Engineered Liver-On-A-Chip Platform to Mimic Liver Functions and Its Biomedical Applications: A Review
Abstract
:1. Introduction
2. Physiological Microenvironment of the Liver
2.1. Cell Types and Composition
2.1.1. Parenchymal Cells
2.1.2. Hepatic Stellate Cells
2.1.3. Hepatic Sinusoidal Endothelial Cells
2.1.4. Kupffer Cells
2.1.5. Biliary Epithelial Cells
2.1.6. Other Non-Parenchymal Cells
2.2. Simulation Objects of a Liver-on-a-Chip
2.2.1. Liver Sinusoid
2.2.2. Liver Lobule
2.2.3. Zonation in the Lobule
3. General Strategies for in Vitro Liver Models
4. Liver-On-A-Chip Technology
4.1. Liver Chips Based on 2D Planar Culture
4.2. Liver Chips Based on Matrixless 3D Spheroid Culture
4.3. Liver Chips Based on Matrix-Dependent 3D Culture
4.4. Liver Chips Based on Layer-by-Layer Deposition
4.5. Liver Chips Based on 3D Bioprinting
4.6. Liver Chips Based on Other Technologies
5. Applications of a Liver-on-a-Chip
5.1. Drug Screening and Toxicity Testing
5.2. Prediction of Metabolism
5.3. Establishment of Liver Disease Models
5.4. Fabrication of Multiple Organs on a Chip
6. Challenges and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Wiśniewski, J.R.; Vildhede, A.; Norén, A.; Artursson, P. In-depth quantitative analysis and comparison of the human hepatocyte and hepatoma cell line HepG2 proteomes. J. Proteomics 2016, 136, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Casotti, V.; Antiga, L.D. Basic principles of liver physiology. In Pediatric Hepatology and Liver Transplantation; D’Antiga, L., Ed.; Springer International Publishing: New York City, NY, USA, 2019; pp. 21–39. [Google Scholar]
- De Boer, Y.S.; Kosinski, A.S.; Urban, T.J.; Zhao, Z.; Long, N.; Chalasani, N.; Kleiner, D.E.; Hoofnagle, J.H. Features of Autoimmune Hepatitis in Patients with Drug-induced Liver Injury. Clin. Gastroenterol. H 2017, 15, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Suzuki, A.; Borlak, J.; Andrade, R.J.; Lucena, M.I. Drug-induced liver injury: Interactions between drug properties and host factors. J. Hepatol. 2015, 63, 503–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Midwoud, P.M.; Verpoorte, E.; Groothuis, G.M.M. Microfluidic devices for in vitro studies on liver drug metabolism and toxicity. Integr. Biol. Uk 2011, 3, 509. [Google Scholar] [CrossRef] [PubMed]
- Olson, H.; Betton, G.; Robinson, D.; Thomas, K.; Monro, A.; Kolaja, G.; Lilly, P.; Sanders, J.; Sipes, G.; Bracken, W.; et al. Concordance of the Toxicity of Pharmaceuticals in Humans and in Animals. Regul. Toxicol. Pharm. 2000, 32, 56–67. [Google Scholar] [CrossRef]
- Choi, W.; Eun, H.S.; Lee, Y.; Kim, S.J.; Kim, M.; Lee, J.; Shim, Y.; Kim, H.; Kim, Y.E.; Yi, H.; et al. Experimental Applications of in situ Liver Perfusion Machinery for the Study of Liver Disease. Mol. Cells 2019, 42, 45–55. [Google Scholar]
- Tomlinson, L.; Hyndman, L.; Firman, J.W.; Bentley, R.; Kyffin, J.A.; Webb, S.D.; McGinty, S.; Sharma, P. In vitro Liver Zonation of Primary Rat Hepatocytes. Front. Bioeng. Biotechnol. 2019, 7, 17. [Google Scholar] [CrossRef]
- Moravcova, A.; Cervinkova, Z.; Kucera, O.; Mezera, V.; Rychtrmoc, D.; Lotkova, H. The effect of oleic and palmitic acid on induction of steatosis and cytotoxicity on rat hepatocytes in primary culture. Physiol. Res. 2015, 64, S627–S636. [Google Scholar]
- Lee, H.; Chae, S.; Kim, J.Y.; Han, W.; Kim, J.; Choi, Y.; Cho, D. Cell-printed 3D liver-on-a-chip possessing a liver microenvironment and biliary system. Biofabrication 2019, 11, 25001. [Google Scholar] [CrossRef]
- Kim, Y.; Kang, K.; Jeong, J.; Paik, S.S.; Kim, J.S.; Park, S.A.; Kim, W.D.; Park, J.; Choi, D. Three-dimensional (3D) printing of mouse primary hepatocytes to generate 3D hepatic structure. Ann. Surg Treat. Res. 2017, 92, 67. [Google Scholar] [CrossRef]
- Broutier, L.; Mastrogiovanni, G.; Verstegen, M.M.; Francies, H.E.; Gavarró, L.M.; Bradshaw, C.R.; Allen, G.E.; Arnes-Benito, R.; Sidorova, O.; Gaspersz, M.P.; et al. Human primary liver cancer–derived organoid cultures for disease modeling and drug screening. Nat. Med. 2017, 23, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef]
- Li, L.; Gokduman, K.; Gokaltun, A.; Yarmush, M.L.; Usta, O.B. A microfluidic 3D hepatocyte chip for hepatotoxicity testing of nanoparticles. Nanomedicine 2019, 14, 16. [Google Scholar] [CrossRef]
- Haque, A.; Gheibi, P.; Stybayeva, G.; Gao, Y.; Torok, N.; Revzin, A. Ductular reaction-on-a-chip: Microfluidic co-cultures to study stem cell fate selection during liver injury. Sci. Rep. UK 2016, 6, 36077. [Google Scholar] [CrossRef] [PubMed]
- Esch, M.B.; Ueno, H.; Applegate, D.R.; Shuler, M.L. Modular, pumpless body-on-a-chip platform for the co-culture of GI tract epithelium and 3D primary liver tissue. Lab Chip 2016, 16, 2719–2729. [Google Scholar] [CrossRef] [PubMed]
- Underhill, G.H.; Khetani, S.R. Bioengineered Liver Models for Drug Testing and Cell Differentiation Studies. Cellul. Mol. Gastroenterol. Hepatol. 2018, 5, 426–439. [Google Scholar] [CrossRef]
- Beckwitt, C.H.; Clark, A.M.; Wheeler, S.; Taylor, D.L.; Stolz, D.B.; Griffith, L.; Wells, A. Liver ‘organ on a chip’. Exp. Cell Res. 2018, 363, 15–25. [Google Scholar] [CrossRef]
- Lauschke, V.M.; Hendriks, D.F.G.; Bell, C.C.; Andersson, T.B.; Ingelman-Sundberg, M. Novel 3D Culture Systems for Studies of Human Liver Function and Assessments of the Hepatotoxicity of Drugs and Drug Candidates. Chem. Res. Toxicol. 2016, 29, 1936–1955. [Google Scholar] [CrossRef]
- Yoon No, D.; Lee, K.; Lee, J.; Lee, S. 3D liver models on a microplatform: well-defined culture, engineering of liver tissue and liver-on-a-chip. Lab Chip 2015, 15, 3822–3837. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Zhang, B.; Korolj, A.; Lai, B.F.L.; Radisic, M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 2018, 3, 257–278. [Google Scholar] [CrossRef]
- Usta, O.B.; McCarty, W.J.; Bale, S.; Hegde, M.; Jindal, R.; Bhushan, A.; Golberg, I.; Yarmush, M.L. Microengineered cell and tissue systems for drug screening and toxicology applications: Evolution ofin-vitro liver technologies. TECHNOLOGY 2015, 3, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.; Hewitt, N.J.; Albrecht, U.; Andersen, M.E.; Ansari, N.; Bhattacharya, S.; Bode, J.G.; Bolleyn, J.; Borner, C.; Böttger, J.; et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch. Toxicol. 2013, 87, 1315–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treyer, A.; Müsch, A. Hepatocyte polarity. Compr. Physiol. 2013, 3, 243. [Google Scholar] [PubMed]
- Laskin, D. Nonparenchymal Cells and Hepatotoxicity. Semin. Liver Dis. 1990, 10, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Poisson, J.; Lemoinne, S.; Boulanger, C.; Durand, F.; Moreau, R.; Valla, D.; Rautou, P. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J. Hepatol. 2017, 66, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, P.; He, K.; Liu, Z.; Gong, J. The role of Kupffer cells in hepatic diseases. Mol. Immunol. 2017, 85, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Boltjes, A.; Movita, D.; Boonstra, A.; Woltman, A.M. The role of Kupffer cells in hepatitis B and hepatitis C virus infections. J. Hepatol. 2014, 61, 660–671. [Google Scholar] [CrossRef] [Green Version]
- Ringelhan, M.; Pfister, D.; O Connor, T.; Pikarsky, E.; Heikenwalder, M. The immunology of hepatocellular carcinoma. Nat. Immunol. 2018, 19, 222–232. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, X.; Chen, Z.; Luo, Y.; Lu, Y.; Liu, T.; Wu, Z.; Jin, Y.; Zhao, W.; Lin, B. A cell lines derived microfluidic liver model for investigation of hepatotoxicity induced by drug-drug interaction. Biomicrofluidics 2019, 13, 24101. [Google Scholar] [CrossRef]
- Adams, D.H.; Ju, C.; Ramaiah, S.K.; Uetrecht, J.; Jaeschke, H. Mechanisms of Immune-Mediated Liver Injury. Toxicol. Sci. 2010, 115, 307–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Wang, Y.; Wang, J.; Wu, J.; Meng, X.; Hu, P.; Mu, X.; Liang, Q.; Luo, G. Design and fabrication of a liver-on-a-chip platform for convenient, highly efficient, and safe in situ perfusion culture of 3D hepatic spheroids. Lab Chip 2018, 18, 2547–2562. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Chang, S.; Shih, M.; Tseng, S.; Lai, C. Scaffold-Free Liver-On-A-Chip with Multiscale Organotypic Cultures. Adv. Mater. 2017, 29, 1701545. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Qu, X.; Zhu, W.; Li, Y.; Yuan, S.; Zhang, H.; Liu, J.; Wang, P.; Lai, C.S.E.; Zanella, F.; et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci. USA 2016, 113, 2206–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.B.; Eo, J.; Mert, S.; Yarmush, M.L.; Usta, O.B. Metabolic Patterning on a Chip: Towards in vitro Liver Zonation of Primary Rat and Human Hepatocytes. Sci. Rep. UK 2018, 8, 8951. [Google Scholar] [CrossRef] [PubMed]
- Madurska, M.J.; Poyade, M.; Eason, D.; Rea, P.; Watson, A.J.M. Development of a Patient-Specific 3D-Printed Liver Model for Preoperative Planning. Surg. Innov. 2017, 24, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Witowski, J.S.; Pędziwiatr, M.; Major, P.; Budzyński, A. Cost-effective, personalized, 3D-printed liver model for preoperative planning before laparoscopic liver hemihepatectomy for colorectal cancer metastases. Int. J. Comput. Ass. Rad. 2017, 12, 2047–2054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramachandran, S.D.; Schirmer, K.; Münst, B.; Heinz, S.; Ghafoory, S.; Wölfl, S.; Simon-Keller, K.; Marx, A.; Øie, C.I.; Ebert, M.P.; et al. In Vitro Generation of Functional Liver Organoid-Like Structures Using Adult Human Cells. PLoS ONE 2015, 10, e139345. [Google Scholar] [CrossRef]
- Matsusaki, M.; Case, C.P.; Akashi, M. Three-dimensional cell culture technique and pathophysiology. Adv. Drug Deliv. Rev. 2014, 74, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Tostões, R.M.; Leite, S.B.; Serra, M.; Jensen, J.; Björquist, P.; Carrondo, M.J.T.; Brito, C.; Alves, P.M. Human liver cell spheroids in extended perfusion bioreactor culture for repeated-dose drug testing. Hepatology 2012, 55, 1227–1236. [Google Scholar] [CrossRef]
- Schanz, S.; Schmalzing, M.; Guenova, E.; Metzler, G.; Ulmer, A.; K Ötter, I.; Fierlbeck, G. Interstitial Granulomatous Dermatitis with Arthritis Responding to Tocilizumab. Arch. Dermatol. 2012, 148, 17–20. [Google Scholar] [CrossRef]
- Kyffin, J.A.; Sharma, P.; Leedale, J.; Colley, H.E.; Murdoch, C.; Mistry, P.; Webb, S.D. Impact of cell types and culture methods on the functionality of in vitro liver systems-A review of cell systems for hepatotoxicity assessment. Toxicol. Vitro 2018, 48, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.M.M.; Salerno, S.; Morelli, S.; Giorno, L.; De Bartolo, L. 3D liver membrane system by co-culturing human hepatocytes, sinusoidal endothelial and stellate cells. Biofabrication 2017, 9, 25022. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.B.A.; Sodunke, T.R.; Lamontagne, J.; Cirillo, J.; Rajiv, C.; Bouchard, M.J.; Noh, M. Liver sinusoid on a chip: Long-term layered co-culture of primary rat hepatocytes and endothelial cells in microfluidic platforms. Biotechnol. Bioeng. 2015, 112, 2571–2582. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; No, D.Y.; Kang, E.; Ju, J.; Kim, D.; Lee, S. Spheroid-based three-dimensional liver-on-a-chip to investigate hepatocyte–hepatic stellate cell interactions and flow effects. Lab Chip 2013, 13, 3529. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, B.; No, D.Y.; Lee, G.; Lee, S.; Oh, H.; Lee, S. A 3D alcoholic liver disease model on a chip. Integr. Biol. UK 2016, 8, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Lauschke, V.M.; Shafagh, R.Z.; Hendriks, D.F.G.; Ingelman Sundberg, M. 3D Primary Hepatocyte Culture Systems for Analyses of Liver Diseases, Drug Metabolism, and Toxicity: Emerging Culture Paradigms and Applications. Biotechnol. J. 2019, 14, 1800347. [Google Scholar] [CrossRef]
- Meyer, K.; Ostrenko, O.; Bourantas, G.; Morales-Navarrete, H.; Porat-Shliom, N.; Segovia-Miranda, F.; Nonaka, H.; Ghaemi, A.; Verbavatz, J.; Brusch, L.; et al. A Predictive 3D Multi-Scale Model of Biliary Fluid Dynamics in the Liver Lobule. Cell Syst. 2017, 4, 277–290. [Google Scholar] [CrossRef]
- Van Grunsven, L.A. 3D in vitro models of liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 133–146. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Ikeuchi, M.; Noguchi, H.; Yagi, T.; Hayashi, S. Spheroid Formation and Evaluation of Hepatic Cells in a Three-Dimensional Culture Device. Cell Med. 2015, 8, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Prieto, A.M.; Skelton, J.K.; Wai, S.N.; Large, E.; Lussignol, M.; Vizcay-Barrena, G.; Hughes, D.; Fleck, R.A.; Thursz, M.; Catanese, M.T.; et al. 3D microfluidic liver cultures as a physiological preclinical tool for hepatitis B virus infection. Nat. Commun. 2018, 9, 682. [Google Scholar] [CrossRef] [PubMed]
- Frey, O.; Misun, P.M.; Fluri, D.A.; Hengstler, J.G.; Hierlemann, A. Reconfigurable microfluidic hanging drop network for multi-tissue interaction and analysis. Nat. Commun. 2014, 5, 4250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bavli, D.; Prill, S.; Ezra, E.; Levy, G.; Cohen, M.; Vinken, M.; Vanfleteren, J.; Jaeger, M.; Nahmias, Y. Real-time monitoring of metabolic function in liver-on-chip microdevices tracks the dynamics of mitochondrial dysfunction. Proc. Natl. Acad. Sci. USA 2016, 113, E2231–E2240. [Google Scholar] [CrossRef] [PubMed]
- Delalat, B.; Cozzi, C.; Rasi Ghaemi, S.; Polito, G.; Kriel, F.H.; Michl, T.D.; Harding, F.J.; Priest, C.; Barillaro, G.; Voelcker, N.H. Microengineered Bioartificial Liver Chip for Drug Toxicity Screening. Adv. Funct. Mater. 2018, 28, 1801825. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, L.; Zhou, E.; Xu, J.; Shen, S.; Wang, J. On-Chip Construction of Liver Lobule-like Microtissue and Its Application for Adverse Drug Reaction Assay. Anal. Chem. 2016, 88, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; George, S.M.; Vernetti, L.; Gough, A.H.; Taylor, D.L. A glass-based, continuously zonated and vascularized human liver acinus microphysiological system (vLAMPS) designed for experimental modeling of diseases and ADME/TOX. Lab Chip 2018, 18, 2614–2631. [Google Scholar] [CrossRef] [PubMed]
- Schepers, A.; Li, C.; Chhabra, A.; Seney, B.T.; Bhatia, S. Engineering a perfusable 3D human liver platform from iPS cells. Lab Chip 2016, 16, 2644–2653. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.; Tseng, H.; Souza, G. Assembly of Hepatocyte Spheroids Using Magnetic 3D Cell Culture for CYP450 Inhibition/Induction. Int. J. Mol. Sci. 2017, 18, 1085. [Google Scholar] [CrossRef]
- Weltin, A.; Hammer, S.; Noor, F.; Kaminski, Y.; Kieninger, J.; Urban, G.A. Accessing 3D microtissue metabolism: Lactate and oxygen monitoring in hepatocyte spheroids. Biosens. Bioelectron. 2017, 87, 941–948. [Google Scholar] [CrossRef]
- Foster, A.J.; Chouhan, B.; Regan, S.L.; Rollison, H.; Amberntsson, S.; Andersson, L.C.; Srivastava, A.; Darnell, M.; Cairns, J.; Lazic, S.E.; et al. Integrated in vitro models for hepatic safety and metabolism: evaluation of a human Liver-Chip and liver spheroid. Arch. Toxicol. 2019, 93, 1021–1037. [Google Scholar] [CrossRef] [Green Version]
- Toh, Y.; Lim, T.C.; Tai, D.; Xiao, G.; van Noort, D.; Yu, H. A microfluidic 3D hepatocyte chip for drug toxicity testing. Lab Chip 2009, 9, 2026. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.E.; Fleming, H.E.; Khetani, S.R.; Bhatia, S.N. Pluripotent stem cell-derived hepatocyte-like cells. Biotechnol. Adv. 2014, 32, 504–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, F.; Liu, G.; Belmonte, J.C.I. Human induced pluripotent stem cells derived hepatocytes: rising promise for disease modeling, drug development and cell therapy. Protein Cell 2012, 3, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.; Lin, R.; Chen, R.; Chin, C.; Gong, S.; Chang, H.; Peng, H.; Hsu, L.; Yew, T.; Chang, S.; et al. Liver-cell patterning Lab Chip: mimicking the morphology of liver lobule tissue. Lab Chip 2013, 13, 3578. [Google Scholar] [CrossRef] [PubMed]
- Misun, P.M.; Rothe, J.; Schmid, Y.R.F.; Hierlemann, A.; Frey, O. Multi-analyte biosensor interface for real-time monitoring of 3D microtissue spheroids in hanging-drop networks. Microsyst. Nanoeng. 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aeby, E.A.; Misun, P.M.; Hierlemann, A.; Frey, O. Microfluidic Hydrogel Hanging-Drop Network for Long-Term Culturing of 3D Microtissues and Simultaneous High-Resolution Imaging. Adv. Biosyst. 2018, 2, 1800054. [Google Scholar] [CrossRef]
- Boos, J.A.; Misun, P.M.; Michlmayr, A.; Hierlemann, A.; Frey, O. Microfluidic Multitissue Platform for Advanced Embryotoxicity Testing In Vitro. Adv. Sci. 2019, 6, 1900294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leite, S.B.; Roosens, T.; El Taghdouini, A.; Mannaerts, I.; Smout, A.J.; Najimi, M.; Sokal, E.; Noor, F.; Chesne, C.; van Grunsven, L.A. Novel human hepatic organoid model enables testing of drug-induced liver fibrosis in vitro. Biomaterials 2016, 78, 1–10. [Google Scholar] [CrossRef]
- Hegde, M.; Jindal, R.; Bhushan, A.; Bale, S.S.; McCarty, W.J.; Golberg, I.; Usta, O.B.; Yarmush, M.L. Dynamic interplay of flow and collagen stabilizes primary hepatocytes culture in a microfluidic platform. Lab Chip 2014, 14, 2033–2039. [Google Scholar] [CrossRef]
- Lu, S.; Cuzzucoli, F.; Jiang, J.; Liang, L.; Wang, Y.; Kong, M.; Zhao, X.; Cui, W.; Li, J.; Wang, S. Development of a biomimetic liver tumor-on-a-chip model based on decellularized liver matrix for toxicity testing. Lab Chip 2018, 18, 3379–3392. [Google Scholar] [CrossRef]
- Jang, M.; Neuzil, P.; Volk, T.; Manz, A.; Kleber, A. On-chip three-dimensional cell culture in phaseguides improves hepatocyte functionsin vitro. Biomicrofluidics 2015, 9, 34113. [Google Scholar] [CrossRef] [PubMed]
- Christoffersson, J.; Aronsson, C.; Jury, M.; Selegård, R.; Aili, D.; Mandenius, C. Fabrication of modular hyaluronan-PEG hydrogels to support 3D cultures of hepatocytes in a perfused liver-on-a-chip device. Biofabrication 2019, 11, 15013. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Fan, X.; Wang, B.; Liu, L.; Yan, X.; Zhou, L.; Zeng, Y.; Poznansky, M.C.; Wang, L.; Chen, H.; et al. Biomechanically primed liver microtumor array as a high-throughput mechanopharmacological screening platform for stroma-reprogrammed combinatorial therapy. Biomaterials 2017, 124, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Yajima, Y.; Lee, C.N.; Yamada, M.; Utoh, R.; Seki, M. Development of a perfusable 3D liver cell cultivation system via bundling-up assembly of cell-laden microfibers. J. Biosci. Bioeng. 2018, 126, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Akagi, T.; Asaoka, T.; Eguchi, H.; Fukuda, Y.; Iwagami, Y.; Yamada, D.; Noda, T.; Wada, H.; Gotoh, K.; et al. Construction of three-dimensional vascularized functional human liver tissue using a layer-by-layer cell coating technique. Biomaterials 2017, 133, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Yi, X.; Du, Z.; Xu, Y.; Sun, W. Construction of a liver sinusoid based on the laminar flow on chip and self-assembly of endothelial cells. Biofabrication 2018, 10, 25010. [Google Scholar] [CrossRef]
- Rennert, K.; Steinborn, S.; Gröger, M.; Ungerböck, B.; Jank, A.; Ehgartner, J.; Nietzsche, S.; Dinger, J.; Kiehntopf, M.; Funke, H.; et al. A microfluidically perfused three dimensional human liver model. Biomaterials 2015, 71, 119–131. [Google Scholar] [CrossRef]
- Prodanov, L.; Jindal, R.; Bale, S.S.; Hegde, M.; McCarty, W.J.; Golberg, I.; Bhushan, A.; Yarmush, M.L.; Usta, O.B. Long-term maintenance of a microfluidic 3D human liver sinusoid. Biotechnol. Bioeng. 2016, 113, 241–246. [Google Scholar] [CrossRef]
- Du, Y.; Li, N.; Yang, H.; Luo, C.; Gong, Y.; Tong, C.; Gao, Y.; Lü, S.; Long, M. Mimicking liver sinusoidal structures and functions using a 3D-configured microfluidic chip. Lab Chip 2017, 17, 782–794. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Ribera, M.; Fernández-Iglesias, A.; Illa, X.; Moya, A.; Molina, V.; Maeso-Díaz, R.; Fondevila, C.; Peralta, C.; Bosch, J.; Villa, R.; et al. Resemblance of the human liver sinusoid in a fluidic device with biomedical and pharmaceutical applications. Biotechnol. Bioeng. 2018, 115, 2585–2594. [Google Scholar] [CrossRef] [Green Version]
- Moya, A.; Ortega-Ribera, M.; Guimerà, X.; Sowade, E.; Zea, M.; Illa, X.; Ramon, E.; Villa, R.; Gracia-Sancho, J.; Gabriel, G. Online oxygen monitoring using integrated inkjet-printed sensors in a liver-on-a-chip system. Lab Chip 2018, 18, 2023–2035. [Google Scholar] [CrossRef] [Green Version]
- Norona, L.M.; Nguyen, D.G.; Gerber, D.A.; Presnell, S.C.; LeCluyse, E.L. Editor’s Highlight: Modeling Compound-Induced FibrogenesisIn Vitro Using Three-Dimensional Bioprinted Human Liver Tissues. Toxicol. Sci. 2016, 154, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.G.; Funk, J.; Robbins, J.B.; Crogan-Grundy, C.; Presnell, S.C.; Singer, T.; Roth, A.B. Bioprinted 3D Primary Liver Tissues Allow Assessment of Organ-Level Response to Clinical Drug Induced Toxicity In Vitro. PLoS ONE 2016, 11, e158674. [Google Scholar] [CrossRef] [PubMed]
- Itai, S.; Tajima, H.; Onoe, H. Double-layer perfusable collagen microtube device for heterogeneous cell culture. Biofabrication 2018, 11, 15010. [Google Scholar] [CrossRef] [PubMed]
- Bhise, N.S.; Manoharan, V.; Massa, S.; Tamayol, A.; Ghaderi, M.; Miscuglio, M.; Lang, Q.; Shrike Zhang, Y.; Shin, S.R.; Calzone, G.; et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 2016, 8, 14101. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Yoshida, T.; Okabe, M.; Goto, M.; Mir, T.A.; Soko, C.; Tsukamoto, Y.; Akaike, T.; Nikaido, T.; Zhou, K.; et al. Fabrication of 3D-culture platform with sandwich architecture for preserving liver-specific functions of hepatocytes using 3D bioprinter. J. Biomed. Mater. Res. A 2017, 105, 1583–1592. [Google Scholar] [CrossRef]
- Grix, T.; Ruppelt, A.; Thomas, A.; Amler, A.; Noichl, B.; Lauster, R.; Kloke, L. Bioprinting Perfusion-Enabled Liver Equivalents for Advanced Organ-on-a-Chip Applications. Genes Basel 2018, 9, 176. [Google Scholar] [CrossRef]
- Roth, A.D.; Lama, P.; Dunn, S.; Hong, S.; Lee, M. Polymer coating on a micropillar chip for robust attachment of PuraMatrix peptide hydrogel for 3D hepatic cell culture. Mater. Sci. Eng. C 2018, 90, 634–644. [Google Scholar] [CrossRef]
- Khetani, S.R.; Bhatia, S.N. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 2008, 26, 120–126. [Google Scholar] [CrossRef]
- Messner, S.; Agarkova, I.; Moritz, W.; Kelm, J.M. Multi-cell type human liver microtissues for hepatotoxicity testing. Arch. Toxicol. 2013, 87, 209–213. [Google Scholar] [CrossRef]
- Massa, S.; Sakr, M.A.; Seo, J.; Bandaru, P.; Arneri, A.; Bersini, S.; Zare-Eelanjegh, E.; Jalilian, E.; Cha, B.; Antona, S.; et al. Bioprinted 3D vascularized tissue model for drug toxicity analysis. Biomicrofluidics 2017, 11, 44109. [Google Scholar] [CrossRef] [PubMed]
- Ware, B.R.; Khetani, S.R. Engineered Liver Platforms for Different Phases of Drug Development. Trends Biotechnol. 2016, 35, 172–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, J.; Qu, Y.; Liu, T.; Jing, B.; Zhang, X.; Chen, Z.; Luo, Y.; Zhao, W.; Lu, Y.; Lin, B. Recent organ-on-a-chip advances toward drug toxicity testing. Microphysiol. Syst. 2018, 2, 4798. [Google Scholar] [CrossRef]
- Yu, F.; Deng, R.; Hao Tong, W.; Huan, L.; Chan Way, N.; IslamBadhan, A.; Iliescu, C.; Yu, H. A perfusion incubator liver chip for 3D cell culture with application on chronic hepatotoxicity testing. Sci. Rep. UK 2017, 7, 14528. [Google Scholar] [CrossRef] [PubMed]
- Prot, J.; Bunescu, A.; Elena-Herrmann, B.; Aninat, C.; Snouber, L.C.; Griscom, L.; Razan, F.; Bois, F.Y.; Legallais, C.; Brochot, C.; et al. Predictive toxicology using systemic biology and liver microfluidic “on chip” approaches: Application to acetaminophen injury. Toxicol. Appl. Pharm. 2012, 259, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Ha, S.K.; Choi, I.; Sung, J.H. 3D gut-liver chip with a PK model for prediction of first-pass metabolism. Biomed. Microdevices 2017, 19, 100. [Google Scholar] [CrossRef] [PubMed]
- Bovard, D.; Sandoz, A.; Luettich, K.; Frentzel, S.; Iskandar, A.; Marescotti, D.; Trivedi, K.; Guedj, E.; Dutertre, Q.; Peitsch, M.C.; et al. A lung/liver-on-a-chip platform for acute and chronic toxicity studies. Lab Chip 2018, 18, 3814–3829. [Google Scholar] [CrossRef] [Green Version]
- Midwoud, P.M.V. An Alternative Approach Based on Microfluidics to Study Drug Metabolism and Toxicity Using Liver and Intestinal Tissue. Ph.D. Thesis, Faculty of Mathematics and Natural Sciences, University of Groningen, Groningen, The Netherlands, 2010. [Google Scholar]
- Choe, A.; Ha, S.K.; Choi, I.; Choi, N.; Sung, J.H. Microfluidic Gut-liver chip for reproducing the first pass metabolism. Biomed. Microdevices 2017, 19, 4. [Google Scholar] [CrossRef]
- Zhou, Q.; Patel, D.; Kwa, T.; Haque, A.; Matharu, Z.; Stybayeva, G.; Gao, Y.; Diehl, A.M.; Revzin, A. Liver injury-on-a-chip: microfluidic co-cultures with integrated biosensors for monitoring liver cell signaling during injury. Lab Chip 2015, 15, 4467–4478. [Google Scholar] [CrossRef] [Green Version]
- Maschmeyer, I.; Hasenberg, T.; Jaenicke, A.; Lindner, M.; Lorenz, A.K.; Zech, J.; Garbe, L.; Sonntag, F.; Hayden, P.; Ayehunie, S.; et al. Chip-based human liver-intestine and liver-skin co-cultures - A first step toward systemic repeated dose substance testing in vitro. Eur. J. Pharm. Biopharm. 2015, 95, 77–87. [Google Scholar] [CrossRef]
- Deng, J.; Chen, Z.; Zhang, X.; Luo, Y.; Wu, Z.; Lu, Y.; Liu, T.; Zhao, W.; Lin, B. A liver-chip-based alcoholic liver disease model featuring multi-non-parenchymal cells. Biomed. Microdevices 2019, 21, 57. [Google Scholar] [CrossRef] [PubMed]
- Gori, M.; Simonelli, M.C.; Giannitelli, S.M.; Businaro, L.; Trombetta, M.; Rainer, A. Investigating Nonalcoholic Fatty Liver Disease in a Liver-on-a-Chip Microfluidic Device. PLoS ONE 2016, 11, e159729. [Google Scholar] [CrossRef] [PubMed]
- Kostrzewski, T.; Cornforth, T.; Snow, S.A.; Ouro-Gnao, L.; Rowe, C.; Large, E.M.; Hughes, D.J. Three-dimensional perfused human in vitro model of non-alcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 204. [Google Scholar] [CrossRef] [PubMed]
- Vernetti, L.A.; Senutovitch, N.; Boltz, R.; DeBiasio, R.; Ying Shun, T.; Gough, A.; Taylor, D.L. A human liver microphysiology platform for investigating physiology, drug safety, and disease models. Exp. Biol. Med. 2015, 241, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Deng, P.; Chen, W.; Guo, Y.; Tao, T.; Qin, J. In situ differentiation and generation of functional liver organoids from human iPSCs in a 3D perfusable chip system. Lab Chip 2018, 18, 3606–3616. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Sung, J.H. Gut-liver on a chip toward an in vitro model of hepatic steatosis. Biotechnol. Bioeng. 2018, 115, 2817–2827. [Google Scholar] [CrossRef]
- Esch, M.B.; Mahler, G.J.; Stokor, T.; Shuler, M.L. Body-on-a-chip simulation with gastrointestinal tract and liver tissues suggests that ingested nanoparticles have the potential to cause liver injury. Lab Chip 2014, 14, 3081–3092. [Google Scholar] [CrossRef]
- Lee, H.; Kim, D.S.; Ha, S.K.; Choi, I.; Lee, J.M.; Sung, J.H. A pumpless multi-organ-on-a-chip (MOC) combined with a pharmacokinetic-pharmacodynamic (PK-PD) model. Biotechnol. Bioeng. 2017, 114, 432–443. [Google Scholar] [CrossRef]
- Theobald, J.; Ghanem, A.; Wallisch, P.; Banaeiyan, A.A.; Andrade-Navarro, M.A.; Taskova, K.; Haltmeier, M.; Kurtz, A.; Becker, H.; Reuter, S.; et al. Liver-Kidney-on-Chip to Study Toxicity of Drug Metabolites. ACS Biomater. Sci. Eng. 2018, 4, 78–89. [Google Scholar] [CrossRef]
- Wagner, I.; Materne, E.; Brincker, S.; S Bier, U.; Fr Drich, C.; Busek, M.; Sonntag, F.; Sakharov, D.A.; Trushkin, E.V.; Tonevitsky, A.G.; et al. A dynamic multi-organ-chip for long-term cultivation and substance testing proven by 3D human liver and skin tissue co-culture. Lab Chip 2013, 13, 3538. [Google Scholar] [CrossRef]
- Maschmeyer, I.; Lorenz, A.K.; Schimek, K.; Hasenberg, T.; Ramme, A.P.; Hubner, J.; Lindner, M.; Drewell, C.; Bauer, S.; Thomas, A.; et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip 2015, 15, 2688–2699. [Google Scholar] [CrossRef] [PubMed] [Green Version]


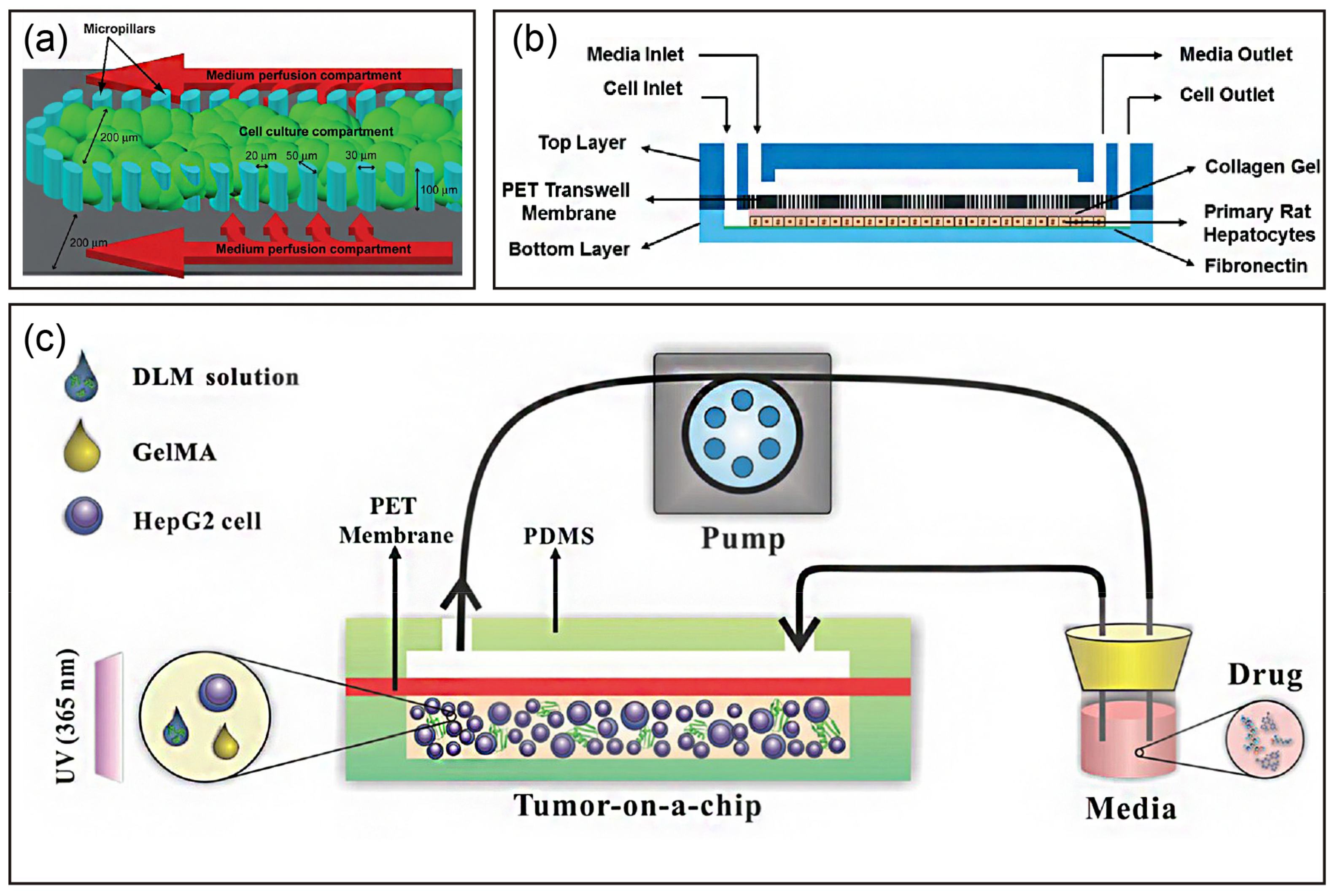
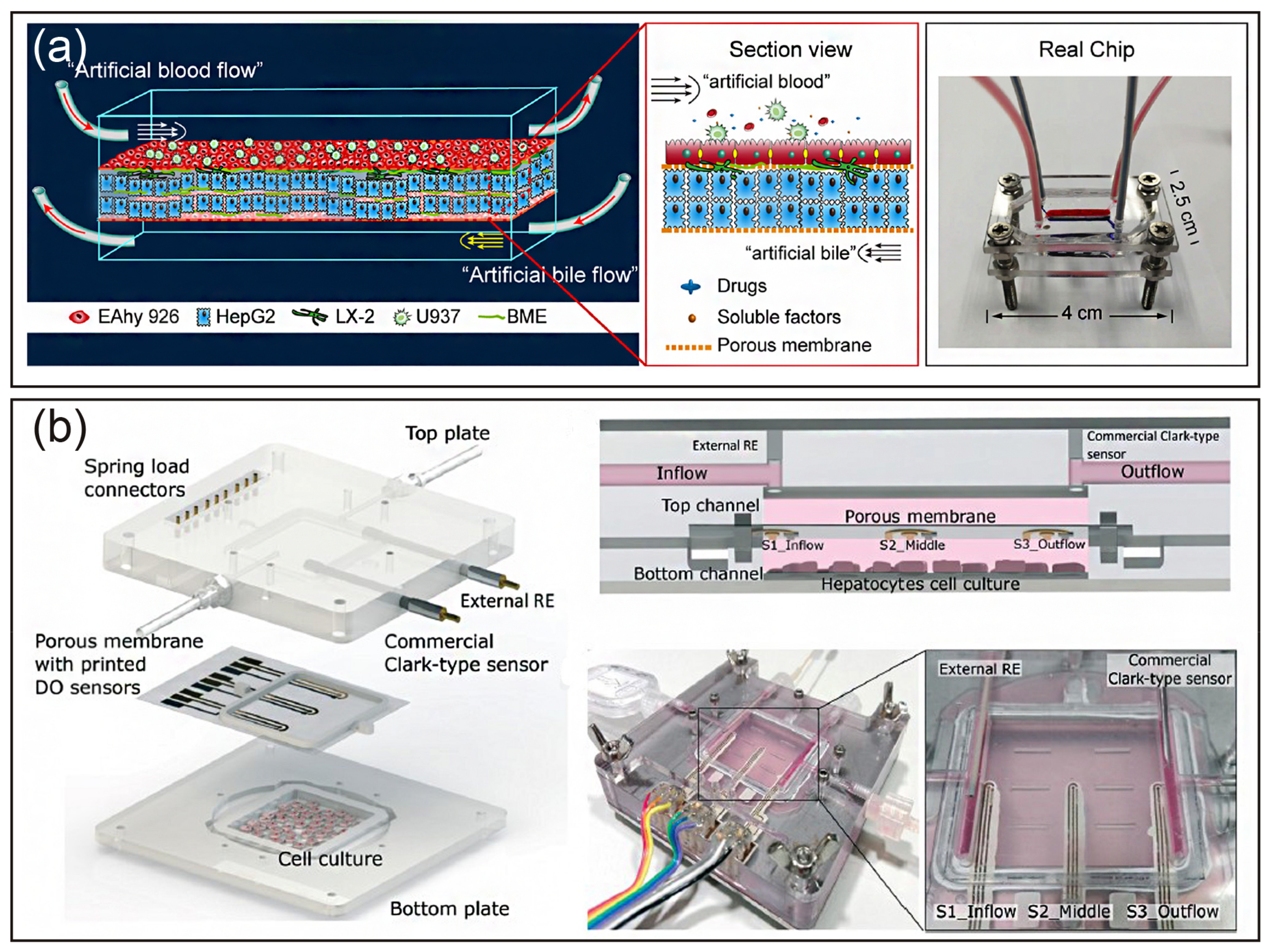
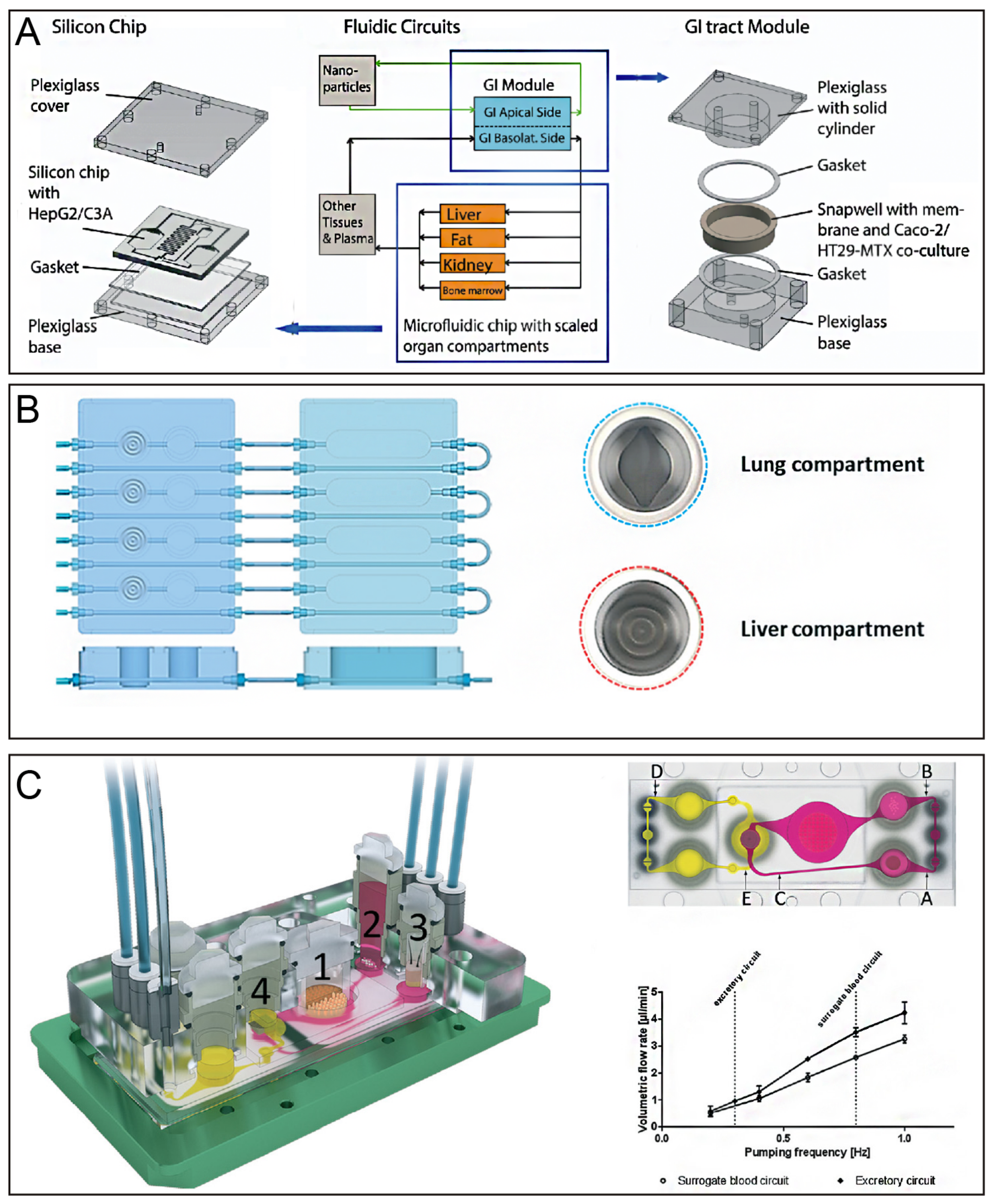
| Cell | Type | Diameter (μm) | Proportion (number) | Features |
|---|---|---|---|---|
| Parenchymal | - | - | - | - |
| hepatocytes | Epithelial | 20–30 | 60%–65% | Large in size, abundant glycogen, mostly double nuclei. |
| Non-parenchymal | - | - | - | - |
| Kupffer cells | Macrophages | 10–13 | ~15% | Irregularly shaped, mobile cells, secretion of mediators. |
| liver sinusoid endothelial cells | Epithelial | 6.5–11 | 16% | SE-1, CD31, fenestrations, none basement membrane. |
| hepatic stellate cells | Fibroblastic | 10.7–11.5 | 8% | Vitamin-storing, |
| Biliary Epithelial Cells | Epithelial | ~10 | Little | Distinct basement membrane. Containing unique proteoglycans, adhesion glycoproteins. |
| In Vitro Approaches | References | Advantages | Limitations |
|---|---|---|---|
| Monolayer | [8,9] | Easily manipulated, low-cost, good repeatability. | Cannot recapitulate in-vivo like cellular morphology and 3D microenvironment, loss of cell polarity, poor function. |
| Co-culture | [44,45,46] | Multi-cellular environment, cell-cell interaction, improve functions and longevity, cellular polarity. | Difficult isolation of NPCs, variations of NPCs, differentiation status and viability are varied depending on culture conditions. |
| 3D culture | [11,47,48,49,50] | Recapitulation of 3D microenvironment and ECM properties, improve gene and protein expression, improve functions and longevity, cellular polarity. | Complicated methods of culture. Necrotic regions within 3D cellular models caused by oxygen diffusion. |
| Spheroids | [41,46,51] | In vivo-like microenvironment, cellular interaction, maintain liver-specific functionality over long term culture, enhanced CYP 450 and transporter expression, formation of secondary structure (e.g., bile canalicular-like structure). | Spheroid size limitation (~200 μm) and variations, necrotic cores, Oxygen and nutrient diffusion through cellular aggregates. |
| Liver-on-a-chip | [35,52,53,54,55,56,57,58] | Dynamic microenvironment, suitable for co-culture, 3D culture, and spheroid, improve liver-specific, functionality, enhanced CYP 450 and transporter expression formation of secondary structure, pattern cells spatially, high through put and low cost. | Complicated methods of operate chip and culture cell in the chip, required perfusion systems, non-specific binding of drugs to chip materials, may wash away molecules in the chamber under perfusion, no standard yet. |
| Cell Type | Advantages | Limitations |
|---|---|---|
| Primary hepatocytes (human, rat) | Liver intrinsic characteristics, including phase I and II metabolic enzyme activity, glucose metabolism, ammonia detoxification | Losing liver specific function; unsuitable for long-term; high cost; donor variation, difficult isolation |
| Hepatic-derived cell lines (HepG2, HepaRG, C3A) | Unlimited lifespan; easily manipulated; stable phenotype; essential for drug metabolism and toxicity response. | Drug reaction are inaccurate; low metabolic competence and rapid loss of expression of liver-specific enzymes/transporters. |
| Stem cell induces hepatocytes | A stable source of hepatocytes; liver organoid; stable functions including albumin secretion, liver-specific gene expression, urea production and metabolic activity. | Hardly manipulated; required specific induce factor; high cost; insufficient maturate. |
| Strategies | References | Characteristics | Culture Period | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Liver chip based on 2D planar culture | [65] | Pattern or capture hepatocytes in 2D form; co-culture with non-parenchymal cells. | Short term | Relatively easy and fast; suitable for high throughput screening. | No polarization; low cell-cell communication; depended on the nature of substrate. |
| Liver chip based on matrixless 3D spheroid culture | [33,41,51,59,60,61] | Hepatocytes form spheroid spontaneously, due to gravity or modification of material surface; also suitable for co-culture. | Medium to long term | Scaffold-free; easy to achieve mass production of uniform size; good part form for stem cell differentiation | Needs special technology, such as cell-repellent plate and hanging drop technique. |
| Liver chip based on matrix-dependent 3D culture | [54,62,70,71,72,73,74,75] | Encapsulate cells within a three-dimensional (3D) matrix, such as hydrogel, BME and collagen, which replicates the supportive functions of the extracellular matrix. | Long term | Provide support and fixation for cells; enhanced cell-cell and cell-matrix interaction; conducive to cell adhesion and regulate dynamic cue of cells | Dependent on matrix, such as stability, stiffness; batch-to-batch variability; potential immunogenicity and presence of biological contaminants; unpredictable effects on signaling pathways. |
| Liver chip based on layer-by-layer deposition | [33,44,45,76,80,85] | Pattern hepatocytes and nonparenchymal cells lay by lay by porous membrane or 3D printing technology, etc. | Long term | Easy to control the position of cell layers to mimic the distribution of liver cells; forming tightly connected endotheliocytes for perfusion; hepatocyte polarization and angiogenesis | Not suitable for organs with unclear cell stratification; depends on other auxiliary tool, such as membrane and bio-ink. |
| Liver chip based on 3D bioprinting | [10,11,84,86,87,88] | Cells and extracellular matrix are laid out according to a preset path through a 3D printer in the form of additive manufacturing. | Long term | Easy to construct complex 3D biological microscale structures with various cell types and biomaterials; time save and high throughput | Limited by printing accuracy, it is difficult to control individual cells; the properties of printed materials are not optimized enough. |
| Liver chip-based cell microarrays such as microwell systems | [74,89,90] | Seed cells in an array of well plates. | Medium to long term | High throughput; miniaturize and parallelize. | Lack of spatial distribution and cellular interactions of cells in vivo. |
| Liver chip-based hanging drops | [68,91] | Form 3D micro-tissues of cells (one type or multi-types) by hanging cells in drop. | Medium term | Controllable and reproducible spheroid formation; no need to use scaffold; each drop served as a culture compartment for a single microtissue that was suitable for high throughput screening. | Not suitable for long-term culture for chronic toxicity and chronic liver disease. |
| Application | Reference | Cells Used | Description | Experimental Specifications |
|---|---|---|---|---|
| Drug screening and toxicity testing | [95] | Primary rat hepatocytes | A perfusion-incubator-liver-chip (PIC) was designed for 3D rat hepatocyte spheroids culture; chronic drug response to repeated dosing of Diclofenac and Acetaminophen were evaluated in PIC. | PIC system structure, functionality and optimization; Maintenance of cell function in PIC; application of PIC-cultured hepatocytes in drug safety testing. |
| Prediction of metabolism | [100] | Caco-2; HepG2 | A microfluidic chip consists of two separate layers for Caco-2 and HepG2 was designed; first pass metabolism of a flavonoid, apigenin was evaluated as a model compound. | Gut-liver chip design for cells proliferation and differentiation; Paracellular permeability of intestinal barrier; first pass metabolism of apigenin. |
| Establishment of liver disease models | [52] | HepDE19; cryopreserved PHH; HepG2 | A 3D microfluidic PHH system permissive to HBV infection; This system enables the recapitulation of all steps of the HBV life cycle, replication of patient-derived HBV and the maintenance of HBV cccDNA. | HBV patient-derived viruses and infections; exogenous stimulation of KC suppresses HBV replication. |
| Fabrication of multi-organ on a chip | [102] | HepaRG; human primary hepatic stellate cells; prepuce | A system for the co-culture of human 3D liver spheroids with human gut barrier and skin toward systemic repeated dose substance testing. | Fourteen-day performance of liver-intestinal co-cultures; 14-day performance of liver-skin co-cultures; repeated dose substance exposure. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.; Wei, W.; Chen, Z.; Lin, B.; Zhao, W.; Luo, Y.; Zhang, X. Engineered Liver-On-A-Chip Platform to Mimic Liver Functions and Its Biomedical Applications: A Review. Micromachines 2019, 10, 676. https://doi.org/10.3390/mi10100676
Deng J, Wei W, Chen Z, Lin B, Zhao W, Luo Y, Zhang X. Engineered Liver-On-A-Chip Platform to Mimic Liver Functions and Its Biomedical Applications: A Review. Micromachines. 2019; 10(10):676. https://doi.org/10.3390/mi10100676
Chicago/Turabian StyleDeng, Jiu, Wenbo Wei, Zongzheng Chen, Bingcheng Lin, Weijie Zhao, Yong Luo, and Xiuli Zhang. 2019. "Engineered Liver-On-A-Chip Platform to Mimic Liver Functions and Its Biomedical Applications: A Review" Micromachines 10, no. 10: 676. https://doi.org/10.3390/mi10100676
APA StyleDeng, J., Wei, W., Chen, Z., Lin, B., Zhao, W., Luo, Y., & Zhang, X. (2019). Engineered Liver-On-A-Chip Platform to Mimic Liver Functions and Its Biomedical Applications: A Review. Micromachines, 10(10), 676. https://doi.org/10.3390/mi10100676





