar] [PubMed]
- Ascenzi, P.; Nardini, M.; Bolognesi, M.; Montfort, W.R. Nitrophorins: Lipocalin-based heme proteins transporting nitric oxide. Biochem. Mol. Biol. Educ. 2002, 30, 68–71. [Google Scholar] [CrossRef]
- Montfort, W.R.; Weichsel, A.; Andersen, J.F. Nitrophorins and related antihemostatic lipocalins from Rhodnius prolixus and other blood-sucking arthropods. Biochim. Biophys. Acta 2000, 1482, 110–118. [Google Scholar] [CrossRef]
- Andersen, J.F.; Gudderra, N.P.; Francischetti, I.M.B.; Ribeiro, J.M.C. The role of salivary lipocalins in blood feeding by Rhodnius prolixus. Arch. Insect Biochem. Physiol. 2005, 58, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Weirauch, C.; Munro, J.B. Molecular phylogeny of the assassin bugs (Hemiptera: Reduviidae), based on mitochondrial and nuclear ribosomal genes. Mol. Phylogenet. Evol. 2009, 53, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Windley, M.J.; Herzig, V.; Dziemborowicz, S.A.; Hardy, M.C.; King, G.F.; Nicholson, G.M. Spider-venom peptides as bioinsecticides. Toxins 2012, 4, 191–227. [Google Scholar] [CrossRef] [PubMed]
- Klint, J.K.; Senff, S.; Saez, N.J.; Seshadri, R.; Lau, H.Y.; Bende, N.S.; Undheim, E.A.; Rash, L.D.; Mobli, M.; King, G.F. Production of recombinant disulfide-rich venom peptides for structural and functional analysis via expression in the periplasm of E. coli. PLoS ONE 2013, 8, e63865. [Google Scholar]
- Schroeder, C.I.; Rash, L.D.; Vila-Farrés, X.; Rosengren, K.J.; Mobli, M.; King, G.F.; Alewood, P.F.; Craik, D.J.; Durek, T. Chemical synthesis, 3D structure, and ASIC binding site of the toxin Mambalgin-2. Angew. Chem. 2014, 53, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Pineda, S.S.; Melgar, S.; Dorn, P.L.; Agreda, E.; Rodas, A.; Monroy, C. Salivary protein profiles distinguish triatomine species and populations of Triatoma dimidiata (Hemiptera: Reduviidae). J. Med. Entomol. 2008, 45, 52–58. [Google Scholar] [CrossRef]
- Schwartz, E.F.; Mourão, C.B.F.; Moreira, K.G.; Camargos, T.S.; Mortari, M.R. Arthropod venoms: A vast arsenal of insecticidal neuropeptides. Biopolymers 2012, 98, 385–405. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, E.; Yanagihara, A.; Karlsson, E.; Tytgat, J. Jellyfish and other cnidarian envenomations cause pain by affecting TRPV1 channels. FEBS Lett. 2006, 580, 5728–5732. [Google Scholar] [CrossRef] [PubMed]
- Siemens, J.; Zhou, S.; Piskorowski, R.; Nikai, T.; Lumpkin, E.A.; Basbaum, A.I.; King, D.; Julius, D. Spider toxins activate the capsaicin receptor to produce inflammatory pain. Nature 2006, 444, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Bohlen, C.J.; Chesler, A.T.; Sharif-Naeini, R.; Medzihradszky, K.F.; Zhou, S.; King, D.; Sanchez, E.E.; Burlingame, A.L.; Basbaum, A.I.; Julius, D. A heteromeric Texas coral snake toxin targets acid-sensing ion channels to produce pain. Nature 2011, 479, 410–414. [Google Scholar] [CrossRef] [PubMed]




| Infraorder | Family | Species | Feeding Strategy a | Phospholipase A2 | Hyaluronidase | Protease | Lipase | Esterase | Invertase | Nuclease | Acid phosphatase | Alkaline phosphatase | Amylase | Pectinase | Refs. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trypsin-like | Chymotrypsin-like | Aminopeptidase | Carboxypeptidase | |||||||||||||||
| Nepo-morpha | Belostomatidae | Lethocerus sp.b | P | yes | yes | yes | yes | yes | yes | yes | yes | no | [39,71] | |||||
| Belostomatidae | Belostoma sp.b | P | yes | yes | yes | yes | ? | no | yes | yes | ? | [39,68,72] | ||||||
| Nepidae | Ranatra elongata | P | yes | no | no | yes | no | [72] | ||||||||||
| Cimicomorpha | Reduviidae | Platymeris rhadamanthus | P | yes | yes | yes | no | no | [6,42] | |||||||||
| Reduviidae | Zelus renardii | P | strong | strong | yes | weak | weak | weak | [31,84,85] | |||||||||
| Reduviidae | Sinea confusa | P | strong | strong | weak | [31,85] | ||||||||||||
| Reduviidae | Rhynocoris marginatusc | P | yes | yes | strong | weak | yes | yes | yes | yes | yes | yes | [86,87,88] | |||||
| Reduviidae | Catamiarus brevipennis | P | yes | yes | yes | yes | [87] | |||||||||||
| Anthocoridae | Orius insidiosus | P | yes | yes | [89] | |||||||||||||
| Nabidae | Nabis alternatus | P,(H) | yes | yes | ? | [31,85] | ||||||||||||
| Miridae | Deraeocoris sp. | P | yes | yes | yes | ? | [85,90] | |||||||||||
| Miridae | Lygus sp. | H,(P) | ? | no | yes | yes | strong | yes | [85,91,92,93,94] | |||||||||
| Miridae | Creontiades dilutus | H,(P) | weak | yes | yes | [95,96] | ||||||||||||
| Pentatom-omorpha | Pentatomidae | Podisus sp.d | P | ? | yes | yes | yes | [31,85,97,98,99] | ||||||||||
| Pentatomidae | Andrallus spinidens | P | yes | yes | yes | yes | [100] | |||||||||||
| Geocoridae | Geocoris punctipes | P,(H) | yes | yes | yes | ? | [31,85,92] | |||||||||||
| Molecule | Protein Family/Molecule Class | Species | Physiological Function a | Molecular Target | Reference |
|---|---|---|---|---|---|
| Nitric oxide | Gas | R. prolixus, C. lectularius | V, PAI | Activates guanylate cyclase | [193,194] |
| Lysophosphatidylcholine | Lipid | Rhodnius prolixus | PAI, other | Unknown | [195] |
| Nitrophorins 1–4 | Lipocalin | Rhodnius prolixus | V, PAI, AI | NO donor, also binds histamine | [159,193,196,197,198,199,200,201,202] |
| Nitrophorin-2 (Prolixin) | Lipocalin | Rhodnius prolixus | AC, V, PAI, AI | Additionally inhibits Tenase complex | [186,203,204,205,206,207] |
| Nitrophorin-7 | Lipocalin | Rhodnius prolixus | AC, V, PAI, AI | Additionally binds anionic phospholipids to prevent activation of clotting factors and platelets | [208,209,210,211,212] |
| Amine Binding Protein | Lipocalin | Rhodnius prolixus | V | Binds serotonin and norepinephrine | [213,214] |
| Triabin | Lipocalin | Triatoma pallidipennis | AC | Inhibits activation of thrombin | [185,215,216] |
| Palladipin | Lipocalin | Triatoma pallidipennis | PAI | Collagen-induced PAI, mechanism unknown | [181,217,218] |
| Rhodnius Platelet Aggregation Inhibitor 1 | Lipocalin | Rhodnius prolixus | PAI | ADP-induced PAI by binding to ADP | [219,220] |
| Triplatin | Lipocalin | Triatoma infestans | V, anti-NET | Binds thromboxane A2 and prostaglandin F2α | [221,222,223] |
| Triafestin-1, -2 | Lipocalin | Triatoma infestans | AC | Inhibits reciprocal activation of Factor XII, prekallikrein | [224] |
| Dipetalodipin | Lipocalin | Dipetalogaster maxima | V, anti-NET | Binds thromboxane A2 and various eicosanoids | [180,222] |
| Dimiconin | Lipocalin | Triatoma dimidiata | AC | Inhibits activation of Factor XII | [225] |
| Antigen-5 | Antigen-5 | D. maxima, T. infestans | PAI | Collagen-induced PAI by scavenging free radicals | [226] |
| Apyrase (Triatomine type) | 5′ Nucleotidase | Triatoma infestans | PAI | Degrades ADP | [227,228] |
| Trialysin | Trialysin | Triatoma infestans | Antimicrobial | Pore formation | [229,230,231] |
| Protease | Trypsin-like | T. infestans, Panstrongylus megistus | AC, unknown | Degrades fibrin nets, other unknown function? | [232,233] |
| Inositol Phosphatase | Inositol phosphatase | Rhodnius prolixus | Unknown | Phosphatidylinositol | [234] |
| Procalin | Lipocalin | Triatoma protracta | Allergen | Unknown | [235] |
| Nitrophorin (Cimex type) | Inositol phosphatase | Cimex lectularius | V, PAI | NO donor | [236,237] |
| Apyrase (Cimex type) | Apyrase (Cimex type) | Cimex lectularius | PAI | Degrades ADP | [183,238] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walker, A.A.; Weirauch, C.; Fry, B.G.; King, G.F. Venoms of Heteropteran Insects: A Treasure Trove of Diverse Pharmacological Toolkits. Toxins 2016, 8, 43. https://doi.org/10.3390/toxins8020043
Walker AA, Weirauch C, Fry BG, King GF. Venoms of Heteropteran Insects: A Treasure Trove of Diverse Pharmacological Toolkits. Toxins. 2016; 8(2):43. https://doi.org/10.3390/toxins8020043
Chicago/Turabian StyleWalker, Andrew A., Christiane Weirauch, Bryan G. Fry, and Glenn F. King. 2016. "Venoms of Heteropteran Insects: A Treasure Trove of Diverse Pharmacological Toolkits" Toxins 8, no. 2: 43. https://doi.org/10.3390/toxins8020043







