Abstract
Toxin producing cyanobacterial blooms have increased globally in recent decades in both frequency and intensity. Despite the recognition of this growing risk, the extent and magnitude of cyanobacterial blooms and cyanotoxin prevalence is poorly characterized in the heavily populated region of southern California. Recent assessments of lentic waterbodies (depressional wetlands, lakes, reservoirs and coastal lagoons) determined the prevalence of microcystins and, in some cases, additional cyanotoxins. Microcystins were present in all waterbody types surveyed although toxin concentrations were generally low across most habitats, as only a small number of sites exceeded California’s recreational health thresholds for acute toxicity. Results from passive samplers (Solid Phase Adsorption Toxin Tracking (SPATT)) indicated microcystins were prevalent throughout lentic waterbodies and that traditional discrete samples underestimated the presence of microcystins. Multiple cyanotoxins were detected simultaneously in some systems, indicating multiple stressors, the risk of which is uncertain since health thresholds are based on exposures to single toxins. Anatoxin-a was detected for the first time from lakes in southern California. The persistence of detectable microcystins across years and seasons indicates a low-level, chronic risk through both direct and indirect exposure. The influence of toxic cyanobacterial blooms is a more complex stressor than presently recognized and should be included in water quality monitoring programs.
Keywords:
cyanotoxins; cyanobacteria; microcystins; cylindrospermopsin; anatoxin-a; California; lakes; estuaries; SPATT 1. Introduction
The global proliferation of toxin producing cyanobacterial blooms has gained international attention in recent years [1,2,3,4,5,6]. These increases have been attributed to a wide variety of environmental factors including nutrient pollution, increased temperature, salinity, water residence time, vertical stratification, and pH, many of which will likely be exacerbated by climate change [6,7,8,9,10,11,12,13,14].
Cyanotoxins pose a significant risk for humans, livestock, pets, and wildlife, causing illness and mortality [15,16,17,18,19,20,21,22,23,24,25]. Cyanotoxin poisoning in pets and livestock is significantly under-recognized and under-reported [16,23,25]. Due to the adverse health effects associated with cyanotoxins, and the growing recognition that cyanobacterial blooms can severely impact water quality [26,27], health advisory thresholds for some cyanotoxins have been developed by many states, including California. The U.S. Environmental Protection Agency has recently released health advisory thresholds for cyanotoxins in drinking water [28,29] and proposed draft thresholds for recreational water and swimming activities [30]. The USGS has recently prioritized 12 cyanotoxins as Tier 1, the highest priority for inclusion in ambient water monitoring in the U.S. (an additional three were listed at intermediate or low priority) [31].
Despite recognition of this growing threat, the extent and magnitude of cyanobacterial blooms in California are poorly characterized, particularly in heavily populated regions such as coastal southern California. Across the continuum of aquatic habitats, lentic waterbodies, such as lakes, reservoirs, depressional wetlands and coastal lagoons, provide conditions that readily support the proliferation of cyanobacterial blooms as they typically include ample supply of nutrients, calm water and stratified conditions, high irradiance and warm water temperatures. In southern California, these lentic habitats are often associated with or downstream of urban and agricultural areas, and therefore subject to further risk of cyanobacteria proliferation due to increased anthropogenic nutrient inputs via point- and non-point source runoff.
Hydrologic alteration is pervasive throughout southern California watersheds, with increased peak flows and altered base flow correlated with total impervious cover [32,33]. Eutrophic habitats are ubiquitous throughout the region. Fetscher et al. [34] found that 60% of southern California streams had elevated stream algal biomass, while McLaughlin et al. [35] found that 83% of southern California coastal lagoons had excessive macroalgal growth. Comprehensive lake surveys have not been undertaken, but regulatory actions to reduce nutrient loads have been enacted for over 13 lakes, and are pending in another 12 lakes that are listed as impaired for nutrients and eutrophication [36].
There are few published studies on cyanotoxins in southern California lentic waterbodies. However, microcystin production was documented from benthic cyanobacteria in several reservoirs [37], and in wadeable streams throughout the State [38]. California Statewide assessments revealed that benthic algae in wadeable streams were a source of cyanotoxin production and supported a high occurrence of potentially toxigenic taxa [38]. Over 90% of stream kilometers in California supported potential toxin-producing genera and 23% supported toxin-producing species [38]. Cyanotoxin analysis from that assessment detected microcystins in 33% of sites statewide, lyngbyatoxin in 21% of sites, and saxitoxin or anatoxin-a was detected in 7% of sites [38]. Cylindrospermopsin and nodularin were not detected in the survey [38]. Similarly, cyanobacteria were ubiquitous in a screening assessment of 30 lakes, depressional wetlands and coastal lagoons in southern California, with Microcystis spp. dominant in 96% of study sites [39]. Quantification of cyanotoxins in that study were limited, however, microcystins were detected in high concentrations at 10% of sites that exceeded both the California recreational health thresholds, and the World Health Organization guidance for human health. Cylindrospermopsin and anatoxin-a were not detected at any sites. The community composition was dominated by potentially toxic cyanobacteria (particularly Microcystis spp.); therefore, these findings generated interest in further characterizing cyanotoxins, and specifically microcystin prevalence, across lentic waterbodies in southern California.
Concerns of ecological and human health risks of cyanotoxins also extend beyond freshwater habitats and into the land-sea interface. Multiple studies in central California and one in Washington State have documented microcystins in marine outflows, with direct impacts in marine ecosystems far downstream of their biological origin [40,41,42,43,44,45,46,47]. The mass mortality of more than 30 marine sea otters in Monterey Bay was attributed to microcystin intoxication from ingestion of contaminated shellfish [45]. Microcystin production originated in Pinto Lake, a eutrophic water body that experiences frequent cyanobacterial blooms and drains to Monterey Bay via a 15-km segment of the Pájaro River [45,48]. Extensive watershed studies conducted in Monterey Bay, California and Washington State showed that this downstream transport of microcystins was a persistent and prevalent issue throughout the watershed (not just from a single waterbody) and that the toxins accumulated in marine shellfish [42,43,47]. These studies underscore an important role of rivers and streams as conduits that can not only produce cyanotoxins, but can also transport intact toxins from inland waters to downstream marine and estuarine waterbodies.
Given the severe and ubiquitous nature of harmful cyanobacterial blooms and cyanotoxins demonstrated by these studies, surveillance and monitoring have become critical for the protection of public health. Traditional monitoring programs for these toxins typically rely on discrete sampling (“grab” samples) from a particular site or sites. These methods are inherently biased if the sampling does not capture the spatial and temporal variability of the system, and ephemeral or episodic events. In response to this challenge, a passive sampling method, Solid Phase Adsorption Toxin Tracking (SPATT), has been developed to monitor toxin concentrations of cyanobacterial and algal toxins in marine, brackish and freshwater environments [45,48,49,50]. These toxins have often been detected using SPATT when simultaneous discrete water samples have failed to detect them in a given waterway or waterbody, making SPATT a more sensitive indicator of the prevalence of toxins than traditional discrete samples [42,48,49]. SPATT was therefore used in the current study as an additional assessment tool to provide insight into the overall toxin prevalence and to provide a time-integrative toxin sample.
The objectives of the current study were to: (1) determine the prevalence of microcystins in lentic waterbodies in southern California to evaluate if they should be included in routine water quality monitoring programs; and (2) evaluate the applicability of a passive sampling technology (SPATT) to detect toxins in monitoring and assessment programs. Microcystins were the focus of the current study due to the dominance of Microcystis spp. in previous screening assessments [38,39], and because microcystins are the most common cyanotoxin detected in lakes in the U.S. [51]. Three separate field surveys were conducted: (1) an ambient probability based assessment of depressional wetlands (2011–2013); (2) a screening assessment of lakes, reservoirs and coastal lagoons determined to be at significant risk for blooms in 2013; and (3) an ad hoc bloom event response survey in 2014.
2. Results
2.1. Depressional Wetlands Assessment
Particulate microcystins were detected at 25% of sites examined during the field study (Table 1) over the whole sampling period (2011–2013), though the percentage of sites with microcystins detected varied widely from year to year, as did concentrations of microcystins (Table 2, Figure 1). Particulate microcystins were detected from 12.5% of all sites sampled in 2011 and 2013 and 47% of all sites sampled in 2012. The range of particulate microcystin concentrations varied from below detection to 2.5 µg L−1 in 2011, up to 0.45 µg L−1 in 2012, and up to 22 µg L−1 in 2013. Interestingly, the highest percentage of positive sites was observed in 2012, although concentrations of particulate microcystins did not exceed the California recreational health thresholds (0.8 µg L−1 OEHHA, 2012; 1.0 µg L−1 http://www.mywaterquality.ca.gov/monitoring_council/cyanohab_network/docs/triggers.pdf). In contrast, there were only two sites with microcystins detected during 2013, but, concentrations at both sites exceeded the California recreational health thresholds. Saxitoxins were detected at very low concentrations (<0.4 µg L−1) at only one site during the 2012–2013 assessment (Santee Lakes Recreation Preserve Lake, site number 44 on Table 1 and Figure 1).

Table 1.
List of study sites from the depressional wetlands assessment survey including year sampled, site name, water regime, wetland function and region location. The site numbers correspond to the numbers listed in Figure 1 and Figure 2 and the asterisk (*) indicates sites in San Diego that were revisited and more intensely sampled in summer 2012.

Table 2.
Summary of depressional wetlands discrete toxin sample results collected during the spring assessments in 2011, 2012 and 2013 throughout Southern California.
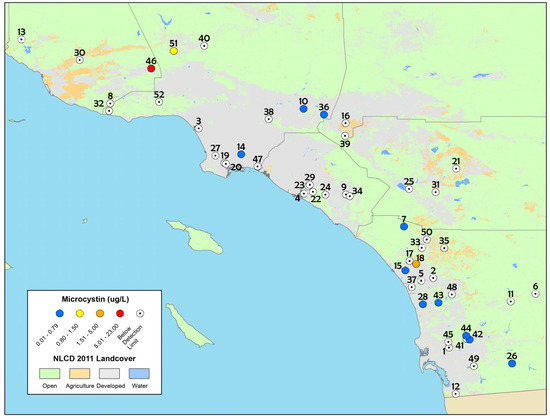
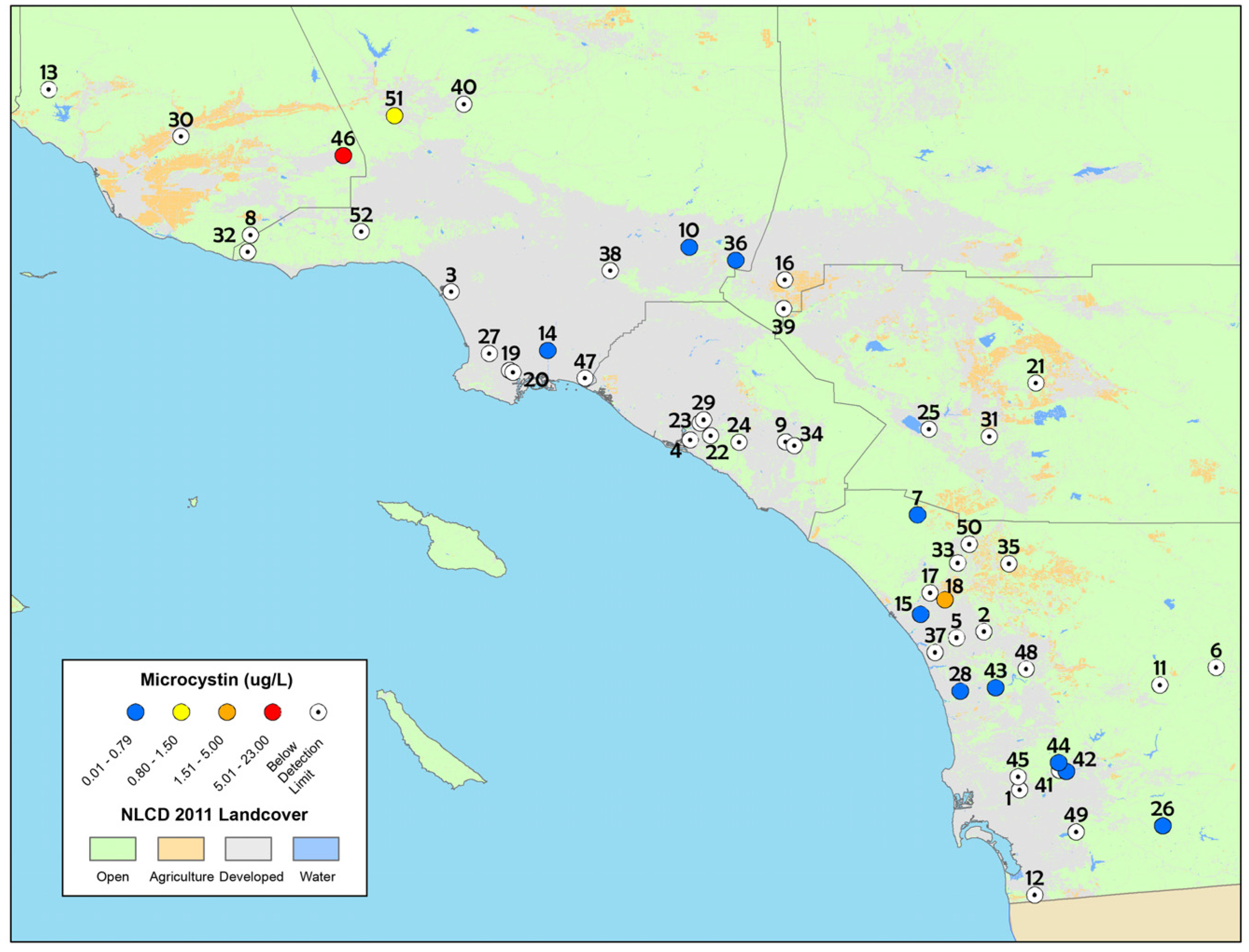
Figure 1.
Map of particulate microcystin concentrations detected from discrete samples in the spring depressional wetlands assessment, 2011–2013. The numbers on the map correspond to sites in Table 1. The white circles with a black dot in the center indicate sites that were below the method limit of detection while blue circles indicate sites that had microcystin concentrations ranging from 0.01 to 0.79 µg L−1. The yellow, orange and red circles indicate microcystin concentrations that exceeded the California recreational health thresholds for microcystins (0.8 µg L−1).
Statistical analysis revealed that chlorophyll-a was not a significant predictor of microcystin concentration (p-value, 0.9), neither across years nor for any individual year. Additionally, no statistically significant relationships were identified between microcystin concentrations and environmental variables, including alkalinity (p-value = 0.7), total nitrogen (p-value = 0.2), total phosphorus (p-value = 0.7), pH (p-value = 0.7), elevation (p-value = 0.4), conductivity (p-value = 0.8), and temperature (p-value = 0.7). Similarly, the landscape disturbance indicators (percentage of agriculture, urban, impervious cover and road density) also were not identified as statistically significant predictors of microcystins, regardless of the area surrounding the wetland that was used in the analysis (30 m, 150 m, 500 m, 1000 m and 3000 m). The three condition indices, macroinvertebrates index, benthic diatom index and CRAM, were also not statistically significant predictors of microcystins (p-values of 0.07, 0.3, and, 0.1 respectively).
The depressional wetlands sites sampled in the spring of 2012 in San Diego County were re-sampled during the summer and early fall (Figure 2) and time-integrating passive samplers (SPATT) were also deployed. During the spring 2012 assessment, particulate microcystins were detected at 60% of the sites in San Diego County based on discrete samples only (Table 3). During the summer and fall seasons when these sites were re-sampled, microcystins were detected at only 29% of sites based on discrete sample results, while time-integrated SPATT results detected dissolved microcystins at 83% of sites (Figure 2, Table 3). The results of SPATT and discrete samples from all seasons in 2012 are summarized in Table 3 and shown in Figure 2.
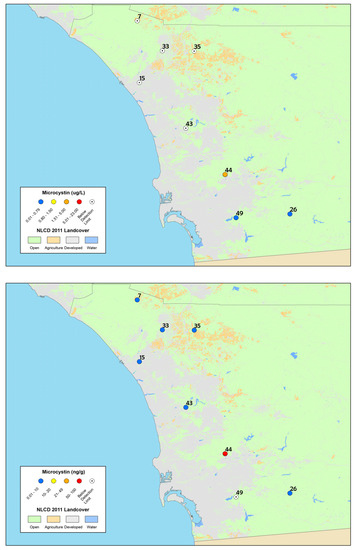
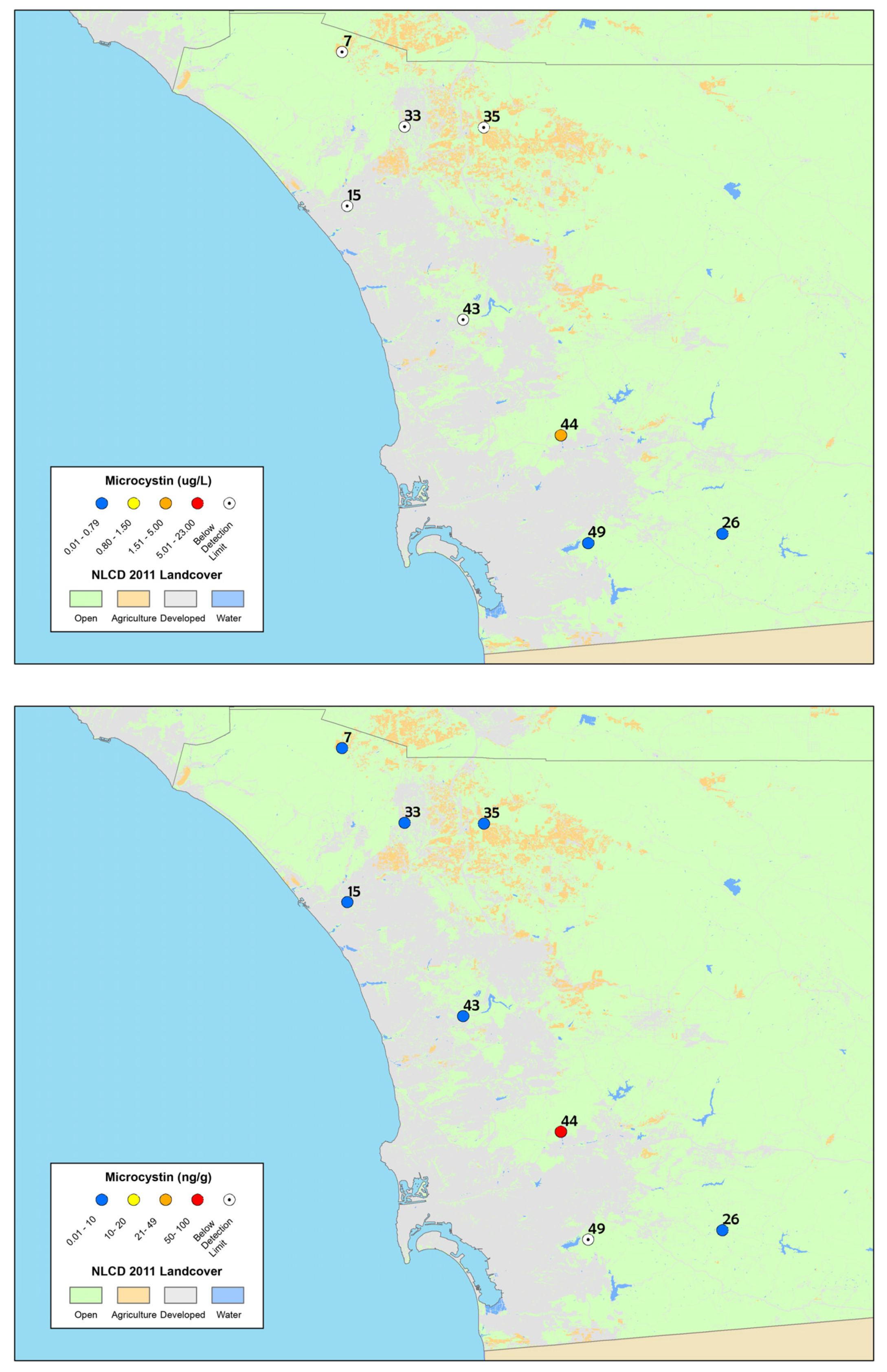
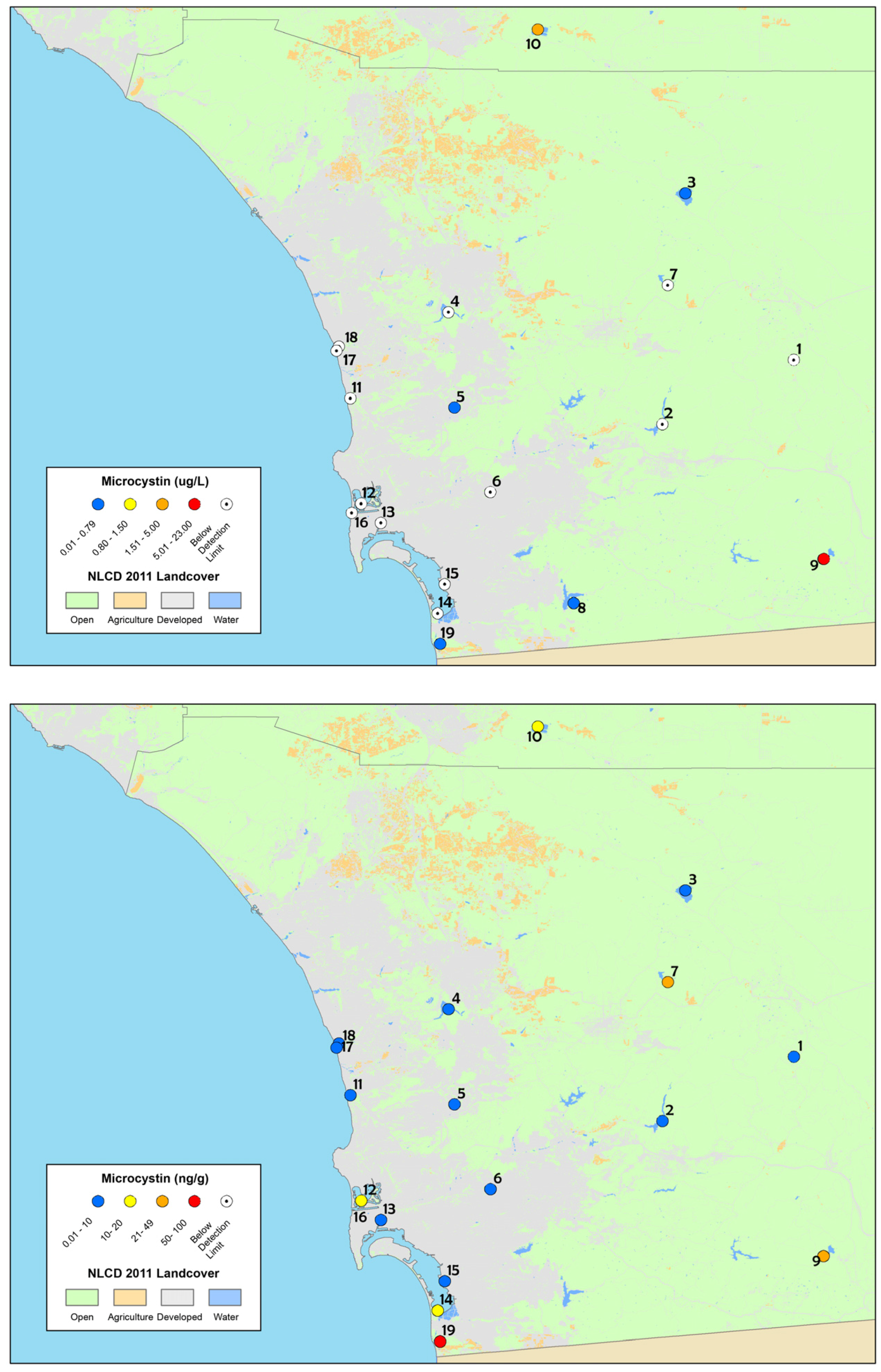
Figure 2.
Maps of: particulate microcystin results (top); and time-integrated dissolved microcystin results from SPATT samples (bottom), collected in summer and fall 2012 from the depressional wetlands assessment sites in San Diego County. The site numbers correspond to Table 1 and the legend is the same as Figure 1. The highest concentrations are shown for discrete samples (sites were sampled twice).

Table 3.
The percentage of depressional wetlands sites where microcystins were detected based on discrete samples and SPATT samples, for collection sites in San Diego County, sampled in 2012.
Particulate saxitoxins were detected at one site (equivalent to 10% of survey sites) during both the spring and summer (Santee Lakes Recreation Preserve lake, site number 44 on the map in Figure 1 and Figure 2), and the concentrations were extremely low (<0.04 µg L−1).
The most common microcystin congener detected from the SPATT samples was MCY-LR, followed by MCY-RR and MCY-LA. MCY-YR was not detected in the samples (Supplementary Materials, Table S1).
2.2. Surveys of Lakes, Reservoirs, and Coastal Lagoons
2.2.1. Screening Assessment Survey 2013
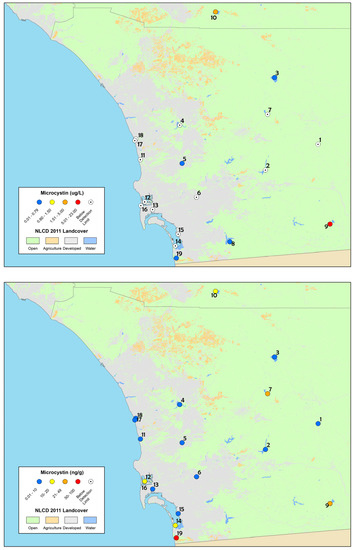
The screening assessment survey of lakes, reservoirs, and coastal lagoons yielded results that, similar to the depressional wetlands assessment, revealed widespread occurrence of micorocystins. Results from time-integrated SPATT samplers indicated that all sites had measurable dissolved microcystins during at least one of the three sampling visits throughout the field survey and seven sites had measurable dissolved microcystins throughout the field survey (Table 4, sites in bold font). In contrast, particulate microcystin results obtained from discrete samples indicated that 26% of the sites had measurable particulate microcystins during the field survey. Two of these sites exceeded the recreational action thresholds for California (OEHHA, 2012; http://www.mywaterquality.ca.gov/monitoring_council/cyanohab_network/docs/triggers.pdf). Morena Reservoir had 23.6 µg L−1 of microcystins detected, indicating the Tier II, Danger threshold and Vail Lake had 2.1 µg L−1, indicating the Caution trigger threshold.

Table 4.
List of study sites and concentrations of microcystins detected from both discrete and SPATT samples collected in the screening assessment survey of lakes, reservoirs and coastal lagoons in 2013. Sites for which microcystins were detected throughout the entire study period are in bold and italics. The site numbers provided correspond to Figure 3.
Particulate microcystin concentrations ranged from below the detection limit of the method (bd) to 23.6 µg L−1 (Table 4; Figure 3) and below the detection limit (bd) to 100.8 ng g−1 for dissolved microsystins detected from SPATT samples (Table 4; Figure 3).
Statistical analysis revealed that chlorophyll-a was a statistically significant predictor of microcystin concentration (p-value = 0.004) during the screening assessment survey of lakes, reservoirs and coastal lagoons. There were no statistically significant environmental predictors (such as alkalinity or nutrients) of microcystins (p-values > 0.05). The pigment results were similar in that neither phycocyanin nor phycoerythrin was a statistically significant predictor of microcystins (p-values > 0.05).
MCY-RR was the most commonly detected dissolved microcystin congener of the four analyzed, was detected at every site, and was present in 64% of the SPATT sample results (Supplementary Materials, Table S2). MCY-LR was only detected at six sites and MCY-LA at five sites (out of the total of 18 for which there are SPATT results). MCY-YR was not detected at any of the sites from the SPATT samples. Particulate microcystin results were positive at only four sites (out of 19 surveyed) for any of the nine microcystin congeners analyzed (Supplementary Materials, Table S3). Within those four sites, MCY-LR and MCY-YR were the most common congeners detected at three of the sites. Two sites exceeded the California recreational action thresholds for microcystins, Vail Lake (2.1 µg L−1) and Morena Reservoir (two time points, August and September, 6.1 µg L−1 and 23.6 µg L−1, respectively).
2.2.2. Ad Hoc Bloom Event Response Survey
Microcystins were detected in half of the sites analyzed for toxins during the ad hoc bloom event response survey in 2014, with concentrations ranging from below detection to 36,549 µg L−1 (results of the discrete samples are shown in Figure 4 and Table 5). Nodularin was not detected in any of the discrete samples. Four sites exhibited microcystin concentrations that exceeded the California recreational health thresholds (OEHHA, 2012; http://www.mywaterquality.ca.gov/monitoring_council/cyanohab_network/docs/triggers.pdf). The results from the San Joaquin Marsh sample were the highest detected within southern California to date (total microcstyins = 36,549 µg L−1) from a whole water sample (i.e., excluding scum and foam samples). Particulate microcystins were also detected at three other sites: Harveston Lake, Lindo Lake and Santee Lake (concentrations of 10 µg L−1, 2.5 µg L−1, and 11.7 µg L−1, respectively). All are highly used recreational sites. These concentrations are likely underestimates of the total microcystins present since only the particulate fraction was measured.
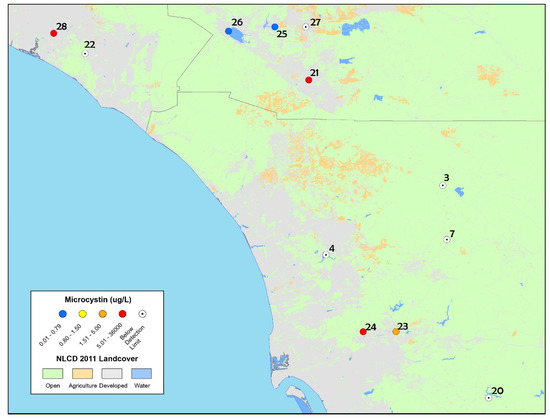
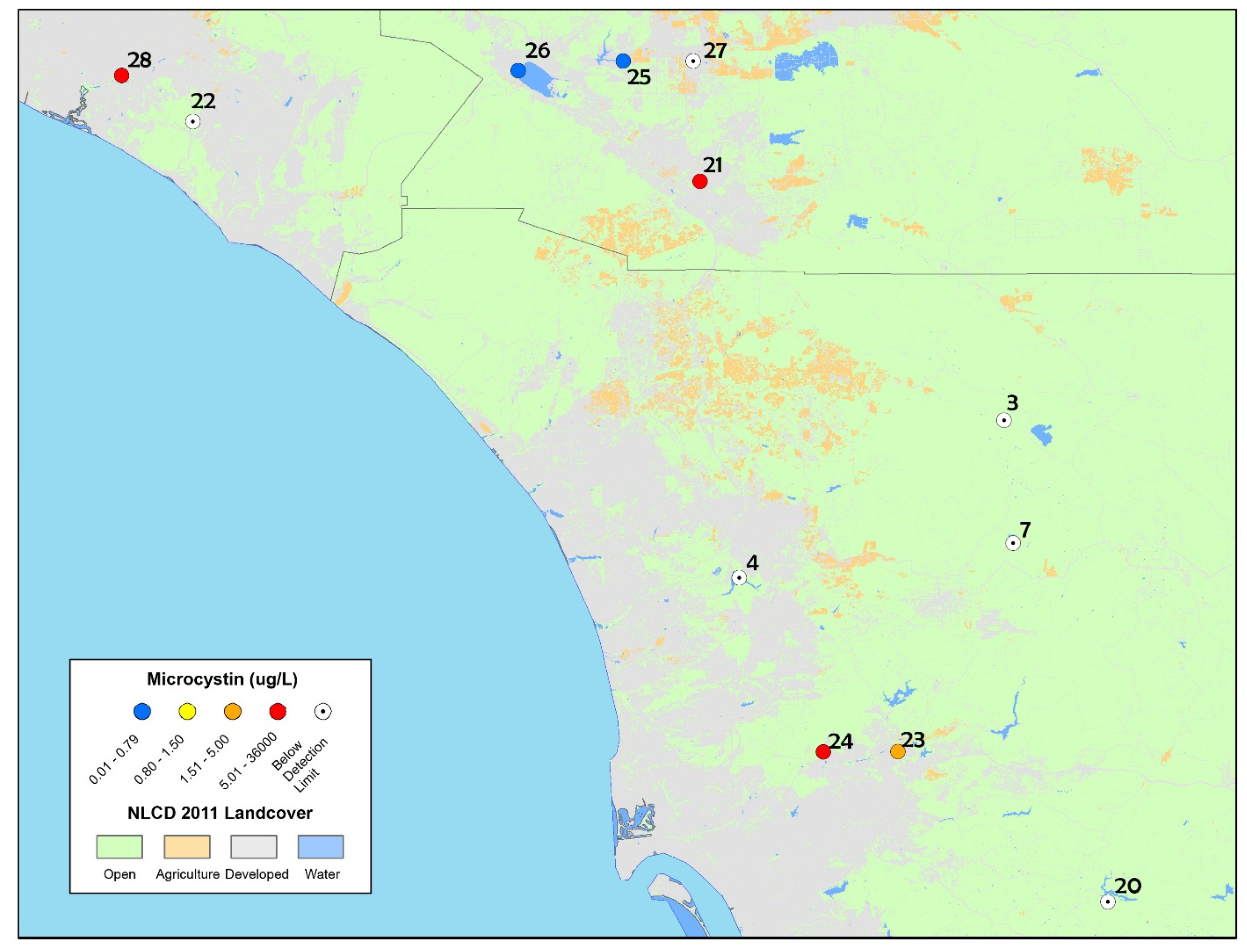
Figure 4.
Map of discrete sample results collected from the ad hoc bloom event response survey in 2014 in San Diego, Riverside and Orange Counties (including one sample collected in 2015 from San Joaquin Marsh). The highest concentrations of microcystins are reported for sites that were re-sampled (and therefore have multiple toxin results).

Table 5.
List of study sites from the ad hoc bloom response survey in 2014 and discrete sample results for cyanobacterial identification and total microcystins (MCY) reported in µg L−1. Sites in bold exceeded the California health advisory thresholds for recreational exposures.
Additional cyanotoxins, cylindrospermopsin and anatoxin-a, were analyzed from samples collected at three lakes, Canyon Lake, Lake Elsinore and Lake Menifee (site numbers 25, 26 and 27, respectively, in Figure 4). Cylindrospermopsin was detected at all three lakes, at concentrations that exceeded the California recreational health thresholds (OEHHA, 2012; http://www.mywaterquality.ca.gov/monitoring_council/cyanohab_network/docs/triggers.pdf). Total cylindrospermosin concentrations detected were 2.7 µg L−1 at Canyon Lake, 4.1 µg L−1 at Lake Elsinore, and 2.9 µg L−1 at Lake Menifee (CA trigger levels for cylindrospermopsin are 1 µg L−1 for caution, 4 µg L−1 for warning and 17 µg L−1 for danger). Total anatoxin-a was detected at Canyon Lake (4.6 µg L−1), and Lake Menifee (3.6 µg L−1), while Lake Elsinore was below the limit of detection. All detectable concentrations exceeded the California recreational health thredholds (OEHHA, 2012; http://www.mywaterquality.ca.gov/monitoring_council/cyanohab_network/docs/triggers.pdf). The detailed results of individual microcystin congeners are summarized in Supplementary Materials, Table S4. MCY-LR and MCY-RR were the most commonly detected congeners.
The potentially toxic cyanobacterial taxa were identified to genus level and where possible to species level and the results are summarized in Table 5. Microcystis sp., Cylindrospermopsis sp., and Anabaena sp. were the three most common genera identified in 40–50% of the sites. The species that were identified included Cylindrospermopsis raciborskii, Anabaena variabilis, A. spiroides. These results indicated the potential for many types of cyanotoxins to be present including microcystins, saxitoxin, cylindrospermopsin, and anatoxin-a.
3. Discussion
3.1. Microcystin Prevalence
Microcystins were detectable and prevalent in all lentic waterbody types surveyed across all land use types in southern California. Particulate microcystin concentrations were generally low across most habitats, exceeding California recreational health thresholds for acute toxicity at only a small number of sites during the depressional wetlands assessment and the screening assessment survey of 2013, similar to findings in the California Sacramento-San Joaquin Delta and San Francisco Bay estuary [44,52,53]. However, the number of waterbodies where microcystins were present was underestimated by discrete particulate samples based on the high number of samples and sites where dissolved microcystins were detected by SPATT samples. The persistence of detectable microcystins across multiple years and seasons indicates a low-level, chronic presence of microcystins in these waterbodies, similar to findings in Monterey Bay watersheds [42]. Chronic exposure of microcystins can have human and wildlife health implications [15,23,54,55,56,57,58,59] and can be transported into riparian food webs [60] and marine shellfish [42,43,45,46,47,61,62]. The sites sampled in this study were chosen due to the designed beneficial uses that include human and wildlife exposure (such as drinking water supply, agricultural water supply and both contact and non-contact recreation) [63]. In contrast to the assessment surveys, the ad hoc bloom event response survey indicated microcystins at half of the sites analyzed, and a quarter of sites had very high acute concentrations, 3–45,000-fold higher than the recreational health thresholds (0.8 µg L−1; OEHHA, 2012; 1.0 µg L−1 http://www. mywaterquality.ca.gov/monitoring_council/cyanohab_network/docs/triggers.pdf), indicating a high risk for immediate impacts to human, wildlife and domestic pet health. Currently, cyanotoxins are not included in routine water quality monitoring programs in California, but cyanotoxin analysis is conducted when visible blooms are reported to the State. The results of the current study detected microcystins (both dissolved and particulate) in waterbodies that had no visible cyanobacterial blooms indicating the current bloom event response efforts in California may be insufficient to protect public health. Cyanotoxins should be added to routine water quality monitoring programs in southern California to ensure protection of public health.
Microcystins produced in freshwater systems have been shown to cause effects far downstream of their biological origin, have been detected in downstream coastal receiving waters and near-shore marine waters and have accumulated in marine shellfish [42,43,45,46,47,61,62]. Multi-year studies of the Monterey Bay watershed have shown that downstream transport of microcystins is a persistent and prevalent issue throughout the watershed [42]. Similar downstream transport of microcystins from a freshwater lake into marine shellfish has been documented in Washington State [47]. These studies underscore an important role of rivers as conduits that can transport intact toxins from inland waters (e.g., lakes, reservoirs and wetlands) to downstream marine and estuarine waterbodies. The widespread and chronic production of microcystins across all waterbody types and the detection of microcystins in all coastal lagoon systems sampled determined from the current study, as well as other studies documenting the transport of toxins from inland to marine waterbodies [38,42,43,45,46,47], suggest microcystins should be added to the list of biotoxins routinely monitored in California coastal waters and marine shellfish.
Summer and fall seasons have been identified as the most conducive to cyanobacteria growth, bloom formation and toxin production [42,53,64]. Interestingly, the San Diego County depressional wetlands sites yielded a higher percentage of microcystins detected in spring (Table 3; 60%), compared with the summer and fall seasons (Table 3; 29%). Similar seasonal differences were observed in the Monterey Bay watershed, with spring providing the highest concentrations of microcystins in some rivers [42]. Our findings indicate that the occurrence of microcystins, and the seasonality of toxin production in California should be examined more comprehensively in future studies. We observed differences in the prevalent microcystin congeners within different waterbody types. For example, MCY-YR was not detected in the depresssional wetlands assessment or the 2013 screening assessment survey, but was detected in the ad hoc survey. These differences are likely due to differences in cyanobacterial species composition, the details of which are beyond the scope of this study. Tatters et al. [62] identified the prevalent potential toxin-producing cyanobacteria genera and species in estuaries and coastal lagoons along the coast of the Southern California Bight. Characterization of the prevalent southern California cyanobacteria species and corresponding toxin profiles should be the focus of future studies.
Chlorophyll-a as a Screening Indicator for Microcystin
Chlorophyll-a has been shown to be a meaningful screening variable in lakes at risk for cyanotoxins, and it is less expensive, easier to collect and analyze (or remotely sense) than cyanotoxins directly. Chlorophyll-a has been linked to pollution management actions such as nutrient load reductions [3,48,65,66,67]. In this study, there was a significant correlation between chlorophyll-a and microcystin concentrations in lakes, reservoirs, and coastal lagoons. Therefore, it is feasible based on these study results that future monitoring could utilize chlorophyll-a as a way to screen for microcystins in these waterbody types. Future studies should determine if this relationship holds for other types of cyanotoxins. However, no such correlative relationship was identified in the depressional wetlands waterbody type. The lack of correlation could be attributed two factors. First, it is possible that a stronger relationship could be found between toxin concentrations and benthic chlorophyll-a, which was not assessed. Many of these depressional wetlands are shallow (<1 m) dominated by benthic algal and cyanobacteria mats rather than planktonic species [68]. Second, the study design, based on one-time discrete sample collection (commonly used to evaluate chemical contaminants), is not ideal for cyanobacterial toxins as they can be sporadically and ephemerally produced, and thus toxin production can change within short time periods. As discussed below, the discrete sample results were not a good indicator of toxin presence in these systems (see Section 3.2 SPATT as an assessment and monitoring tool).
3.2. SPATT as an Assessment and Monitoring Tool
Traditional monitoring programs for cyanotoxins typically rely on discrete samples for toxin analysis, and health advisory thresholds have been based around this method of sampling. These methods can effectively measure toxin presence on the day and at the time of sample collection, but they are less effective at capturing ephemeral or episodic toxic events. In response to this challenge, a passive sampling method, SPATT, has been developed to monitor toxin concentrations in marine, brackish and freshwater environments. SPATT was first proposed for harmful algal bloom monitoring in 2004 as a means by which disadvantages associated with shellfish or other indicator organisms might be circumvented [50]. The approach has recently been refined as an assessment and monitoring tool to provide a time-integrated indicator of dissolved toxin presence with a waterbody [48,49,69,70,71]. SPATT has been shown to be a more sensitive indicator of toxin presence than discrete samples [42,48,49], however, it cannot currently be compared with health advisory thresholds established by California and US EPA. SPATT, was successfully used in this study as a monitoring and assessment tool to determine the prevalence and persistence of dissolved microcystins. The results indicated a high prevalence throughout the region, indicating the probability of a low-level but chronic exposure via direct as well as indirect pathways and indication that dissolved microcystins are prevalent and should be included in monitoring and event response programs.
3.3. Other Cyanotoxins and Multiple Stressors
Cyanobacteria genera and species identifications indicated a high risk for multiple cyanotoxins to be routinely produced and co-occur in these systems. The co-occurrence of multiple cyanotoxins at a single location has been documented in other studies, both within and outside of the U.S. [62,72,73,74,75,76] and in lagoons and estuaries within our study region in southern California [62]. There were a wide variety of potential toxin producing cyanobacteria identified in our study, but the most common genera were Microcystis sp., Cylindrospermopsis spp., and Anabaena spp., suggesting microcystins cylindrospermopsin, anatoxins and saxitoxin could be widespread. As such, a subset of lake samples was analyzed for additional cyanotoxins, and the results revealed the first detection of anatoxin-a from lakes in southern California as well as simultaneous detection of multiple cyanotoxins.
Lake Elsinore, Lake Menifee, and Canyon Lake were tested for additional cyanotoxins and were positive for 2–3 cyanotoxins. Lake Menifee and Canyon Lake had concentrations of cylindrospermopsin and anatoxin-a that exceeded recreational health thresholds. Cylindrospermopsin concentrations detected in Lake Elsinore exceeded recreational health thresholds. Previous studies of cyanobacteria species identification in 2003 and 2010 in Lake Elsinore indicate the potential for a historical presence of multiple cyanotoxins, since potential toxin producing species identified in 2003 and 2010 included Cylindrospermopsis raciborskii, Cylindrospermopsis c.f. catemaco, Aphanizomenon, Pseudanabaena limnetica, Pseudanabaena c.f. acicularis, Pseudanabaena catenata, and Planktothrix agardhii [77,78].
The combination of multiple cyanotoxin producing species identified, and the detection of multiple cyanotoxins simultaneously, highlights an important data gap in our understanding of the commonly occurring cyanotoxins, and cyanobacteria in these lentic systems. As with the interplay between acute and chronic risk, the interaction of multiple stressors on humans and other wildlife make the risk uncertain, because most recreational and drinking water health thresholds are typically based on exposures to single toxins and single organisms. The health consequences and implications of exposure to co-occurring cyanotoxins is poorly characterized. These biotoxins have different mechanisms of toxicity that could have synergistic effects and act as different, but additive, physiological stressors.
3.4. Conclusions and Recommendations
The dominance of cyanobacteria in the lentic waterbodies in the present study and the ubiquitous and persistent detection of microcystins in these heavily utilized aquatic habitats, suggest that cyanobacteria characterization and cyanotoxin detection should be included in routine and systematic monitoring programs of these systems. Future studies need to characterize the cyanobacteria species that are producing toxins and determine the concentration ranges routinely present in these systems. Passive sampling devices, such as SPATT, were successfully used in this study to determine prevalence of microcystins and should be included in future ambient monitoring and assessment programs. These sampling devices are particularly useful at capturing ephemeral events that traditional discrete samples do not capture, and therefore provide a more comprehensive view of cyanotoxins in a waterbody or region but are not at this time a replacement for traditional grab sampling, required for public health monitoring.
The potential for bioaccumulation of multiple cyanotoxins into the food web suggests that the influence of toxic cyanobacterial blooms is a much more complex stressor than presently recognized and should be considered a high priority measurement to include in condition assessments, water quality monitoring programs and additional risk assessments. Both high acute concentrations and low chronic concentrations of cyanotoxins should be considered a predominant stressor within lentic waterbodies. Since many of these waterbodies and watersheds are ultimately connected to the coastal ocean, these studies underscore the importance of inland waters as potential conduits for transfer of freshwater toxins to the marine environment, as evidenced by the occurrence of these toxins within the marine food web [43,45,47].
4. Methods
4.1. Study Area
The field surveys were all conducted within the coastal regions of the Southern California Bight (SCB), which is an open embayment that stretches from Point Conception, California, to Cabo Colnette, Baja California. The climate of this region is Mediterranean, with an average annual rainfall range of 10 to 100 cm, concentrated largely over the winter months of December-March, followed by hot, dry summers [79]. The SCB is a highly developed urban environment with a heavily altered landscape due to the approximately 20 million residents and 100 million visitors to the area annually [80]. This urbanization has extensively altered the natural landscape, watersheds and waterbodies, and converted significant amounts of open land to impervious surfaces, thereby changing both the timing and the rate of runoff releases to downstream waterbodies [80,81,82,83]. The majority of the nutrient loading is derived from non-point sources, since municipal wastewater is discharged as point sources into ocean outfalls directly to the SCB [84]. The region has few natural lakes and open-water depressional wetlands. Their abundance on the landscape can be attributed to type-conversion of riverine and palustrine habitats to create drinking water and recreational reservoirs, and stormwater detention basins [85].
4.2. Depressional Wetlands Assessment
The first ever systematic condition assessment of southern California depressional wetlands was conducted as part of the regional monitoring and assessment program led by California’s Surface Water Ambient Monitoring Program (SWAMP) [68]. The goals of the condition assessment were to determine: (1) the extent and distribution; and (2) the condition of depressional wetlands; and (3) to identify major stressors (such as urban and agricultural contaminants, invasive species, nutrient loading etc.). Cyanotoxins were not the focus of the regional condition assessment but samples were collected opportunistically as part of the field sampling program associated with the assessment. Particulate material only (i.e., plankton in the water column) was collected for cyanotoxin analysis, along with chlorophyll-a samples, following the guidelines in the Standard Operating Procedures (SOP) [86]. This approach excluded analysis of dissolved toxins, and therefore, underestimated the total concentration of cyanotoxins in the water.
4.2.1. Site Selection and Sampling Approach for Discrete Samples
The sites were randomly selected using a probabilistic sampling design by the Generalized Random Tessellation Stratified (GRTS) technique [87] in a spatially balanced approach. The assessment was conducted from 2011–2013, and each site was revisited once (May/June) after the initial reconnaissance survey. The 52 sites are summarized and categorized in Table 1.
Discrete samples were collected from composite surface water samples and a detailed version of the sample collection methodology is described in the SWAMP Depressional Wetlands Standard Operating Procedures [86]. Briefly, composite water samples were collected from 10 sampling “nodes” evenly spaced throughout the entire wetland perimeter. Field crews counted how many paces are required to walk the perimeter of the wetland at the water’s edge, then that number of paces was divided by 10, to yield the distance between adjacent sampling nodes. The field crews paced the perimeter of the wetland again, and placed an orange flag at each sampling node. They collected 200 mL sample from each sampling node, using an extended sampler device that allowed the collection of undisturbed samples approximately an arm’s length from the water’s edge, and placed the water into 2 L aluminum foil-covered bottles. Discrete samples were collected from the 2 L composite sample bottle after gentle mixing (inverted bottle upside-down five times) [86]. Particulate samples of chlorophyll-a and cyanotoxins were filtered onto 47 mm Whatman GF/F filters and frozen immediately in the field. Cyanotoxins were not the focus of the regional condition assessment, therefore the approach was to collect only filtered particulate fraction samples in the same way as chlorophyll-a samples [86] to ensure the samples would be collected with ease in a timely manner that allowed for completion of all of assessment samples. This collection approach also added a concentration step which allowed for a lower limit of detection. Additional water samples were collected and filtered through 0.45 µm polytetrafluoroethylene (PTFE) filters for later analysis of dissolved nutrients (ammonia, nitrate, nitrite, orthophosphate) and whole water samples were collected for total nitrogen, total Kjeldahl nitrogen (TKN), total phosphorus and alkalinity. All samples were frozen immediately in the field [86].
A more thorough cyanotoxin assessment was conducted in San Diego County in 2012 as both the particulate and dissolved fractions were measured. The 8 sites from the depressional wetlands assessment (sampled in May) were revisited twice between July and September (indicated by an asterisk (*) next to the site number in Table 1). Passive sampling devices, SPATT [48,49,50], were deployed continuously between site visits and were used as a screening assessment tool that provided time-integrated dissolved toxin samples of microcystin presence in the waterbodies. SPATT were deployed in 1 location of the wetland (the easiest to access and deploy) using a PVC tube and a rope tied to the SPATT sampler for approximately 1-month intervals between July and September 2012. Discrete samples were collected during each SPATT deployment and retrieval, for a total of two discrete sampling events per site. Discrete samples included particulate chlorophyll-a, particulate cyanotoxins (microcystins and saxitoxin), dissolved nutrients (ortho-phosphate, nitrate, nitrite, ammonium), total (whole water) phosphorus and nitrogen, TKN, and particulate nitrogen and were collected as described above.
4.2.2. Laboratory Analysis of Microcystin and Saxitoxin Samples
Discrete toxin samples were analyzed using enzyme-linked immunosorbent assay (ELISA) for both microcystins and saxitoxins. Microcystins were analyzed using the Envirologix QuantiPlateTM kit (Envirologix, Portland, Me, USA, Cat. No. EP 022, as described in [48]). The Envirologix ELISA kit has been compared with liquid chromatography/mass spectrometry (LC-MS) analysis at University of California, Santa Cruz [48]. The BIOO Scientific MaxSignalTM Saxitoxin (PSP) test kit (BIOO Scientific Corp., Austin, TX, USA, Cat. No. 1034) was used for saxitoxin analysis. These plates provide some cross-reactivity with other toxin congeners, but primarily target STX and dcSTX. Prior to analysis, the sample filters were extracted in 3 mL of Milli-Q™ water, sonicated for 30 s to ensure cell disruption, and centrifuged for 10 min at 2147 g (as described in [88]). The extract was then analyzed per the manufacturer’s instructions for both toxins (Envirologix: http://www.envirologix.com/wp-content/uploads/2016/04/EP022Microcystin.pdf and BIOO: http://www.biooscientific.com/Phycotoxin-test-kits/MaxSignal-Saxitoxin-PSP-ELISA-Test-Kit). The limit of detection for the microcystin and saxitoxin ELISA’s are 0.10 ppb and 0.4 ppb respectively.
SPATT samples were analyzed at the University of California, Santa Cruz for four microcystin congeners (MCY-LA, MCY-LR, MCY-RR, MCY-YR) by LC-MS with electrospray ionization (ESI) with selected ion monitoring (SIM) on an Agilent 6130 with a Phenomenex Kinetix (100 X 2.10) C18 column. The method was adapted from [89] with minor modifications to account for the choice of column and LCMS/SIM instead of tandem mass spectrometry [48]. The samples were prepared as described in [48]. Analysis included replicates and matrix-additions, with the quantification based on external standards. The method detection limit was 0.05 ng g−1 for all congeners. Percent recovery is reported in [48], and was ~58–100% for the congeners using a standardized recovery method, with MCY-RR being lowest followed by MCY-LR (~88%), MCY-YR (~100%), and MCY-LA (~100%).
4.2.3. Laboratory Analysis of Discrete Samples
Chlorophyll-a samples were analyzed at the Southern California Coastal Water Research Project following the method EPA 445.0. Nutrient samples were analyzed at Physis Environmental Laboratories, in Anaheim, California using the following methods: Total phosphorus (SM 4500-P E), dissolved nitrate + nitrite (EPA 300.0), TKN (EPA 351.2), dissolved ortho-phosphate (EPA 300.0) and alkalinity (SM 2320B).
4.3. Surveys of Lakes, Reservoirs, and Coastal Lagoons
4.3.1. Site Selection, Sampling Approach and Discrete Sample Collection
There were two types of surveys conducted of lakes, reservoirs and coastal lagoons: (a) a screening assessment survey in 2013; and (b) an ad hoc bloom event response survey in 2014. The screening assessment study was conducted during the summer and fall in 2013 in San Diego and Orange Counties (the two southern-most counties in coastal California). The sites were chosen based on several criteria including the following: (1) high number of beneficial uses of the water body; (2) high frequency of use by the public; (3) impaired nutrient status based on the Clean Water Act Section 303(d) list; and (4) high risk of cyanobacterial blooms. There were 19 sites sampled three times in the screening assessment survey in 2013 between July and October including 10 lakes and reservoirs and 9 estuaries (see Table 4 for list of sites).
The screening assessment survey in 2013 collected discrete samples from composite surface water samples as described in the Depressional Wetlands Assessment (see Section 4.2.1 Site Selection and Sampling Approach for Discrete Samples). SPATT bags were also deployed for two 1-month intervals, typically from July to August and from August to September, as described above in the Depressional Wetlands Assessment (Section 4.2). Discrete samples were collected at the time of each SPATT deployment and retrieval, for a total of three discrete sampling events per site. Discrete samples were collected as described above (Section 4.2.1 Site Selection and Sampling Approach for Discrete Samples), and consisted of particulate chlorophyll-a, particulate microcystins and particulate pigments (phycoerythrin and phycocyanin pigments). The pigment samples were collected on 1 µm Whatman polycarbonate filters, covered in aluminum foil and frozen immediately. In situ measurements of temperature, pH, dissolved oxygen (DO), conductivity, alkalinity and salinity were also collected at each site.
Due to reports of visible blooms, an ad hoc bloom event response survey was conducted in the summer of 2014 in San Diego, Orange and Riverside Counties. Discrete samples of surface water were collected in glass bottles from one location at the edge of the lake within the visible bloom. There were 17 lakes sampled between May and August for discrete samples of cyanobacterial species identification (whole water samples) and particulate cyanotoxins (see Table 5 for list of sites). The samples for cyanobacterial identification were stored in an incubator overnight and analyzed live the following day. The particulate cyanotoxin samples were filtered onto a Whatmann GF/F and frozen immediately. The samples for cyanobacterial identification were used to determine if cyanotoxin sample analysis was needed due to potential toxin-producing genera present in the samples.
The cyanotoxin discrete samples collected from four lakes sampled in May 2014 (Lake Elsinore, Canyon Lake, San Joaquin Marsh, and Lake Menifee) entailed analysis of whole water, and therefore results are representative of total toxins (both particulate and dissolved).
4.3.2. Laboratory Analysis of Cyanotoxin Samples
The discrete samples collected from the screening assessment study of lakes, reservoirs and coastal lagoons during 2013 and most of the ad hoc bloom response survey in 2014 were analyzed for 9 microcystin congeners (MCY-LA, MCY-LR, MCY-RR, MCY-YR, MCY-LW, MCY-LY, MC-desmethyl-LR, MC-desmethyl-RR, and MCY-LF) and nodularin at the California Fish and Wildlife Water Pollution Control Lab (WPCL) using LC-ESI-MS/MS as described in [89]. The discrete cyanotoxin samples collected at Lake Menifee, Lake Elsinore, Canyon Lake and San Joaquin Marsh were analyzed at University of California Santa Cruz for four microcystin congeners (MCY-LA, MCY-LR, MCY-RR, MCY-YR), anatoxin-a and cylindrospermopsin, by liquid chromatography/mass spectrometry (LCMS) with electrospray ionization (ESI) with selected ion monitoring (SIM) on an Agilent 6130 with a Phenomenex Kinetix (100 X 2.10) C18 column. The method was adapted from [89] with minor modifications to account for the choice of column and LCMS/SIM instead of tandem mass spectrometry [48].
SPATT samples were analyzed at University of California, Santa Cruz as described previously (see Section 4.2.2 Laboratory Analysis of Microcystin and Saxitoxin Samples).
4.3.3. Discrete Sample Analysis
Cyanobacterial genera and species identification samples were collected from all of the ad hoc bloom event response samples (2014) and analyzed at the University of Southern California. Samples were examined live within one day of collection. Briefly, homogenized water was aliquoted into shallow plastic tissue culture dishes and allowed to settle for approximately 30 min. The subsamples (5 mL) were viewed with an Accu-Scope (Commack, New York, NY, USA) 3032 inverted microscope and cyanobacteria were identified to genus and when possible, the species was identified. The San Joaquin Marsh sample was viewed using the CellScope Aquatic (Berkeley, CA, USA) field microscope at SCCWRP.
Phycoerythrin and phycocyanin pigment samples were sent to the laboratory of Dr. Gregory Boyer at the State University of New York and analyzed using a Milton-Roy MR3000 UV-VIS Spectrophotometer.
4.4. Statistical Analysis
The relationships between environmental variables, microcystins and chlorophyll-a were determined for the depressional wetlands assessment and the screening assessment survey in 2013 (no statistical analysis was performed on the ad hoc bloom response survey in 2014). Statistical packages of RStudio, (version 0.96.122 2009-2011, Boston, MA, USA) were used and the significance was set at 0.05 for all statistical analysis. Logistic regression was used to determine if chlorophyll-a (log 10 transformed), was a significant predictor of microcystin and additional environmental variables were included when data were available. The variables from the depressional wetlands assessment include the following: Landscape disturbance indicators (including percentage of agriculture, percentage of urban, percentage of agriculture and urban, impervious cover and road density) derived from the 2001 National Land Cover Database, total nitrogen and phosphorus, temperature, pH, alkalinity, elevation, conductivity and 3 condition indices developed for depressional wetlands [68]; (1) assemblages of macroinvertebrates [90]; (2) benthic diatom index [34]; and (3) the California Rapid Assessment Method (CRAM), which is a visual assessment of the plants and physical habitat [91].
The environmental variables used in the screening assessment survey in 2013 included alkalinity, total nitrogen, total phosphorus, cyanobacteria pigments (phycocyanin and phycoerythrin), temperature, salinity, conductivity, DO, and pH.
Supplementary Materials
The following are available online at www.mdpi.com/2072-6651/9/7/231/s1, Table S1: SPATT sample results for all microcystin congeners (in ng g−1) analyzed from the depressional wetlands sites that were revisited in summer and fall 2012 in San Diego, Table S2: SPATT results for all microcystin congeners (in ng g−1) analyzed from the 2013 screening assessment survey of lakes, reservoirs and coastal lagoons, Table S3: Discrete sample results for all microcystin congeners analyzed (in µg L−1) from the screening assessment survey of lakes, reservoirs and coastal lagoons in 2013, Table S4: Discrete sample results of all microcystin congeners (in μg L−1) analyzed from the ad hoc bloom event response survey in 2014.
Author Contributions
M.D.A.H., C.N., L.B., J.B., E.D.S. conceived and designed the study; M.D.A.H., C.N., K.H., A.O.T., R.K., and J.B. collected and analyzed samples; M.D.A.H., A.O.T., D.A.C., M.S. and those acknowledged analyzed the data; M.D.A.H. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carmichael, W. A world overview—One-hundred-twenty-seven years of research on toxic cyanobacteria—Where do we go from here? In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Hudnell, H.K., Ed.; Springer: New York, NY, USA, 2008; pp. 105–125. [Google Scholar]
- Hudnell, H.K.; Dortch, Q. A synopsis of research needs identified at the interagency, international symposium on cyanobacterial harmful algal blooms (isoc-hab). In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Hudnell, H.K., Ed.; Springer: New York, NY, USA, 2008; pp. 17–43. [Google Scholar]
- Otten, T.G.; Xu, H.; Qin, B.; Zhu, G.; Paerl, H.W. Spatiotemporal patterns and ecophysiology of toxigenic Microcystis blooms in Lake Taihu, China: Implications for water quality management. Environ. Sci. Technol. 2012, 46, 3480–3488. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Paerl, H.W. Nutrient and other environmental controls of harmful cyanobacterial blooms along the freshwater-marine continuum. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Hudnell, H.K., Ed.; Springer: New York, NY, USA, 2008; pp. 217–238. [Google Scholar]
- Paerl, H.W. Nuisance phytoplankton blooms in coastal, estuarine, and inland waters. Limnol. Oceanogr. 1988, 33, 823–847. [Google Scholar] [CrossRef]
- Paerl, H.W. A comparison of cyanobacterial bloom dynamics in freshwater, estuarine and marine environments. Phycologia 1996, 35 (Suppl. 6), 25–35. [Google Scholar] [CrossRef]
- Paerl, H.W.; Fulton, R.S. Ecology of harmful cyanobacteria. In Ecology of Harmful Marine Algae; Graneli, T., Ed.; Springer: Berlin, Germany, 2006; pp. 95–107. [Google Scholar]
- Paerl, H.W.; Hall, N.S.; Calandrino, E.S. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total Environ. 2011, 409, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Otten, T. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Paerl, H.W.; Qin, B.; Zhu, G.; Gao, G. Nitrogen and phosphorus inputs control phytoplankton growth in eutrophic Lake Taihu, China. Limnol. Oceanogr. 2010, 55, 420–432. [Google Scholar] [CrossRef]
- Backer, L.C.; Carmichael, W.; Kirkpatrick, B.; Williams, C.; Irvin, M.; Zhou, Y.; Johnson, T.B.; Nierenberg, K.; Hill, V.R.; Kieszak, S.M.; et al. Recreational exposure to low concentrations of microcystins during an algal bloom in a small lake. Mar. Drugs 2008, 6, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Backer, L.C.; Landsberg, J.H.; Miller, M.; Keel, K.; Taylor, T.K. Canine cyanotoxin poisonings in the United States (1920s–2012): Review of suspected and confirmed cases from three data sources. Toxins 2013, 5, 1597–1628. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W. Peer Review of Cyanotoxin Toxicity Criteria and Health Based Water Concentrations to Protect Human Swimmers, Dogs and Cattle; Wright State University: Dayton, OH, USA, 2001. [Google Scholar]
- Edwards, C.; Beattie, K.A.; Scrimgeour, C.M.; Codd, G.A. Identification of anatoxin-a in benthic cyanobacteria (blue-green algae) and in associated dog poisonings at Loch Insh, Scotland. Toxicon 1992, 30, 1165–1175. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.A.; Zhao, Q.; Pu, C.; Qiu, Z.; Zhang, R.; Shu, W. A cross-sectional investigation of chronic exposure to microcystin in relationship to childhood liver damage in the three Gorges Reservoir Region, China. Environ. Health Perspect. 2011, 119, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Mez, K.; Beattie, K.; Codd, G.; Hanselmann, K.; Hauser, B.; Naegeli, H.; Preisig, H. Identification of a microcystin in benthic cyanobacteria linked to cattle deaths on alpine pastures in switzerland. Eur. J. Phycol. 1997, 32, 111–117. [Google Scholar] [CrossRef]
- Pouria, S.; de Andrade, A.; Barbosa, J.; Cavalcanti, R.L.; Barreto, V.T.; Ward, C.J.; Preiser, W.; Poon, G.K.; Neild, G.H.; Codd, G.A. Fatal microcystin intoxication in haemodialysis unit in Caruaru, Brazil. Lancet 1998, 352, 21–26. [Google Scholar] [CrossRef]
- Stewart, I.; Seawright, A.A.; Shaw, G.R. Cyanobacterial poisoning in livestock, wild mammals and birds—An overview. Adv. Exp. Med. Biol. 2008, 619, 613–637. [Google Scholar] [PubMed]
- Trevino-Garrison, I.; DeMent, J.; Ahmed, F.S.; Haines-Lieber, P.; Langer, T.; Menager, H.; Neff, J.; van der Merwe, D.; Carney, E. Human illnesses and animal deaths associated with freshwater harmful algal blooms-kansas. Toxins 2015, 7, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Van Halderen, A.; Harding, W.R.; Wessels, J.C.; Schneider, D.J.; Heine, E.W.; Van der Merwe, J.; Fourie, J.M. Cyanobacterial (blue-green algae) poisoning of livestock in the western cape province of South Africa. J. S. Afr. Vet. Assoc. 1995, 66, 260–264. [Google Scholar] [PubMed]
- Wood, S.A.; Heath, M.W.; Holland, P.T.; Munday, R.; McGregor, G.B.; Ryan, K.G. Identification of a benthic microcystin-producing filamentous cyanobacterium (Oscillatoriales) associated with a dog poisoning in New Zealand. Toxicon 2010, 55, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.W.; Lazorchak, J.; Howard, M.D.A.; Johnson, M.V.; Morton, S.; Perkins, D.; Reavie, E.; Scott, G.; Smith, S.; Steevens, J.A. Are harmful algal blooms becoming the greatest inland water quality threat to public health and aquatic ecosystems? Environ. Toxicol. Chem. 2016, 35, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.W.; Lazorchak, J.; Howard, M.D.A.; Johnson, M.V.; Morton, S.; Perkins, D.; Reavie, E.; Scott, G.; Smith, S.; Steevens, J.A. In some places, in some cases, and at some times, harmful algal blooms are the greatest threat to inland water quality. Environ. Toxicol. Chem. 2017, 36, 1125–1127. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. Drinking Water Health Advisory for the Cyanobacterial Toxin Cylindrospermopsin; United States Environmental Protection Agency: Washington, DC, USA, 2015; p. 52.
- U.S. EPA. Drinking Water Health Advisory for the Cyanobacterial Microcystin Toxins; United States Environmental Protection Agency: Washington, DC, USA, 2015; p. 75.
- U.S. EPA. Human Health Recreational Ambient Water Quality Criteria or Swimming Advisories for Microcystins and Cylindrospermopsin; U.S. EPA Office of Water: Washington, DC, USA, 2016; p. 185.
- Olsen, L.D.; Valder, J.F.; Carter, J.M.; Zogorski, J.S. Prioritization of Constituents for National- and Regional-Scale Ambient Monitoring of Water and Sediment in the United States: U.S. Geological Survey; U.S. Geological Survey: Reston, VA, USA, 2013.
- Mazor, R.D. Bioassessment of Streams in Southern California: A Report on the First Five Years of the Stormwater Monitoring Coalition’s Regional Stream Survey; Technical Report #844; Southern California Coastal Water Research Project: Costa Mesa, CA, USA, 2015. [Google Scholar]
- Mazor, R.D.; May, J.; Sengupta, A.; McCune, K.; Stein, E.D. Setting hydrologic targets to support biological integrity. Freshw. Biol. submitted.
- Fetscher, A.E.; Stancheva, R.; Kociolek, J.P.; Sheath, R.G.; Mazor, R.D.; Stein, E.D.; Ode, P.R.; Busse, L.B. Development and comparison of stream indices of biotic integrity using diatoms vs. Non-diatom algae. J. Appl. Phycol. 2014, 26, 433–450. [Google Scholar] [CrossRef]
- McLaughlin, K.; Sutula, M.; Busse, L.; Anderson, S.; Crooks, J.; Dagit, R.; Gibson, D.; Johnston, K.; Stratton, L. A regional survey of the extent and magnitude of eutrophication in mediterranean estuaries of southern California, USA. Estuar. Coasts 2013, 37, 259–278. [Google Scholar] [CrossRef]
- State Water Resources Control Board. Impaired Water Bodies; State Water Resources Control Board: Sacramento, CA, USA, 2012.
- Izaguirre, G.; Jungblut, A.D.; Neilan, B.A. Benthic cyanobacteria (Oscillatoriaceae) that produce microcystin-LR, isolated from four reservoirs in southern California. Water Res. 2007, 41, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Fetscher, A.E.; Howard, M.D.A.; Stancheva, R.; Kudela, R.M.; Stein, E.D.; Sutula, M.A.; Busse, L.B.; Sheath, R.C. Wadeable streams as widespread sources of benthic cyanotoxins in California, USA. Harmful Algae 2015, 49, 105–116. [Google Scholar] [CrossRef]
- Magrann, T.; Howard, M.D.A.; Dunbar, S.G.; Sutula, M.; Boskovic, S.; Hayes, W.K. Screening assessment of cyanobacteria and cyanotoxins in southern California lentic habitats. Environ. Manag. Sustain. Dev. 2015, 4, 2164–7682. [Google Scholar] [CrossRef]
- Drake, J.L.; Carpenter, E.J.; Cousins, M.; Nelson, K.L.; Guido-Zarate, A.; Loftin, K. Effects of light and nutrients on seasonal phytoplankton succession in a temperate eutrophic coastal lagoon. Hydrobiol 2010, 654, 177–192. [Google Scholar] [CrossRef]
- Fetcho, K. Klamath River Blue-Green Algae Summary Report; Yurok Tribe: Klamath, CA, USA, 2007. [Google Scholar]
- Gibble, C.M.; Kudela, R.M. Detection of persistent microcystin toxins at the land-sea interface in Monterey Bay, California. Harmful Algae 2014, 39, 146–153. [Google Scholar] [CrossRef]
- Gibble, C.M.; Peacock, M.B.; Kudela, R.M. Evidence of freshwater algal toxins in marine shellfish: Implications for human and aquatic health. Harmful Algae 2016, 59, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Lehman, P.W.; Boyer, G.; Hall, C.; Waller, S.; Gehrts, K. Distribution and toxicity of a new colonial Microcystis aeruginosa bloom in the San Francisco Bay estuary, California. Hydrobiol 2005, 541, 87–99. [Google Scholar] [CrossRef]
- Miller, M.A.; Kudela, R.M.; Mekebri, A.; Crane, D.; Oates, S.C.; Tinker, M.T.; Staedler, M.; Miller, W.A.; Toy-Choutka, S.; Dominik, C.; et al. Evidence for a novel marine harmful algal bloom: Cyanotoxin (microcystin) transfer from land to sea otters. PLoS ONE 2010, 5, e12576. [Google Scholar] [CrossRef] [PubMed]
- Preece, E.P.; Hardy, F.J.; Moore, B.C.; Bryan, M. A review of microcystin detections in estuarine and marine waters: Environmental implications and human health risk. Harmful Algae 2017, 61, 31–45. [Google Scholar] [CrossRef]
- Preece, E.P.; Moore, B.C.; Hardy, F.J. Transfer of microcystin from freshwater lakes to Puget Sound, WA and toxin accumulation in marine mussels (mytilus trossulus). Ecotoxicol. Environ. 2015, 122, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Kudela, R.M. Characterization and deployment of solid phase adsorption toxin tracking (SPATT) resin for monitoring of microcystins in fresh and saltwater. Harmful Algae 2011, 11, 117–125. [Google Scholar] [CrossRef]
- Lane, J.Q.; Roddam, C.M.; Langlois, G.W.; Kudela, R.M. Application of solid phase adsorption toxin tracking (SPATT) for field detection of the hydrophilic phycotoxins domoic acid and saxitoxin in coastal California. Limnol. Oceanogr. Methods 2010, 8, 645–660. [Google Scholar] [CrossRef]
- MacKenzie, L.; Beuzenberg, V.; Holland, P.; McNabb, P.; Selwood, A. Solid phase adsorption toxin tracking (SPATT): A new monitoring tool that simulates the biotoxin contamination of filter feeding bivalves. Toxicon 2004, 44, 901–918. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. National Lakes Assessment: A Collaborative Survey of the Nation’s Lakes; United States Environmental Protection Agency: Washington, DC, USA, 2009.
- Berg, M.; Sutula, M. Factors Affecting the Growth of Cyanobacteria with Special Emphasis on the Sacramento-San Joaquin Delta; Technical Report 869 for Southern California Coastal Water Research Project: Costa Mesa, CA, USA, August 2015. [Google Scholar]
- Lehman, P.W.; Boyer, G.; Satchwell, M.; Waller, S. The influence of environmental conditions on the seasonal variation of Microcystis cell density and microcystins concentration in San Francisco Estuary. Hydrobiol 2008, 600, 187–204. [Google Scholar] [CrossRef]
- Backer, L.C.; McNeel, S.V.; Barber, T.; Kirkpatrick, B.; Williams, C.; Irvin, M.; Zhou, Y.; Johnson, T.B.; Nierenberg, K.; Aubel, M.; et al. Recreational exposure to microcystins during algal blooms in two California lakes. Toxicon 2009, 55, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Bury, N.R.; Eddy, F.B.; Codd, G.A. The effects of the cyanobacterium Microcystis aeruginosa, the cyanobacterial hepatotoxin microcystin–LR, and ammonia on growth rate and ionic regulation of brown trout. J. Fish Biol. 1995, 46, 1042–1054. [Google Scholar] [CrossRef]
- De Figueiredo, D.R.; Azeiteiro, U.M.; Esteves, S.M.; Goncalves, F.J.; Pereira, M.J. Microcystin-producing blooms--a serious global public health issue. Ecotoxicol. Environ. 2004, 59, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, C.; Thermes, V.; de Luze, A.; Puiseux-Dao, S.; Bernard, C.; Joly, J.S.; Bourrat, F.; Edery, M. Effects of microcystin-LR on development of medaka fish embryos (Oryzias latipes). Toxicon 2004, 43, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Malbrouk, C.; Kestemont, P. Effects of microcystins on fish. Environ. Toxicol. Chem. 2006, 25, 72–86. [Google Scholar] [CrossRef]
- Wiegand, C.; Pflugmacher, S.; Giese, M.; Frank, H.; Steinberg, C. Uptake, toxicity, and effects on detoxication enzymes of atrazine and trifluoroacetate in embryos of zebrafish. Ecotoxicol. Environ. 2000, 45, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Moy, N.J.; Dodson, J.; Tassone, S.J.; Bukaveckas, P.A.; Bulluck, L.P. Biotransport of algal toxins to riparian food webs. Environ. Sci. Technol. 2016, 50, 10007–10014. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Z.; Boland, M.P.; Smillie, M.A.; Klix, H.; Ptak, C.; Andersen, R.J.; Holmes, C.F. Identification of protein phosphatase inhibitors of the microcystin class in the marine environment. Toxicon 1993, 31, 1407–1414. [Google Scholar] [CrossRef]
- Tatters, A.O.; Howard, M.D.; Nagoda, C.; Busse, L.; Gellene, A.G.; Caron, D.A. Multiple stressors at the land-sea interface: Cyanotoxins at the land-sea interface in the Southern California Bight. Toxins 2017, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.T.; Schwall, K.K.; Pardy, L.L. Water Quality Control Plan for the San Diego Basin (9); California Regional Water Quality Control Board: San Diego Region, CA, USA, 1994; Volume 8.
- Moisander, P.H.; Ochiai, M.; Lincoff, A. Nutrient limitation of Microcystis aeruginosa in northern california klamath river reservoirs. Harmful Algae 2009, 8, 889–897. [Google Scholar] [CrossRef]
- Rinta-Kanto, J.M.; Konopko, E.A.; DeBruyn, J.M.; Bourbonniere, R.A.; Boyer, G.L.; Wilhelm, S.W. Lake Erie Microcystis: Relationship between microcystin production, dynamics of genotypes and environmental parameters in a large lake. Harmful Algae 2009, 8, 665–673. [Google Scholar] [CrossRef]
- Yuan, L.L.; Pollard, A.I. Deriving nutrient targets to prevent excessive cyanobacterial densities in U.S. Lakes and reservoirs. Freshw. Biol. 2015, 60, 1901–1916. [Google Scholar] [CrossRef]
- Yuan, L.L.; Pollard, A.I.; Pather, S.; Oliver, J.L.; D’Anglada, L. Managing microcystin: Identifying national-scale thresholds for total nitrogen and chlorophyll a. Freshw. Biol. 2014, 59, 1970–1981. [Google Scholar] [CrossRef]
- Brown, J.S.; Stein, E.D.; Solek, C.; Fetscher, A.E. Assessment of the Condition of Southern California Depressional Wetlands: Application of Macroinvertebrate, Diatom and Overall Condition Indices for Assessing Southern California Depressional Wetlands; State Water Resources Control Board Surface Water Ambient Monitoring Program: Sacramento, CA, USA, 2016.
- Fux, E.; Marcaillou, C.; Mondeguer, F.; Bire, R.; Hess, P. Field and mesocosm trials on passive sampling for the study of adsorption and desorption behavior of lipophilic toxins with a focus on OA and DTX1. Harmful Algae 2008, 7, 574–583. [Google Scholar] [CrossRef]
- Fux, E.; Bire, R.; Hess, P. Comparative accumulation and composition of lipophilic marine biotoxins in passive samplers and in mussels (M. Edulis) on the west coast of Ireland. Harmful Algae 2009, 8, 523–537. [Google Scholar] [CrossRef]
- Wood, S.A.; Holland, P.T.; MacKenzie, L. Development of solid phase adsorption toxin tracking (SPATT) for monitoring anatoxin-a and homoanatoxin-a in river water. Chemosphere 2011, 82, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Gkelis, S.; Zaoutsos, N. Cyanotoxin occurrence and potentially toxin producing cyanobacteria in freshwaters of Greece: A multi-disciplinary approach. Toxicon 2014, 78, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.L.; Loftin, K.A.; Meyer, M.T.; Ziegler, A.C. Cyanotoxin mixtures and taste-and-odor compounds in cyanobacterial blooms from the midwestern United States. Environ. Sci. Technol. 2010, 44, 7361–7368. [Google Scholar] [CrossRef] [PubMed]
- Pekar, H.; Westerberg, E.; Bruno, O.; Laane, A.; Persson, K.M.; Sundstrom, L.F.; Thim, A.M. Fast, rugged and sensitive ultra high pressure liquid chromatography tandem mass spectrometry method for analysis of cyanotoxins in raw water and drinking water--first findings of anatoxins, cylindrospermopsins and microcystin variants in Swedish source waters and infiltration ponds. J. Chromatogr. A 2016, 1429, 265–276. [Google Scholar] [PubMed]
- Rodriguez, I.; Rodriguez, C.; Alfonso, A.; Otero, P.; Meyer, T.; Breitenbach, U.; Botana, L.M. Toxin profile in samples collected in fresh and brackish water in Germany. Toxicon 2014, 91, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Sabart, M.; Crenn, K.; Perrière, F.; Abila, A.; Leremboure, M.; Colombet, J.; Jousse, C.; Latour, D. Co-occurrence of microcystin and anatoxin-a in the freshwater Lake Aydat (France): Analytical and molecular approaches during a three-year survey. Harmful Algae 2015, 48, 12–20. [Google Scholar] [CrossRef]
- Oza, H.I. Nutrient Levels and Phytoplankton Abundance in Canyon Lake and Lake Elsinore, CA; University of California, Riverside: Riverside, CA, USA, 2003. [Google Scholar]
- Tobin, M. A Characterization of the Phytoplankton, Zooplankton and Benthic Invertebrate Communities of Lake Elsinore; Masters, University of California, Riverside: Riverside, CA, USA, 2011. [Google Scholar]
- Schiff, K.; Allen, M.J.; Zeng, E.Y.; Bay, S.M. Southern California. Mar. Pollut. Bull. 2000, 41, 76–93. [Google Scholar] [CrossRef]
- National Research Council (NRC). Monitoring Southern California’s Coastal Waters; National Academy Press: Washington, DC, USA, 1990. [Google Scholar]
- Brownlie, W.R.; Taylor, B.D. Sediment Management for Southern California Mountains, Coastal Plains and Shoreline; Part C, Coastal Sediment Delivery by Major Rivers in Southern California; Environmental Quality Laboratory Report No. 17-C; California Institute of Technology: Pasadena, CA, USA, 1981. [Google Scholar]
- Horn, M.H.; Allen, L.G. Fish Community Ecology in Southern California Baysand Estuaries; UNAM Press: Mexico City, Mexico, 1985; pp. 169–190. [Google Scholar]
- Zedler, J.B. Coastal mitigation in southern California: The need for a regional restoration strategy. Ecol. Appl. 1996, 6, 84–93. [Google Scholar] [CrossRef]
- Howard, M.; Sutula, M.; Caron, D.A.; Chao, Y.; Farrara, J.D.; Jones, H.F.B.; Robertson, G.; McLaughlin, K.; Sengupta, A. Anthropogenic nutrient sources rival natural sources on small scales in the coastal waters of the Southern California Bight. Limnol. Oceanogr. 2014, 59, 285–297. [Google Scholar] [CrossRef]
- Brown, J.; Sutula, M.; Stransky, C.; Rudolph, J.; Byron, E. Sediment contaminant chemistry and toxicity of freshwater in urban wetlands in southern California. J. Am. Water Res. Assoc. 2010, 46, 367–384. [Google Scholar] [CrossRef]
- Fetscher, A.; Lunde, K.; Stein, E.D.; Brown, J.S. Standard Operating Procedures (SOP) for Collection of Macroinvertebrates, Benthic Algae, and Associated Physical Habitat Data in California Depressional Wetlands; Technical Report 832; Southern California Coastal Water Research Project: Costa Mesa, CA, USA, 2015; p. 62. [Google Scholar]
- Stevens, D.L.; Olsen, A.R. Spatially balanced sampling of natural resources. J. Am. Stat. Assoc. 2004, 99, 262–278. [Google Scholar] [CrossRef]
- Seubert, E.L.; Howard, M.D.; Kudela, R.M.; Stewart, T.N.; Litaker, R.W.; Evans, R.; Caron, D.A. Development, comparison, and validation using elisas for the determination of domoic acid in California sea lion body fluids. J. AOAC Int. 2014, 97, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Mekebri, A.; Blondina, G.J.; Crane, D.B. Method validation of microcystins in water and tissue by enhanced liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 3147–3155. [Google Scholar] [CrossRef] [PubMed]
- Lunde, K.B.; Resh, V.H. Development and validation of a macroinvertebrate index of biotic integrity (ibi) for assessing urban impacts to northern California freshwater wetlands. Environ. Monit. Assess. 2012, 184, 3653–3674. [Google Scholar] [CrossRef] [PubMed]
- California Wetland Monitoring Workgroup. California Rapid Assessment Method (CRAM) for Wetlands, Version 6.1. California Rapid Assessment Method Web Site, 2013. Available online: http://www.cramwetlands.org/documents (accessed on 14 February 2013).
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).