The Sulfur Metabolite Lanthionine: Evidence for a Role as a Novel Uremic Toxin
Abstract
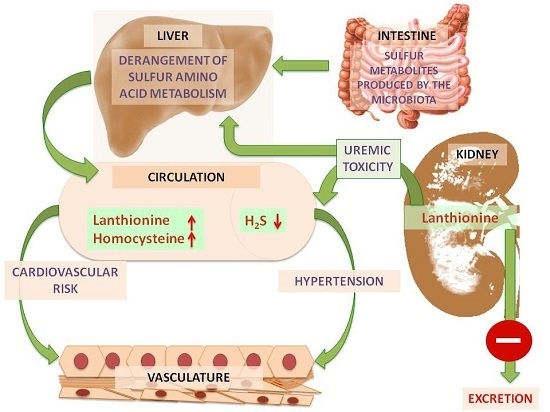
:1. Introduction
2. Lanthionine, A Side Product of H2S Biosynthesis and A Prospective Uremic Toxin
3. Possible Causes of Increased Lanthionine
4. Toxic Effects of Lanthionine
4.1. Lanthionine Hampers H2S Release In Vitro
4.2. Lanthionine Synthetase C-Like Protein (LanCL)
4.3. Lanthionine Concentrations as a Prospective Uremic Toxin
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Duranton, F.; Cohen, G.; de Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A.; European Uremic Toxin Work Group. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Li, J.; Qin, X.; Huang, Y.; Wang, X.; Gottesman, R.F.; Tang, G.; Wang, B.; Chen, D.; He, M.; et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: The CSPPT randomized clinical trial. JAMA 2015, 313, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Qin, X.; Li, Y.; Sun, D.; Wang, J.; Linag, M.; Wang, B.; Huo, Y.; Huo, F.F. Efficacy of folic acid therapy on the progression of chronic kidney disease: The Renal Substudy of the China Stroke Primary Prevention Trial. JAMA Intern. Med. 2016, 176, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Elmariah, S.; Daher, M.; Shi, X.; Keyes, M.J.; Cain, C.H.; Pomerantsev, E.; Vlahakes, G.J.; Inglessis, I.; Passeri, J.J.; Palacios, I.F.; et al. Metabolite Profiles Predict Acute Kidney Injury and Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.; Rodríguez-Soriano, J.; Prieto, J.A.; Aguirre, M.; Ariceta, G.; Lage, S.; Azcona, I.; Prado, C.; Sanjurjo, P.; Aldámiz-Echevarría, L. Methylation cycle, arginine-creatine pathway and asymmetric dimethylarginine in paediatric renal transplant. Nephrol. Dial. Transplant. 2011, 26, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Klawitter, J.; Reed-Gitomer, B.Y.; McFann, K.; Pennington, A.; Klawitter, J.; Abebe, K.Z.; Klepacki, J.; Cadnapaphornchai, M.A.; Brosnahan, G.; Chonchol, M.; et al. Endothelial dysfunction and oxidative stress in polycystic kidney disease. Am. J. Physiol. Renal Physiol. 2014, 307, F1198–F1206. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; Koury, M.J. S-Adenosylhomocysteine: A better indicator of vascular disease than homocysteine? Am. J. Clin. Nutr. 2007, 86, 1581–1585. [Google Scholar] [PubMed]
- Perna, A.F.; Lanza, D.; Sepe, I.; Conzo, G.; Altucci, L.; Ingrosso, D. Altered folate receptor 2 expression in uraemic patients on haemodialysis: Implications for folate resistance. Nephrol. Dial. Transplant. 2013, 28, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Kabil, O.; Yadav, V.; Banerjee, R. Heme-dependent Metabolite Switching Regulates H2S Synthesis in Response to Endoplasmic Reticulum (ER) Stress. J. Biol. Chem. 2016, 291, 16418–16423. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.F.; di Nunzio, A.; Amoresano, A.; Pane, F.; Fontanarosa, C.; Pucci, P.; Vigorito, C.; Cirillo, G.; Zacchia, M.; Trepiccione, F.; et al. Divergent behavior of hydrogen sulfide pools and of the sulfur metabolite lanthionine, a novel uremic toxin, in dialysis patients. Biochimie 2016, 126, 97–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiku, T.; Padovani, D.; Zhu, W.; Singh, S.; Vitvitsky, V.; Banerje, R. H2S biogenesis by human cystathionine γ-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J. Biol. Chem. 2009, 284, 11601–11612. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Padovani, D.; Leslie, R.A.; Chiku, T.; Banerjee, R. The relative contributions of cystathionine β-synthase and γ-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J. Biol. Chem. 2009, 284, 22457–22466. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.F.; Ingrosso, D. Low hydrogen sulphide and chronic kidney disease: A dangerous liaison. Nephrol. Dial. Transplant. 2012, 27, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Westhof, T.; Vaziri, N.D.; Ingrosso, D.; Perna, A.F. Gases as uremic toxins: Is there something in the air? Semin. Nephrol. 2014, 34, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.F.; Luciano, M.G.; Ingrosso, D.; Pulzella, P.; Sepe, I.; Lanza, D.; Violetti, E.; Capasso, R.; Lombardi, C.; de Santo, N.G. Hydrogen sulphide-generating pathways in haemodialysis patients: A study on relevant metabolites and transcriptional regulation of genes encoding for key enzymes. Nephrol. Dial. Transplant. 2009, 24, 3756–3763. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.F.; Ingrosso, D. Atherosclerosis determinants in renal disease: How much is homocysteine involved? Nephrol. Dial. Transplant. 2016, 31, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, A.E.; Goryachenkova, E.V. The beta-replacement-specific pyridoxal-P-dependent lyases. Adv. Enzymol. Relat. Areas Mol. Biol. 1984, 56, 1–89. [Google Scholar] [PubMed]
- Aminzadeh, M.A.; Vaziri, N.D. Downregulation of the renal and hepatic hydrogen sulfide (H2S)-producing enzymes and capacity in chronic kidney disease. Nephrol. Dial. Transplant. 2012, 27, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Draper, L.A.; Cotter, P.D.; Hill, C.; Ross, R.P. Lantibiotic resistance. Microbiol. Mol. Biol. Rev. 2015, 79, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Pahl, M.V.; Vaziri, N.D. The Chronic Kidney Disease—Colonic Axis. Semin. Dial. 2015, 28, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Glorieux, G. The intestine and the kidneys: A bad marriage can be hazardous. Clin. Kidney J. 2015, 8, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Wadman, S.K.; de Bree, P.K.; Kamerling, J.P. Lanthionine detected in human urine. Clin. Chim. Acta 1978, 82, 281–284. [Google Scholar] [CrossRef]
- DeRatt, B.N.; Ralat, M.A.; Kabil, O.; Chi, Y.Y.; Banerjee, R.; Gregory, J.F., 3rd. Vitamin B-6 restriction reduces the production of hydrogen sulfide and its biomarkers by the transsulfuration pathway in cultured human hepatoma cells. J. Nutr. 2014, 144, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.B.; Colaço, H.G.; Sarti, P.; Leandro, P.; Giuffrè, A. S-Adenosyl-l-methionine Modulates CO and NO• Binding to the Human H2S-generating Enzyme Cystathionine β-Synthase. J. Biol. Chem. 2016, 291, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.X.; Wang, Y.B.; Peng, L.; Ge, X.Z.; Zhang, J.; Liu, S.S.; Zhang, X.N.; Xu, Z.H.; Chen, Z.; Luo, J.H. Lanthionine synthetase C-like protein 1 interacts with and inhibits cystathionine β-synthase: A target for neuronal antioxidant defense. J. Biol. Chem. 2012, 287, 34189–34201. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.N.; Yadav, P.K.; Adamec, J.; Banerjee, R. S-glutathionylation enhances human cystathionine β-synthase activity under oxidative stress conditions. Antioxid. Redox Signal. 2015, 22, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Knerr, P.J.; van der Donk, W.A. Discovery, biosynthesis, and engineering of lantipeptides. Annu. Rev. Biochem. 2012, 81, 479–505. [Google Scholar] [CrossRef] [PubMed]
- Mayer, H.; Salzer, U.; Breuss, J.; Ziegler, S.; Marchler-Bauer, A.; Prohaska, R. Isolation, molecular characterization, and tissue-specific expression of a novel putative G protein-coupled receptor. Biochim. Biophys. Acta 1998, 1395, 301–308. [Google Scholar] [CrossRef]
- Chung, C.H.; Kurien, B.T.; Mehta, P.; Mhatre, M.; Mou, S.; Pye, Q.N.; Stewart, C.; West, M.; Williamson, K.S.; Post, J.; et al. Identification of lanthionine synthase C-like protein-1 as a prominent glutathione binding protein expressed in the mammalian central nervous system. Biochemistry 2007, 46, 3262–3269. [Google Scholar] [CrossRef] [PubMed]
- Dupree, J.L.; Polak, P.E.; Hensley, K.; Pelligrino, D.; Feinstein, D.L. Lanthionine ketimine ester provides benefit in a mouse model of multiple sclerosis. J. Neurochem. 2015, 134, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Hensley, K.; Christov, A.; Kamat, S.; Zhang, X.C.; Jackson, K.W.; Snow, S.; Post, J. Proteomic identification of binding partners for the brain metabolite lanthionine ketimine (LK) and documentation of LK effects on microglia and motoneuron cell cultures. J. Neurosci. 2010, 30, 2979–2988. [Google Scholar] [CrossRef] [PubMed]
- Harris-White, M.E.; Ferbas, K.G.; Johnson, M.F.; Eslami, P.; Poteshkina, A.; Venkova, K.; Christov, A.; Hensley, K. A cell-penetrating ester of the neural metabolite lanthionine ketimine stimulates autophagy through the mTORC1 pathway: Evidence for a mechanism of action with pharmacological implications for neurodegenerative pathologies. Neurobiol. Dis. 2015, 84, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Sturla, L.; Guida, L.; Bruzzone, S.; Scarfì, S.; Usai, C.; Fruscione, F.; Magnone, M.; Millo, E.; Basile, G.; Grozio, A.; et al. LANCL2 is necessary for abscisic acid binding and signaling in human granulocytes and in rat insulinoma cells. J. Biol. Chem. 2009, 284, 28045–28057. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; James, C.D. Lanthionine synthetase components C-like 2 increases cellular sensitivity to adriamycin by decreasing the expression of P-glycoprotein through a transcription-mediated mechanism. Cancer Res. 2003, 63, 723–727. [Google Scholar] [PubMed]
- Landlinger, C.; Salzer, U.; Prohaska, R. Myristoylation of human LanC-like protein 2 (LANCL2) is essential for the interaction with the plasma membrane and the increase in cellular sensitivity to adriamycin. Biochim. Biophys. Acta 2006, 1758, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; van der Donk, W.A.; Chen, J. Lanthionine synthetase C-like protein 2 (LanCL2) is a novel regulator of Akt. Mol. Biol. Cell 2014, 25, 3954–3961. [Google Scholar] [CrossRef] [PubMed]
- Kabil, O.; Vitvitsky, V.; Xie, P.; Banerjee, R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid. Redox Signal. 2011, 15, 363–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitvitsky, V.; Thomas, M.; Ghorpade, A.; Gendelman, H.E.; Banerjee, R. A functional transsulfuration pathway in the brain links to glutathione homeostasis. J. Biol. Chem. 2006, 281, 35785–35793. [Google Scholar] [CrossRef] [PubMed]
- Bearden, S.E.; Beard, R.S., Jr.; Pfau, J.C. Extracellular transsulfuration generates hydrogen sulfide from homocysteine and protects endothelium from redox stress. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1568–H1576. [Google Scholar] [CrossRef] [PubMed]




© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perna, A.F.; Zacchia, M.; Trepiccione, F.; Ingrosso, D. The Sulfur Metabolite Lanthionine: Evidence for a Role as a Novel Uremic Toxin. Toxins 2017, 9, 26. https://doi.org/10.3390/toxins9010026
Perna AF, Zacchia M, Trepiccione F, Ingrosso D. The Sulfur Metabolite Lanthionine: Evidence for a Role as a Novel Uremic Toxin. Toxins. 2017; 9(1):26. https://doi.org/10.3390/toxins9010026
Chicago/Turabian StylePerna, Alessandra F., Miriam Zacchia, Francesco Trepiccione, and Diego Ingrosso. 2017. "The Sulfur Metabolite Lanthionine: Evidence for a Role as a Novel Uremic Toxin" Toxins 9, no. 1: 26. https://doi.org/10.3390/toxins9010026
APA StylePerna, A. F., Zacchia, M., Trepiccione, F., & Ingrosso, D. (2017). The Sulfur Metabolite Lanthionine: Evidence for a Role as a Novel Uremic Toxin. Toxins, 9(1), 26. https://doi.org/10.3390/toxins9010026