A Silicon-based Coral-like Nanostructured Microfluidics to Isolate Rare Cells in Human Circulation: Validation by SK-BR-3 Cancer Cell Line and Its Utility in Circulating Fetal Nucleated Red Blood Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
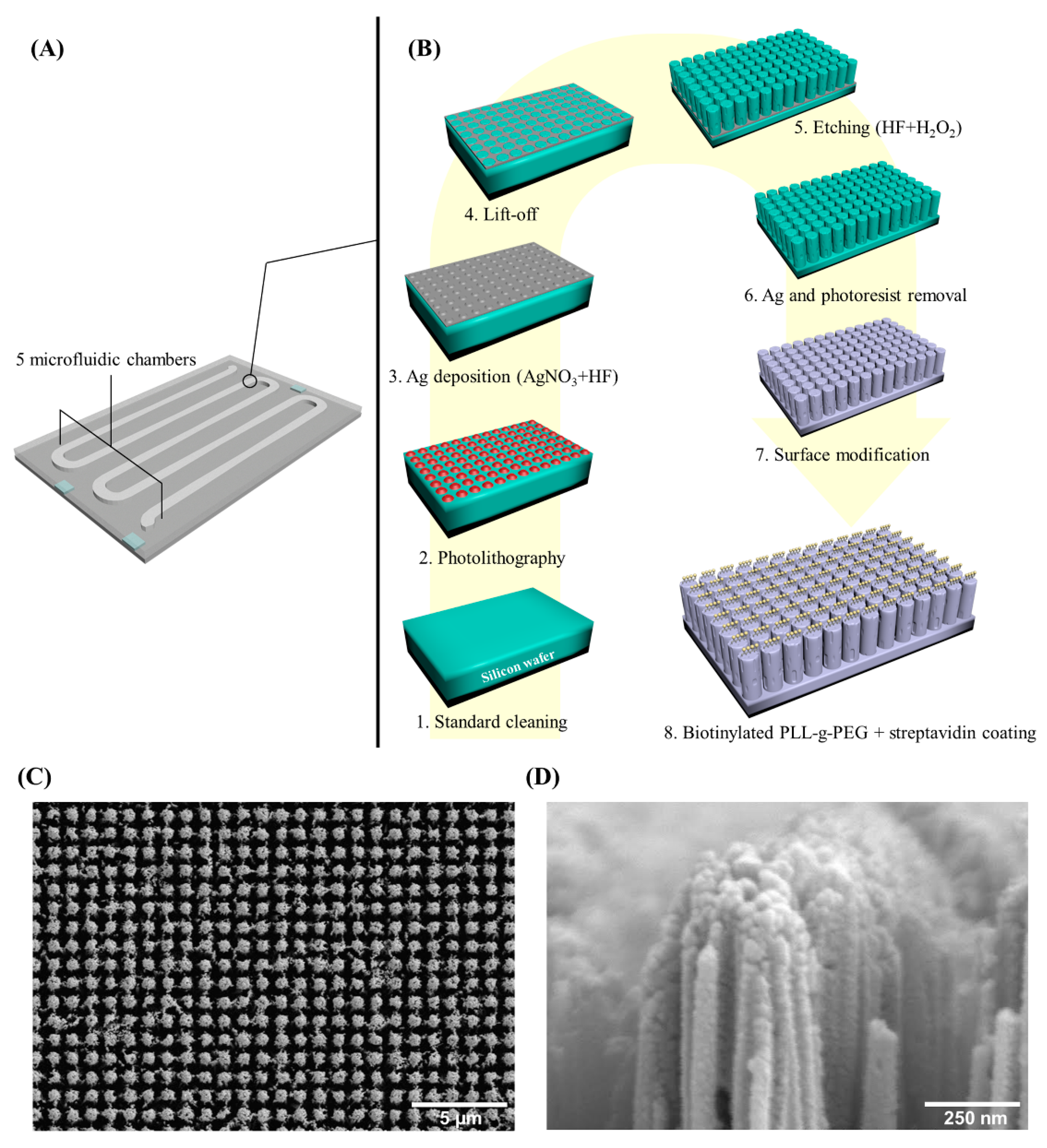
2.2. Coral Chip Manufacture
2.3. Cell Spiking Test
2.4. Fetal Nucleated Red Blood Cells (fnRBCs) Capture
2.5. Fluorescence in Situ Hybridization (FISH)
2.6. Captured Cells Recovery
2.7. Whole Genome Amplification (WGA)
2.8. Array Comparative Genomic Hybridization (aCGH)
2.9. Next Generation Sequencing (NGS)
2.10. Short Tandem Repeat (STR) Analysis
3. Results
3.1. Capture Efficiency Estimated by Cell Spiking Test
3.2. Circulating fnRBC Captured by Coral Chip
3.3. FISH
3.4. Captured Cells Recovery
3.5. WGA
3.6. aCGH and NGS Analysis
3.7. STR Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zipursky, A.; Hull, A.; White, F.D.; Israels, L.G. Foetal erythrocytes in the maternal circulation. Lancet 1959, 1, 451–452. [Google Scholar] [CrossRef]
- Norwitz, E.R.; Levy, B. Noninvasive prenatal testing: The future is now. Rev. Obstet. Gynecol. 2013, 6, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Beaudet, A.L. Using fetal cells for prenatal diagnosis: History and recent progress. Am. J. Med. Genet. C Semin. Med. Genet. 2016, 172, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.J.; Ma, G.C.; Lee, M.H.; Chen, Y.C.; Chen, M. Ultrasonography for prognosis in case of trisomy 14 confined placental mosaicism developing after preimplantation genetic screening. Ultrasound Obstet. Gynecol. 2017, 50, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.J.; Ma, G.C.; Lin, Y.S.; Yeang, C.H.; Ni, Y.H.; Li, W.C.; Tsai, H.D.; Shur-Fen Gau, S.; Chen, M. Detection of 22q11.2 microduplication by cell-free DNA screening and chromosomal microarray in fetus with multiple anomalies. Ultrasound Obstet. Gynecol. 2016, 48, 530–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.H.; Ma, G.C.; Tsai, C.C.; Wu, W.J.; Lan, K.C.; Hsu, T.Y.; Yang, C.W.; Chen, M. Confined placental mosaicism of double trisomies 9 and 21: Discrepancy between noninvasive prenatal testing.; chorionic villus sampling and postnatal confirmation. Ultrasound Obstet. Gynecol. 2016, 48, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Gregg, A.R.; Skotko, B.G.; Benkendorf, J.L.; Monaghan, K.G.; Bajaj, K.; Best, R.G.; Klugman, S.; Watson, M.S. Noninvasive prenatal screening for fetal aneuploidy.; 2016 update: A position statement of the American College of Medical Genetics and Genomics. Genet. Med. 2016, 18, 1056–1065. [Google Scholar] [CrossRef]
- Sinkey, R.G.; Odibo, A.O. Cost-effectiveness of old and new technology for aneuploidy screening. Clin. Lab Med. 2016, 36, 237–248. [Google Scholar] [CrossRef]
- Chitty, L.S.; Lo, Y.M. Noninvasive Prenatal Screening for Genetic Diseases Using Massively Parallel Sequencing of Maternal Plasma DNA. Cold Spring Harb Perspect. Med. 2015, 5, a023085. [Google Scholar] [CrossRef] [Green Version]
- Norton, M.E.; Jacobsson, B.; Swamy, G.K.; Laurent, L.C.; Ranzini, A.C.; Brar, H.; Tomlinson, M.W.; Pereira, L.; Spitz, J.L.; Hollemon, D.; et al. Cell-free DNA analysis for noninvasive examination of trisomy. N. Engl. J. Med. 2015, 372, 1589–1597. [Google Scholar] [CrossRef]
- Wapner, R.J.; Babiarz, J.E.; Levy, B.; Stosic, M.; Zimmermann, B.; Sigurjonsson, S.; Wayham, N.; Ryan, A.; Banjevic, M.; Lacroute, P.; et al. Expanding the scope of noninvasive prenatal testing: detection of fetal microdeletion syndromes. Am. J. Obstet. Gynecol. 2015, 212, 332.e1-9. [Google Scholar] [CrossRef]
- Yeang, C.H.; Ma, G.C.; Hsu, H.W.; Lin, Y.S.; Chang, S.M.; Cheng, P.J.; Chen, C.A.; Ni, Y.H.; Chen, M. Genome-wide normalized score: a novel algorithm to detect fetal trisomy 21 during noninvasive prenatal testing. Ultrasound Obstet. Gynecol. 2014, 44, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Sayres, L.C.; Cho, M.K.; Cook-Deegan, R.; Chandrasekharan, S. Commercial landscape of noninvasive prenatal testing in the United States. Prenat. Diagn. 2013, 33, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Grati, F.R.; Malvestiti, F.; Ferreira, J.C.; Bajaj, K.; Gaetani, E.; Agrati, C.; Grimi, B.; Dulcetti, F.; Ruggeri, A.M.; De Toffol, S.; et al. Fetoplacental mosaicism: potential implications for false-positive and false-negative noninvasive prenatal screening results. Genet. Med. 2014, 16, 620–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meck, J.M.; Dugan, E.K.; Matyakhina, L.; Aviram, A.; Trunca, C.; Pineda-Alvarez, D.; Aradhya, S.; Klein, R.T.; Cherry, A.M. Noninvasive prenatal screening for aneuploidy: positive predictive values based on cytogenetic findings. Am. J. Obstet. Gynecol. 2015, 213, 214.e1-5. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Chen, J.F.; Song, M.; Zhu, Y.; Jan, Y.J.; Chen, S.H.; Weng, T.H.; Ling, D.A.; Chen, S.F.; Ro, T.; et al. Imprinted nanoVelcro microchips for isolation and characterization of circulating fetal trophoblasts: toward noninvasive prenatal diagnostics. ACS Nano 2017, 11, 8167–8177. [Google Scholar] [CrossRef] [PubMed]
- Breman, A.M.; Chow, J.C.; U'Ren, L.; Normand, E.A.; Qdaisat, S.; Zhao, L.; Henke, D.M.; Chen, R.; Shaw, C.A.; Jackson, L.; et al. Evidence for feasibility of fetal trophoblastic cell-based noninvasive prenatal testing. Prenat. Diagn. 2016, 36, 1009–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kølvraa, S.; Singh, R.; Normand, E.A.; Qdaisat, S.; van den Veyver, I.B.; Jackson, L.; Hatt, L.; Schelde, P.; Uldbjerg, N.; Vestergaard, E.M.; et al. Genome-wide copy number analysis on DNA from fetal cells isolated from the blood of pregnant women. Prenat. Diagn. 2016, 36, 1127–1134. [Google Scholar] [CrossRef]
- Huang, C.E.; Ma, G.C.; Jou, H.J.; Lin, W.H.; Lee, D.J.; Lin, Y.S.; Ginsberg, N.A.; Chen, H.F.; Chang, F.M.; Chen, M. Noninvasive prenatal diagnosis of fetal aneuploidy by circulating fetal nucleated red blood cellsand extravillous trophoblasts using silicon-based nanostructured microfluidics. Mol. Cytogenet. 2017, 10, 44. [Google Scholar] [CrossRef]
- Calabrese, G.; Fantasia, D.; Alfonsi, M.; Morizio, E.; Celentano, C.; Guanciali Franchi, P.; Sabbatinelli, G.; Palka, C.; Benn, P.; Sitar, G. Aneuploidy screening using circulating fetal cells in maternal blood by dual-probe FISH protocol: a prospective feasibility study on a series of 172 pregnant women. Mol. Genet. Genomic Med. 2016, 4, 634–640. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.; He, Z.; Cai, B.; Peng, J.; Song, J.; Yu, X.; Sun, Y.; Yuan, J.; Zhao, X.; Zhang, Y. Noninvasive Prenatal Diagnosis of Chromosomal Aneuploidies and Microdeletion Syndrome Using Fetal Nucleated Red Blood Cells Isolated by Nanostructure Microchips. Theranostics 2018, 8, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Ao, Z.; Cheng, L.; He, Z.; Huang, Q.; Cai, B.; Rao, L.; Meng, Q.; Wang, Z.; Sun, Y.; et al. Highly sensitive and rapid isolation of fetal nucleated red blood cells with microbead-based selective sedimentation for noninvasive prenatal diagnostics. Nanotechnology 2018, 29, 43401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, Y.; Li, X.; Shi, Y.; Hu, B.; An, Y.; Zhu, Z.; Hong, G.; Yang, C.J. Frequency-enhanced transferrin receptor antibody-labelled microfluidic chip (FETAL-Chip) enables efficient enrichment of circulating nucleated red blood cells for noninvasive prenatal diagnosis. Lab Chip 2018, 18, 2749–2756. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Ki, C.S.; Han, K.H. Isolation of nucleated red blood cells in maternal blood for Noninvasive prenatal diagnosis. Biomed Microdevices 2015, 17, 118. [Google Scholar] [CrossRef] [PubMed]
- Kanda, E.; Yura, H.; Kitagawa, M. Practicability of prenatal testing using lectin-based enrichment of fetal erythroblasts. J. Obstet. Gynecol. Res. 2016, 42, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Hatt, L.; Brinch, M.; Singh, R.; Møller, K.; Lauridsen, R.H.; Schlütter, J.M.; Uldbjerg, N.; Christensen, B.; Kølvraa, S. A new marker set that identifies fetal cells in maternal circulation with high specificity. Prenat. Diagn. 2014, 34, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.; Hollmann, C.; Stachelhaus, S.A. Unique monoclonal antibodies specifically bind surface structures on human fetal erythroid blood cells. Exp. Cell Res. 2013, 319, 2700–2707. [Google Scholar] [CrossRef] [Green Version]
- Kruckow, S.; Schelde, P.; Hatt, L.; Ravn, K.; Petersen, O.B.; Uldbjerg, N.; Vogel, I.; Singh, R. Does maternal body mass index affect the quantity of circulating fetal cells available to use for cell-based noninvasive prenatal test in high-risk pregnancies? Fetal Diagn. Ther. 2018, 1–4. [Google Scholar] [CrossRef]
- Schlütter, J.M.; Kirkegaard, I.; Petersen, O.B.; Larsen, N.; Christensen, B.; Hougaard, D.M.; Kølvraa, S.; Uldbjerg, N. Fetal gender and several cytokines are associated with the number of fetal cells in maternal blood--an observational study. PLoS ONE 2014, 9, e106934. [Google Scholar] [CrossRef]
- Jan, Y.J.; Chen, J.F.; Zhu, Y.; Lu, Y.T.; Chen, S.H.; Chung, H.; Smalley, M.; Huang, Y.W.; Dong, J.; Chen, L.C.; et al. NanoVelcro rare-cell assays for detection and characterization of circulating tumor cells. Adv. Drug Deliv. Rev. 2018, 125, 78–93. [Google Scholar] [CrossRef]
- Laget, S.; Broncy, L.; Hormigos, K.; Dhingra, D.M.; BenMohamed, F.; Capiod, T.; Osteras, M.; Farinelli, L.; Jackson, S.; Paterlini-Br échot, P. Technical Insights into Highly Sensitive Isolation and Molecular Characterization of Fixed and Live Circulating Tumor Cells for Early Detection of Tumor Invasion. PLoS ONE 2017, 12, e0169427. [Google Scholar] [CrossRef] [PubMed]
- Schreier, S.; Sawaison, P.; Udomsanpetch, R.; Triampo, W. Advances in rare cell isolation: an optimization and evaluation study. J. Transl. Med. 2017, 15, 6. [Google Scholar] [CrossRef]
- Ferreira, M.M.; Ramani, V.C.; Jeffrey, S.S. Circulating tumor cell technologies. Mol. Oncol. 2016, 10, 374–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, A.; Schoendube, J.; Zimmermann, S.; Steeb, M.; Zengerle, R.; Koltay, P. Technologies for single-cell isolation. Int. J. Mol. Sci. 2015, 16, 16897–16919. [Google Scholar] [CrossRef] [PubMed]
- Sahmani, M.; Vatanmakanian, M.; Goudarzi, M.; Mobarra, N.; Azad, M. Microchips and their significance in isolation of circulating tumor cells and monitoring of cancers. Asian Pac. J. Cancer. Prev. 2016, 17, 879–894. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.M.; Fehm, T.; Orsini, M.; Cayrefourcq, L.; Maudelonde, T.; Pantel, K.; Alix-Panabières, C. Prognostic relevance of viable circulating tumor cells detected by EPISPOT in metastatic breast cancer patients. Clin. Chem. 2014, 60, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Seppo, A.; Frisova, V.; Ichetovkin, I.; Kim, Y.; Evans, M.I.; Antsaklis, A.; Nicolaides, K.H.; Tafas, T.; Tsipouras, P.; Kilpatrick, M.W. Detection of circulating fetal cells utilizing automated microscopy: potential for noninvasiveprenatal diagnosis of chromosomal aneuploidies. Prenat. Diagn. 2008, 28, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Al-Mufti, R.; Hambley, H.; Farzaneh, F.; Nicolaides, K.H. Fetal erythroblasts in maternal blood in relation to gestational age. J. Matern. Fetal Neonatal. Med. 2003, 14, 392–397. [Google Scholar] [CrossRef]
- Bianchi, D.W.; Zickwolf, G.K.; Yih, M.C.; Flint, A.F.; Geifman, O.H.; Erikson, M.S.; Williams, J.M. Erythroid-specific antibodies enhance detection of fetal nucleated erythrocytes in maternal blood. Prenat. Diagn. 1993, 13, 293–300. [Google Scholar] [CrossRef]
- Ziegler, B.L.; Müller, R.; Valtieri, M.; Lamping, C.P.; Thomas, C.A.; Gabbianelli, M.; Giesert, C.; Bühring, H.J.; Kanz, L.; Peschle, C. Unicellular-unilineage erythropoietic cultures: Molecular analysis of regulatory gene expression at sibling cell level. Blood 1999, 93, 3355–3368. [Google Scholar]
- Sørensen, M.D.; Gonzalez Dosal, R.; Jensen, K.B.; Christensen, B.; Kølvraa, S.; Jensen, U.B.; Kristensen, P. Epsilon haemoglobin specific antibodies with applications in noninvasive prenatal diagnosis. J. Biomed. Biotechnol. 2009, 2009, 659219. [Google Scholar] [CrossRef] [PubMed]
- Babayan, A.; Alawi, M.; Gormley, M.; Müller, V.; Wikman, H.; Mcmullin, R.P.; Smirnov, D.A.; Li, W.; Geffken, M.; Pantel, K.; Joosse, S.A. Comparative study of whole genome amplification and next generation sequencing performanceof single cancer cells. Oncotarget 2016, 8, 56066–56080. [Google Scholar] [CrossRef]
- Ma, G.C.; Wu, W.J.; Lee, M.H.; Lin, Y.S.; Chen, M. The use of low molecular weight heparin reduced the fetal fraction and rendered the cell-free DNA testing for fetal trisomy 21 false negative. Ultrasound Obstet. Gynecol. 2018, 51, 276–277. [Google Scholar] [CrossRef] [PubMed]
- Trola, L.; Al-Kouatly, H.B.; McKurdy, R.; Konchak, P.S.; Weiner, S.; Berghella, V. The recurrent risk of fetomaternal hemorrhage. Fetal Diagn. Ther. 2019, 45, 1–12. [Google Scholar] [CrossRef]






| Sample No. | No. of Captured SK-BR-3 cells | No. of Falsely Captured Jurkat Cells | Capture Rate for SK-BR-3 Cells (%) | False Capture Rate for Jurkat Cells (%) |
|---|---|---|---|---|
| 1 | 4012 | 7 | 80.24 | 0.0007 |
| 2 | 4241 | 0 | 84.82 | 0 |
| 3 | 4683 | 0 | 93.06 | 0 |
| 4 | 4728 | 1 | 94.56 | 0.0001 |
| Mean | 4405 | 2 | 88.17 | 0.0002 |
| Case No. | MA (Year) | GA (Week+day) | Pre-acquired Fetal Genetic Condition | cbNIPD | ||
|---|---|---|---|---|---|---|
| No. of fnRBCs Captured (in 2 mL Maternal Blood) | Non-maternal Genomic Markers Used to Confirm the Fetal Origin of Captured Cells | Validated * Method | ||||
| 1 | 30 | 27+5 | arr[GRCh37] 9p24.2p23 (2267812_13374304) × 1 dn | 10 | 1. 9p24.2p23 deletion 2. Chr Y | aCGH [pooled 8] |
| 2 | 38 | 20+6 | arr[GRCh37] 10q25.2q26.12 (114393625_121720948) × 1 dn | 47 | 1. 10q25.2q26.12 deletion 2. Chr Y | aCGH [pooled 13] |
| 3 | 31 | 25 | arr[GRCh37] 21q22.11q22.3 (35703384_48056450) × 1 dn | 47 | 1. 21q22.11q22.3 deletion 2. Chr Y | aCGH [pooled 15] |
| 4 | 40 | 18 | arr[GRCh37] 22q11.21 (18894835_21505417) × 1 dn | 18 | 22q11.21 deletion | aCGH [pooled 10] |
| 5 | 28 | 15+6 | 48,XXY,+18 | 7 | T18 | FISH [4] |
| 6 | 37 | 13+4 | 47,XY,+18 | 25 | T18 | FISH [10] |
| 7 | 29 | 16 | 47,XY,+18 | 3 | T18 | FISH [3] |
| 8 | 34 | 20+6 | 47,XY,+21 | 14 | T21 | FISH [6] |
| 9 | 43 | 25+6 | 46,XY | 3 | Chr Y | FISH [3] |
| 10 | 32 | 19 | 46,XY | 2 | Chr Y | FISH [2] |
| 11 | 29 | 24+6 | 46,XY | 10 | Chr Y | FISH [6] |
| 12 | 37 | 15 | 46,XY | 10 | Chr Y | FISH [4] |
| 13 | 28 | 24 | 46,XY | 71 | Chr Y | FISH [22] |
| 14 | 42 | 24 | 46,XY | 6 | Chr Y | STR analysis [pooled 5] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, G.-C.; Lin, W.-H.; Huang, C.-E.; Chang, T.-Y.; Liu, J.-Y.; Yang, Y.-J.; Lee, M.-H.; Wu, W.-J.; Chang, Y.-S.; Chen, M. A Silicon-based Coral-like Nanostructured Microfluidics to Isolate Rare Cells in Human Circulation: Validation by SK-BR-3 Cancer Cell Line and Its Utility in Circulating Fetal Nucleated Red Blood Cells. Micromachines 2019, 10, 132. https://doi.org/10.3390/mi10020132
Ma G-C, Lin W-H, Huang C-E, Chang T-Y, Liu J-Y, Yang Y-J, Lee M-H, Wu W-J, Chang Y-S, Chen M. A Silicon-based Coral-like Nanostructured Microfluidics to Isolate Rare Cells in Human Circulation: Validation by SK-BR-3 Cancer Cell Line and Its Utility in Circulating Fetal Nucleated Red Blood Cells. Micromachines. 2019; 10(2):132. https://doi.org/10.3390/mi10020132
Chicago/Turabian StyleMa, Gwo-Chin, Wen-Hsiang Lin, Chung-Er Huang, Ting-Yu Chang, Jia-Yun Liu, Ya-Jun Yang, Mei-Hui Lee, Wan-Ju Wu, Yun-Shiang Chang, and Ming Chen. 2019. "A Silicon-based Coral-like Nanostructured Microfluidics to Isolate Rare Cells in Human Circulation: Validation by SK-BR-3 Cancer Cell Line and Its Utility in Circulating Fetal Nucleated Red Blood Cells" Micromachines 10, no. 2: 132. https://doi.org/10.3390/mi10020132
APA StyleMa, G.-C., Lin, W.-H., Huang, C.-E., Chang, T.-Y., Liu, J.-Y., Yang, Y.-J., Lee, M.-H., Wu, W.-J., Chang, Y.-S., & Chen, M. (2019). A Silicon-based Coral-like Nanostructured Microfluidics to Isolate Rare Cells in Human Circulation: Validation by SK-BR-3 Cancer Cell Line and Its Utility in Circulating Fetal Nucleated Red Blood Cells. Micromachines, 10(2), 132. https://doi.org/10.3390/mi10020132






