Porous Alginate Scaffolds Assembled Using Vaterite CaCO3 Crystals
Abstract
:1. Introduction
2. Ca2+-Alginate Gel Based Scaffolds
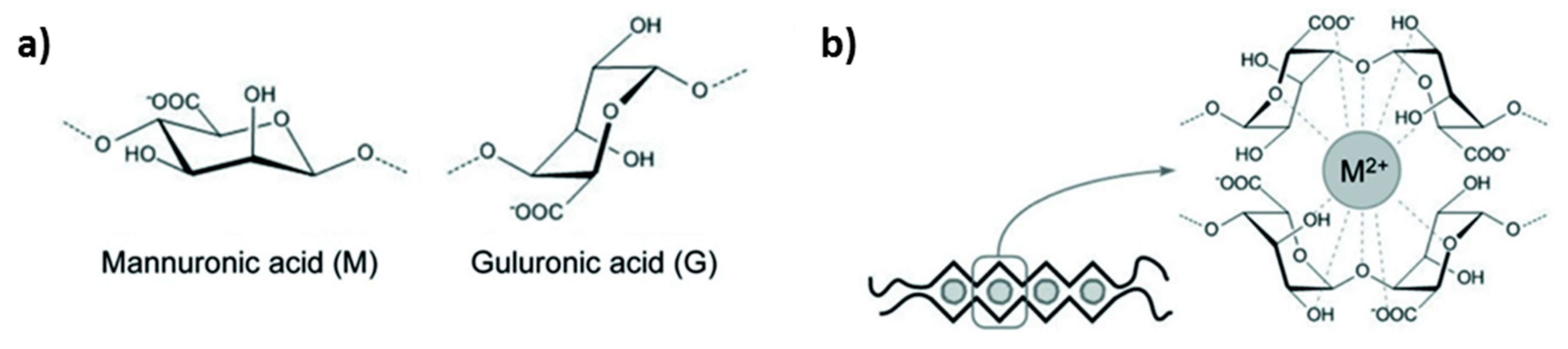
2.1. Chemistry of Alginate
2.2. Alginate Hydrogels: Formation and Structure
2.3. Alginate Gels as Drug Carriers: Encapsulation and Release
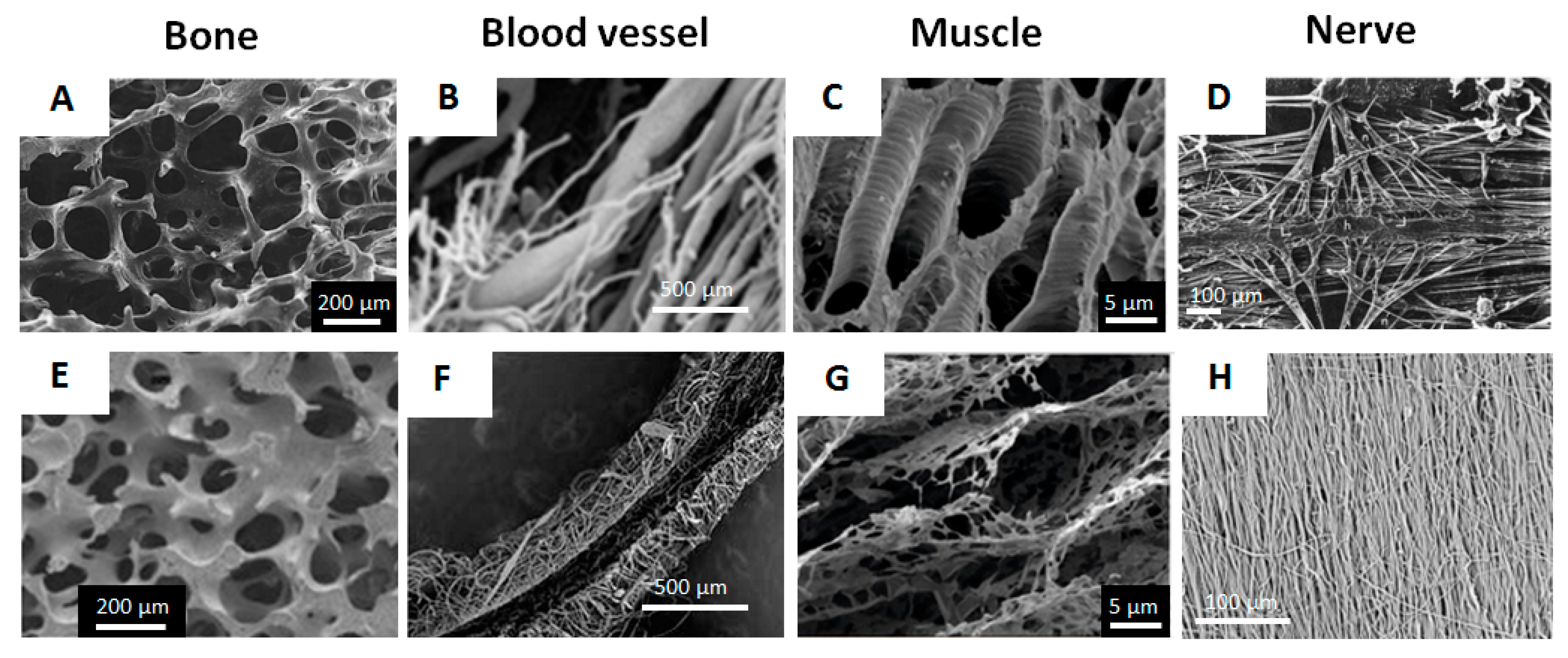
2.4. Alginate Gels for the Design of Porous Scaffolds
3. CaCO3 Vaterite Crystals: Loading and Release Opportunities
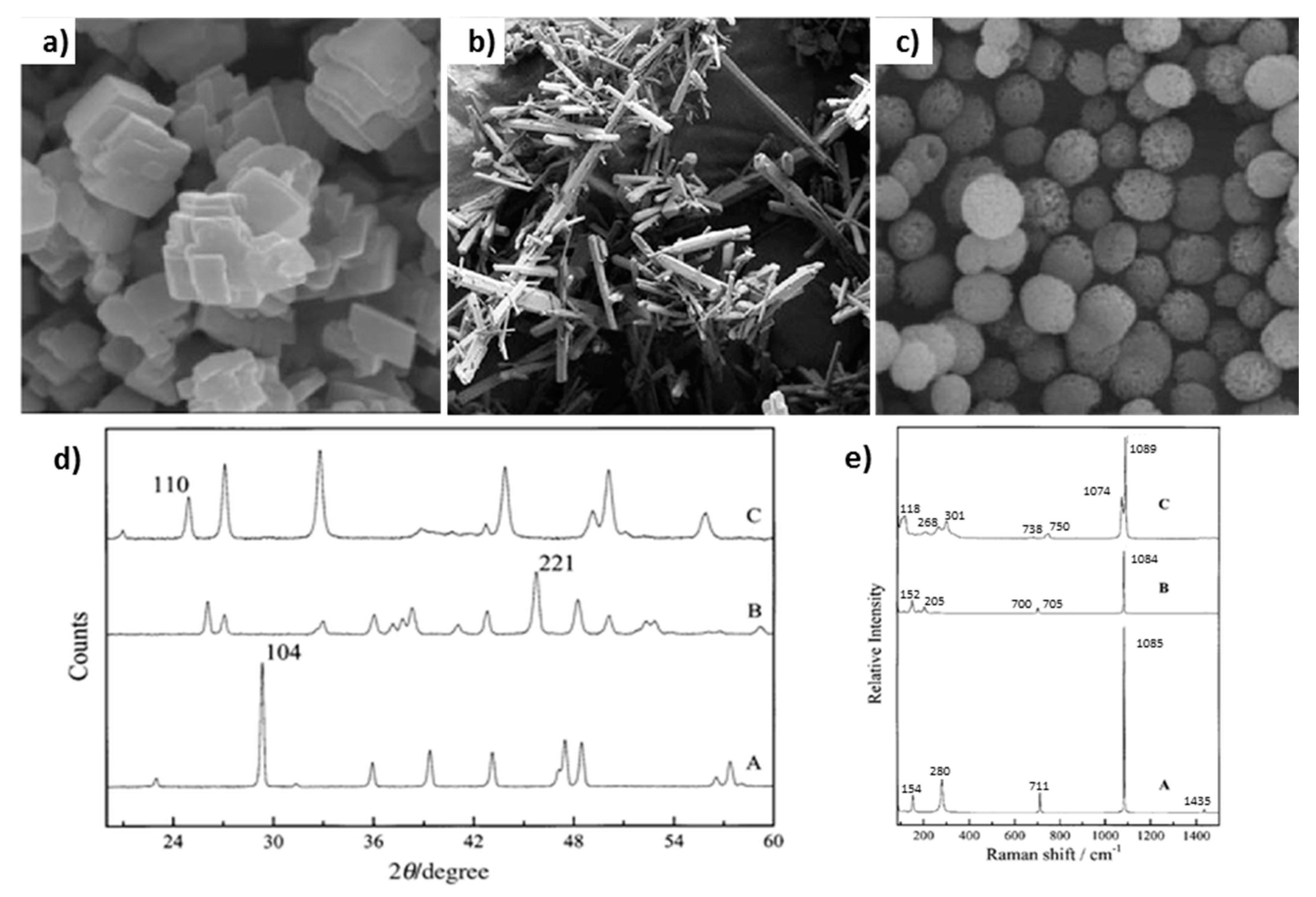
3.1. Morphology of Vaterite Caco3 Crystals
3.2. Vaterite CaCO3 as Decomposable Templates for Microencapsulation
3.3. Release from Vaterite Caco3 Crystal: Dissolution and Recrystallization
4. Vaterite CaCO3-Assistant Porous Alginate Scaffolds (PAS)
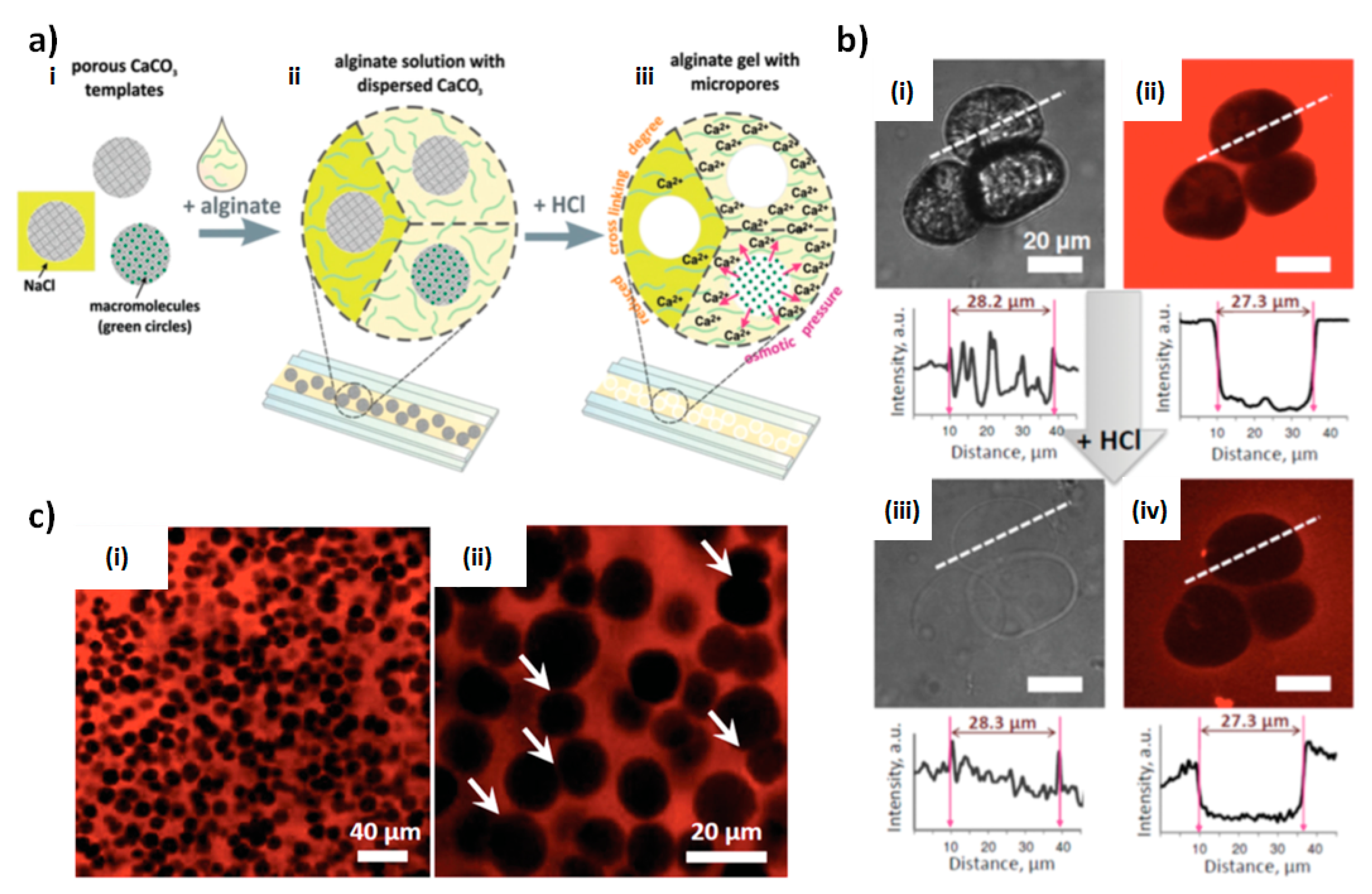
4.1. Fabrication Strategy
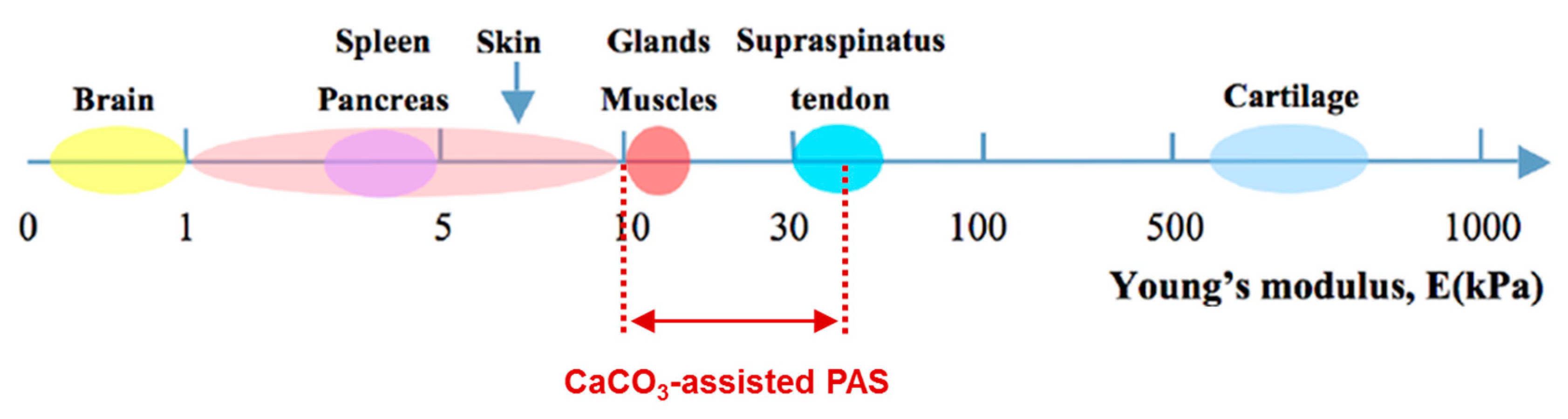
4.2. PAS Porosity and Mechanical Properties
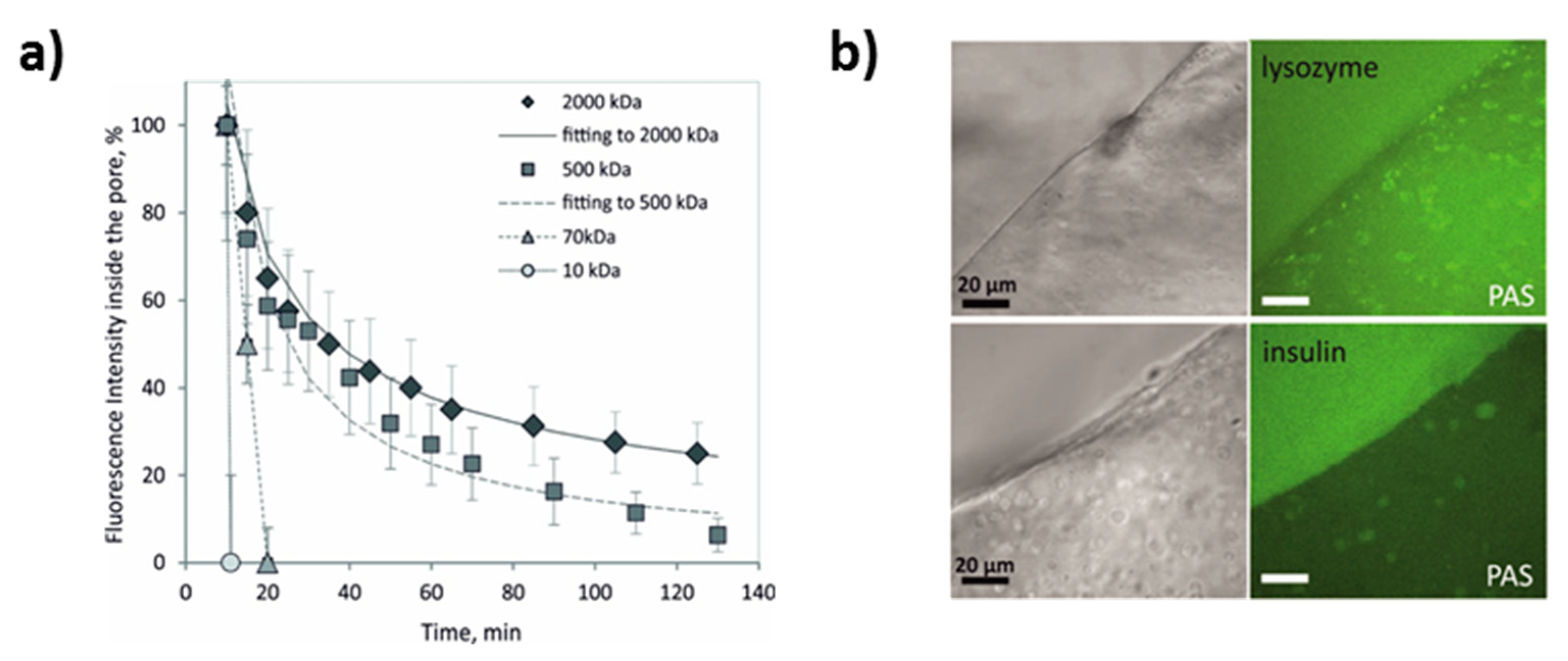
4.3. PAS as Reservoirs for Encapsulation and Controlled Release
5. Summary and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R. Future Prospects for Scaffolding Methods and Biomaterials in Skin Tissue Engineering: A Review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Raeisdasteh Hokmabad, V.; Davaran, S.; Ramazani, A.; Salehi, R. Design and fabrication of porous biodegradable scaffolds: A strategy for tissue engineering. J. Biomater. Sci. Polym. Ed. 2017, 28, 1797–1825. [Google Scholar] [CrossRef] [PubMed]
- Van Bochove, B.; Grijpma, D.W. Photo-crosslinked synthetic biodegradable polymer networks for biomedical applications. J. Biomater. Sci. Polym. Ed. 2019, 30, 77–106. [Google Scholar] [CrossRef] [Green Version]
- Chappard, D.; Baslé, M.F.; Legrand, E.; Audran, M. New laboratory tools in the assessment of bone quality. Osteoporos. Int. 2011, 22, 2225–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santamaría, V.A.; Deplaine, H.; Mariggió, D.; Villanueva-Molines, A.R.; García-Aznar, J.M.; Ribelles, J.G.; Doblaré, M.; Ferrer, G.G.; Ochoa, I. Influence of the macro and micro-porous structure on the mechanical behavior of poly(l-lactic acid) scaffolds. J. Non-Cryst. Solids 2012, 358, 3141–3149. [Google Scholar] [CrossRef]
- Walocha, J.A.; Miodoński, A.J.; Szczepański, W.; Skrzat, J.; Stachura, J. Two types of vascularisation of intramural uterine leiomyomata revealed by corrosion casting and immunohistochemical study. Folia Morphol. 2004, 63, 37–41. [Google Scholar]
- Vaz, C.M.; van Tuijl, S.; Bouten, C.V.C.; Baaijens, F.P.T. Design of scaffolds for blood vessel tissue engineering using a multi-layering electrospinning technique. Acta Biomater. 2005, 1, 575–582. [Google Scholar] [CrossRef]
- Ivashchenko, O.; Przysiecka, Ł.; Peplińska, B.; Jarek, M.; Coy, E.; Jurga, S. Gel with silver and ultrasmall iron oxide nanoparticles produced with Amanita muscaria extract: Physicochemical characterization, microstructure analysis and anticancer properties. Sci. Rep. 2018, 8, 13260. [Google Scholar] [CrossRef]
- Wasserthal, L.; Wasserthal, W. Innervation of heart and alary muscles in Sphinx ligustri L. (Lepidoptera). Cell Tissue Res. 1977, 184. [Google Scholar] [CrossRef]
- Baklaushev, V.P.; Bogush, V.G.; Kalsin, V.A.; Sovetnikov, N.N.; Samoilova, E.M.; Revkova, V.A.; Sidoruk, K.V.; Konoplyannikov, M.A.; Timashev, P.S.; Kotova, S.L.; et al. Tissue Engineered Neural Constructs Composed of Neural Precursor Cells, Recombinant Spidroin and PRP for Neural Tissue Regeneration. Sci. Rep. 2019, 9, 3161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozawa, F.; Ino, K.; Arai, T.; Ramón-Azcón, J.; Takahashi, Y.; Shiku, H.; Matsue, T. Alginate gel microwell arrays using electrodeposition for three-dimensional cell culture. Lab Chip 2013, 13, 3128–3135. [Google Scholar] [CrossRef] [PubMed]
- Zawko, S.A.; Schmidt, C.E. Simple benchtop patterning of hydrogel grids for living cell microarrays. Lab Chip 2010, 10, 379–383. [Google Scholar] [CrossRef]
- Sharma, C.; Dinda, A.K.; Mishra, N.C. Fabrication and characterization of natural origin chitosan-gelatin-alginate composite scaffold by foaming method without using surfactant. J. Appl. Polym. Sci. 2013, 127, 3228–3241. [Google Scholar] [CrossRef]
- Despang, F.; Börner, A.; Dittrich, R.; Tomandl, G.; Pompe, W.; Gelinsky, M. Alginate/calcium phosphate scaffolds with oriented, tube-like pores. Mater. Und Werkst. 2005, 36, 761–767. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Gao, Q.; Liu, X.; Tong, Z. Alginate–calcium carbonate porous microparticle hybrid hydrogels with versatile drug loading capabilities and variable mechanical strengths. Carbohydr. Polym. 2008, 71, 476–480. [Google Scholar] [CrossRef]
- Roberts, J.R.; Ritter, D.W.; McShane, M.J. A Design Full of Holes: Functional Nanofilm-Coated Microdomains in Alginate Hydrogels. J. Mater. Chem. B 2013, 107, 3195–3201. [Google Scholar] [CrossRef]
- Sergeeva, A.; Feoktistova, N.; Prokopovic, V.; Gorin, D.; Volodkin, D. Design of Porous Alginate Hydrogels by Sacrificial CaCO3 Templates: Pore Formation Mechanism. Adv. Mater. Interfaces 2015, 2, 1500386. [Google Scholar] [CrossRef]
- Sergeeva, A.S.; Gorin, D.A.; Volodkin, D.V. In-situ assembly of Ca-alginate gels with controlled pore loading/release capability. Langmuir 2015, 31, 10813–10821. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles-A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Gombotz, W. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 1998, 31, 267–285. [Google Scholar] [CrossRef]
- Paques, J.P.; van der Linden, E.; van Rijn, C.J.M.; Sagis, L.M.C. Preparation methods of alginate nanoparticles. Adv. Colloid Interface Sci. 2014, 209, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Rehm, B.H.A. Alginates: Biology and Applications; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-92678-8. [Google Scholar]
- Li, L.; Fang, Y.; Vreeker, R.; Appelqvist, I.; Mendes, E. Reexamining the egg-box model in calcium-alginate gels with X-ray diffraction. Biomacromolecules 2007, 8, 464–468. [Google Scholar] [CrossRef]
- Sikorski, P.; Mo, F.; Skjåk-Braek, G.; Stokke, B.T. Evidence for egg-box-compatible interactions in calcium-alginate gels from fiber X-ray diffraction. Biomacromolecules 2007, 8, 2098–2103. [Google Scholar] [CrossRef] [PubMed]
- Lim, F.; Sun, A. Microencapsulated islets as bioartificial endocrine pancreas. Science 1980, 210, 908–910. [Google Scholar] [CrossRef]
- Higham, A.K.; Bonino, C.A.; Raghavan, S.R.; Khan, S.A. Photo-activated ionic gelation of alginate hydrogel: Real-time rheological monitoring of the two-step crosslinking mechanism. Soft Matter 2014, 10, 4990–5002. [Google Scholar] [CrossRef]
- Larobina, D.; Cipelletti, L. Hierarchical cross-linking in physical alginate gels: A rheological and dynamic light scattering investigation. Soft Matter 2013, 9, 10005. [Google Scholar] [CrossRef]
- Alexander, B.R.; Murphy, K.E.; Gallagher, J.; Farrell, G.F.; Taggart, G. Gelation time, homogeneity, and rupture testing of alginate-calcium carbonate-hydrogen peroxide gels for use as wound dressings. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.; Bouhadir, K.H.; Mansour, J.M.; Alsberg, E. Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials 2009, 30, 2724–2734. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Arha, M.; Choudhary, S.; Ashton, R.S.; Bhatia, S.R.; Schaffer, D.V.; Kane, R.S. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials 2009, 30, 4695–4699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purcell, E.K.; Singh, A.; Kipke, D.R. Alginate composition effects on a neural stem cell-seeded scaffold. Tissue Eng. Part C Methods 2009, 15, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Østberg, T.; Vesterhus, L.; Graffner, C. Calcium alginate matrices for oral multiple unit administration: II. Effect of process and formulation factors on matrix properties. Int. J. Pharm. 1993, 97, 183–193. [Google Scholar] [CrossRef]
- Østberg, T.; Graffner, C. Calcium alginate matrices for oral multiple unit administration: III. Influence of calcium concentration, amount of drug added and alginate characteristics on drug release. Int. J. Pharm. 1994, 111, 271–282. [Google Scholar] [CrossRef]
- Østberg, T.; Lund, E.M.; Graffner, C. Calcium alginate matrices for oral multiple unit administration: IV. Release characteristics in different media. Int. J. Pharm. 1994, 112, 241–248. [Google Scholar] [CrossRef]
- Dodoo, S.; Steitz, R.; Laschewsky, A.; von Klitzing, R. Effect of ionic strength and type of ions on the structure of water swollen polyelectrolyte multilayers. Phys. Chem. Chem. Phys. 2011, 13, 10318–10325. [Google Scholar] [CrossRef] [Green Version]
- Burmistrova, A.; Richter, M.; Eisele, M.; Üzüm, C.; von Klitzing, R. The Effect of Co-Monomer Content on the Swelling/Shrinking and Mechanical Behaviour of Individually Adsorbed PNIPAM Microgel Particles. Polymers 2011, 3, 1575–1590. [Google Scholar] [CrossRef] [Green Version]
- Karg, M.; Pastoriza-Santos, I.; Rodriguez-González, B.; von Klitzing, R.; Wellert, S.; Hellweg, T. Temperature, pH, and ionic strength induced changes of the swelling behavior of PNIPAM-poly(allylacetic acid) copolymer microgels. Langmuir 2008, 24, 6300–6306. [Google Scholar] [CrossRef]
- Micciulla, S.; Dodoo, S.; Chevigny, C.; Laschewsky, A.; von Klitzing, R. Short versus long chain polyelectrolyte multilayers: A direct comparison of self-assembly and structural properties. Phys. Chem. Chem. Phys. 2014, 16, 21988–21998. [Google Scholar] [CrossRef] [PubMed]
- Volodkin, D.; von Klitzing, R. Competing mechanisms in polyelectrolyte multilayer formation and swelling: Polycation–polyanion pairing vs. polyelectrolyte–ion pairing. Curr. Opin. Colloid Interface Sci. 2014, 19, 25–31. [Google Scholar] [CrossRef]
- Schelero, N.; Hedicke, G.; Linse, P.; Klitzing, R.V. Effects of counterions and co-ions on foam films stabilized by anionic dodecyl sulfate. J. Phys. Chem. B 2010, 114, 15523–15529. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Md Akil, H. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Grulova, I.; Slovinska, L.; Blaško, J.; Devaux, S.; Wisztorski, M.; Salzet, M.; Fournier, I.; Kryukov, O.; Cohen, S.; Cizkova, D. Delivery of Alginate Scaffold Releasing Two Trophic Factors for Spinal Cord Injury Repair. Sci. Rep. 2015, 5, 13702. [Google Scholar] [CrossRef]
- Hashimoto, T.; Suzuki, Y.; Tanihara, M.; Kakimaru, Y.; Suzuki, K. Development of alginate wound dressings linked with hybrid peptides derived from laminin and elastin. Biomaterials 2004, 25, 1407–1414. [Google Scholar] [CrossRef]
- Saha, S.; Chhatbar, M.U.; Mahato, P.; Praveen, L.; Siddhanta, A.K.; Das, A. Rhodamine-alginate conjugate as self indicating gel beads for efficient detection and scavenging of Hg2+ and Cr3+ in aqueous media. Chem. Commun. 2012, 48, 1659–1661. [Google Scholar] [CrossRef]
- Mierisch, C.M.; Cohen, S.B.; Jordan, L.C.; Robertson, P.G.; Balian, G.; Diduch, D.R. Transforming growth factor-β in calcium alginate beads for the treatment of articular cartilage defects in the rabbit. Arthrosc. J. Arthrosc. Relat. Surg. 2002, 18, 892–900. [Google Scholar] [CrossRef]
- Mazutis, L.; Vasiliauskas, R.; Weitz, D.A. Microfluidic Production of Alginate Hydrogel Particles for Antibody Encapsulation and Release. Macromol. Biosci. 2015, 15, 1641–1646. [Google Scholar] [CrossRef]
- Bouhadir, K.H.; Alsberg, E.; Mooney, D.J. Hydrogels for combination delivery of antineoplastic agents. Biomaterials 2001, 22, 2625–2633. [Google Scholar] [CrossRef]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Liu, M.; Chen, J.; Zhang, X. Preparation and controlled degradation of oxidized sodium alginate hydrogel. Polym. Degrad. Stab. 2009, 94, 1405–1410. [Google Scholar] [CrossRef]
- Chueh, B.-H.; Zheng, Y.; Torisawa, Y.-S.; Hsiao, A.Y.; Ge, C.; Hsiong, S.; Huebsch, N.; Franceschi, R.; Mooney, D.J.; Takayama, S. Patterning alginate hydrogels using light-directed release of caged calcium in a microfluidic device. Biomed. Microdevices 2010, 12, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Esser, E.; Tessmar, J.K.V. Preparation of well-defined calcium cross-linked alginate films for the prevention of surgical adhesions. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Nunamaker, E.A.; Otto, K.J.; Kipke, D.R. Investigation of the material properties of alginate for the development of hydrogel repair of dura mater. J. Mech. Behav. Biomed. Mater. 2011, 4, 16–33. [Google Scholar] [CrossRef]
- Shi, X.-W.; Tsao, C.-Y.; Yang, X.; Liu, Y.; Dykstra, P.; Rubloff, G.W.; Ghodssi, R.; Bentley, W.E.; Payne, G.F. Electroaddressing of Cell Populations by Co-Deposition with Calcium Alginate Hydrogels. Adv. Funct. Mater. 2009, 19, 2074–2080. [Google Scholar] [CrossRef]
- Costantini, M.; Colosi, C.; Mozetic, P.; Jaroszewicz, J.; Tosato, A.; Rainer, A.; Trombetta, M.; Święszkowski, W.; Dentini, M.; Barbetta, A. Correlation between porous texture and cell seeding efficiency of gas foaming and microfluidic foaming scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 668–677. [Google Scholar] [CrossRef]
- You, F.; Wu, X.; Chen, X. 3D printing of porous alginate/gelatin hydrogel scaffolds and their mechanical property characterization. Int. J. Polym. Mater. Polym. Biomater. 2016, 66, 299–306. [Google Scholar] [CrossRef]
- Chen, H.; Xie, S.; Yang, Y.; Zhang, J.; Zhang, Z. Multiscale regeneration scaffold in vitro and in vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1218–1225. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, G. Three-dimensional electrospun polycaprolactone (PCL)/alginate hybrid composite scaffolds. Carbohydr. Polym. 2014, 114, 213–221. [Google Scholar] [CrossRef]
- Kuznetsov, K.A.; Stepanova, A.O.; Kvon, R.I.; Douglas, T.E.L.; Kuznetsov, N.A.; Chernonosova, V.S.; Zaporozhchenko, I.A.; Kharkova, M.V.; Romanova, I.V.; Karpenko, A.A.; et al. Electrospun Produced 3D Matrices for Covering of Vascular Stents: Paclitaxel Release Depending on Fiber Structure and Composition of the External Environment. Materials 2018, 11, 2176. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Ke, C.-J.; Yen, K.-C.; Hsieh, H.-C.; Sun, J.-S.; Lin, F.-H. 3D porous calcium-alginate scaffolds cell culture system improved human osteoblast cell clusters for cell therapy. Theranostics 2015, 5, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Mao, A.S.; Desai, R.M.; Wang, H.; Weitz, D.A.; Mooney, D.J. Controlled self-assembly of alginate microgels by rapidly binding molecule pairs. Lab Chip 2017, 17, 2481–2490. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Yang, K.-C.; Lin, K.-H.; Liu, H.-C.; Lin, F.-H. A highly organized three-dimensional alginate scaffold for cartilage tissue engineering prepared by microfluidic technology. Biomaterials 2011, 32, 7118–7126. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Farouz, Y.; Nesmith, A.P.; Deravi, L.F.; McCain, M.L.; Parker, K.K. Micropatterning Alginate Substrates for in vitro Cardiovascular Muscle on a Chip. Adv. Funct. Mater. 2013, 23, 3738–3746. [Google Scholar] [CrossRef] [PubMed]
- Alsberg, E.; Anderson, K.W.; Albeiruti, A.; Franceschi, R.T.; Mooney, D.J. Cell-interactive alginate hydrogels for bone tissue engineering. J. Dent. Res. 2001, 80, 2025–2029. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.W.; Yang, J.; Akaike, T.; Cho, K.Y.; Nah, J.W.; Kim, S.I.; Cho, C.S. Preparation of alginate/galactosylated chitosan scaffold for hepatocyte attachment. Biomaterials 2002, 23, 2827–2834. [Google Scholar] [CrossRef]
- Dvir-Ginzberg, M.; Gamlieli-Bonshtein, I.; Agbaria, R.; Cohen, S. Liver tissue engineering within alginate scaffolds: Effects of cell-seeding density on hepatocyte viability, morphology, and function. Tissue Eng. 2003, 9, 757–766. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1. [Google Scholar] [CrossRef]
- Shahriari, D.; Koffler, J.; Lynam, D.A.; Tuszynski, M.H.; Sakamoto, J.S. Characterizing the degradation of alginate hydrogel for use in multilumen scaffolds for spinal cord repair. J. Biomed. Mater. Res. A 2016, 104, 611–619. [Google Scholar] [CrossRef]
- Yan, H.; Huang, D.; Chen, X.; Liu, H.; Feng, Y.; Zhao, Z.; Dai, Z.; Zhang, X.; Lin, Q. A novel and homogeneous scaffold material: Preparation and evaluation of alginate/bacterial cellulose nanocrystals/collagen composite hydrogel for tissue engineering. Polym. Bull. 2018, 75, 985–1000. [Google Scholar] [CrossRef]
- Lee, S.-H.; Jo, A.R.; Choi, G.P.; Woo, C.H.; Lee, S.J.; Kim, B.-S.; You, H.-K.; Cho, Y.-S. Fabrication of 3D alginate scaffold with interconnected pores using wire-network molding technique. Tissue Eng. Regen. Med. 2013, 10, 53–59. [Google Scholar] [CrossRef]
- Johann, R.M.; Renaud, P. Microfluidic patterning of alginate hydrogels. Biointerphases 2007, 2, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Bruchet, M.; Mendelson, N.; Melman, A. Photochemical Patterning of Ionically Cross-Linked Hydrogels. Processes 2013, 1, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Barbetta, A.; Barigelli, E.; Dentini, M. Porous alginate hydrogels: Synthetic methods for tailoring the porous texture. Biomacromolecules 2009, 10, 2328–2337. [Google Scholar] [CrossRef]
- Bots, P.; Benning, L.G.; Rodriguez-Blanco, J.-D.; Roncal-Herrero, T.; Shaw, S. Mechanistic Insights into the Crystallization of Amorphous Calcium Carbonate (ACC). Cryst. Growth Des. 2012, 12, 3806–3814. [Google Scholar] [CrossRef]
- Gebauer, D.; Völkel, A.; Cölfen, H. Stable prenucleation calcium carbonate clusters. Science 2008, 322, 1819–1822. [Google Scholar] [CrossRef]
- Trushina, D.B.; Bukreeva, T.V.; Antipina, M.N. Size-Controlled Synthesis of Vaterite Calcium Carbonate by the Mixing Method: Aiming for Nanosized Particles. Cryst. Growth Des. 2016, 16, 1311–1319. [Google Scholar] [CrossRef]
- Trushina, D.B.; Sulyanov, S.N.; Bukreeva, T.V.; Kovalchuk, M.V. Size control and structure features of spherical calcium carbonate particles. Crystallogr. Rep. 2015, 60, 570–577. [Google Scholar] [CrossRef]
- Sergeeva, A.; Sergeev, R.; Lengert, E.; Zakharevich, A.; Parakhonskiy, B.; Gorin, D.; Sergeev, S.; Volodkin, D. Composite Magnetite and Protein Containing CaCO3 Crystals. External Manipulation and Vaterite → Calcite Recrystallization-Mediated Release Performance. ACS Appl. Mater. Interfaces 2015, 7, 21315–21325. [Google Scholar] [CrossRef]
- Feoktistova, N.; Rose, J.; Prokopović, V.Z.; Vikulina, A.S.; Skirtach, A.; Volodkin, D. Controlling the Vaterite CaCO3 Crystal Pores. Design of Tailor-Made Polymer Based Microcapsules by Hard Templating. Langmuir 2016, 32, 4229–4238. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Feng, L.; Zhu, W.; Sun, X.; Gao, M.; Zhao, H.; Chao, Y.; Liu, Z. CaCO3 nanoparticles as an ultra-sensitive tumor-pH-responsive nanoplatform enabling real-time drug release monitoring and cancer combination therapy. Biomaterials 2016, 110, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Keowmaneechai, E.; McClements, D.J. Influence of EDTA and Citrate on Physicochemical Properties of Whey Protein-Stabilized Oil-in-Water Emulsions Containing CaCl2. J. Agric. Food Chem. 2002, 50, 7145–7153. [Google Scholar] [CrossRef] [PubMed]
- Al Omari, M.M.H.; Rashid, I.S.; Qinna, N.A.; Jaber, A.M.; Badwan, A.A. Calcium Carbonate. Profiles Drug Subst. Excip. Relat. Methodol. 2016, 41, 31–132. [Google Scholar] [CrossRef] [PubMed]
- Kontoyannis, C.G.; Vagenas, N.V. Calcium carbonate phase analysis using XRD and FT-Raman spectroscopy. Analyst 2000, 125, 251–255. [Google Scholar] [CrossRef]
- Sergeeva, A.S.; Gorin, D.A.; Volodkin, D.V. Polyelectrolyte Microcapsule Arrays: Preparation and Biomedical Applications. Bionanosci. 2014, 4, 1–14. [Google Scholar] [CrossRef]
- Parakhonskiy, B.V.; Foss, C.; Carletti, E.; Fedel, M.; Haase, A.; Motta, A.; Migliaresi, C.; Antolini, R. Tailored intracellular delivery via a crystal phase transition in 400 nm vaterite particles. Biomater. Sci. 2013, 1, 1273. [Google Scholar] [CrossRef]
- Sukhorukov, G.B.; Volodkin, D.V.; Günther, A.M.; Petrov, A.I.; Shenoy, D.B.; Möhwald, H. Porous calcium carbonate microparticles as templates for encapsulation of bioactive compounds. J. Mater. Chem. 2004, 14, 2073–2081. [Google Scholar] [CrossRef]
- Trushina, D.B.; Bukreeva, T.V.; Kovalchuk, M.V.; Antipina, M.N. CaCO₃ vaterite microparticles for biomedical and personal care applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 644–658. [Google Scholar] [CrossRef]
- Volodkin, D.V.; Schmidt, S.; Fernandes, P.; Larionova, N.I.; Sukhorukov, G.B.; Duschl, C.; Möhwald, H.; von Klitzing, R. One-Step Formulation of Protein Microparticles with Tailored Properties: Hard Templating at Soft Conditions. Adv. Funct. Mater. 2012, 22, 1914–1922. [Google Scholar] [CrossRef]
- Parakhonskiy, B.V.; Svenskaya, Y.I.; Yashchenok, A.M.; Fattah, H.A.; Inozemtseva, O.A.; Tessarolo, F.; Antolini, R.; Gorin, D.A. Size controlled hydroxyapatite and calcium carbonate particles: Synthesis and their application as templates for SERS platform. Colloids Surf. B Biointerfaces 2014, 118, 243–248. [Google Scholar] [CrossRef]
- Kulak, A.N.; Semsarilar, M.; Kim, Y.-Y.; Ihli, J.; Fielding, L.A.; Cespedes, O.; Armes, S.P.; Meldrum, F.C. One-pot synthesis of an inorganic heterostructure: Uniform occlusion of magnetite nanoparticles within calcite single crystals. Chem. Sci. 2014, 5, 738–743. [Google Scholar] [CrossRef]
- Fakhrullin, R.F.; Bikmullin, A.G.; Nurgaliev, D.K. Magnetically responsive calcium carbonate microcrystals. ACS Appl. Mater. Interfaces 2009, 1, 1847–1851. [Google Scholar] [CrossRef] [PubMed]
- Lengert, E.; Kozlova, A.; Pavlov, A.M.; Atkin, V.; Verkhovskii, R.; Kamyshinsky, R.; Demina, P.; Vasiliev, A.L.; Venig, S.B.; Bukreeva, T.V. Novel type of hollow hydrogel microspheres with magnetite and silver nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Stetciura, I.Y.; Markin, A.V.; Ponomarev, A.N.; Yakimansky, A.V.; Demina, T.S.; Grandfils, C.; Volodkin, D.V.; Gorin, D.A. New surface-enhanced Raman scattering platforms: Composite calcium carbonate microspheres coated with astralen and silver nanoparticles. Langmuir 2013, 29, 4140–4147. [Google Scholar] [CrossRef] [PubMed]
- Shchukin, D.G.; Sukhorukov, G.B.; Price, R.R.; Lvov, Y.M. Halloysite nanotubes as biomimetic nanoreactors. Small 2005, 1, 510–513. [Google Scholar] [CrossRef]
- Peng, C.; Zhao, Q.; Gao, C. Sustained delivery of doxorubicin by porous CaCO3 and chitosan/alginate multilayers-coated CaCO3 microparticles. Colloids Surf. A Physicochem. Eng. Asp. 2010, 353, 132–139. [Google Scholar] [CrossRef]
- Svenskaya, Y.; Parakhonskiy, B.; Haase, A.; Atkin, V.; Lukyanets, E.; Gorin, D.; Antolini, R. Anticancer drug delivery system based on calcium carbonate particles loaded with a photosensitizer. Biophys. Chem. 2013, 182, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Balabushevich, N.G.; Sholina, E.A.; Mikhalchik, E.V.; Filatova, L.Y.; Vikulina, A.S.; Volodkin, D. Self-Assembled Mucin-Containing Microcarriers via Hard Templating on CaCO3 Crystals. Micromachines 2018, 9, 307. [Google Scholar] [CrossRef]
- Balabushevich, N.G.; Kovalenko, E.A.; Mikhalchik, E.V.; Filatova, L.Y.; Volodkin, D.; Vikulina, A.S. Mucin adsorption on vaterite CaCO3 microcrystals for the prediction of mucoadhesive properties. J. Colloid Interface Sci. 2019, 545, 330–339. [Google Scholar] [CrossRef]
- Volodkin, D.V.; von Klitzing, R.; Möhwald, H. Pure protein microspheres by calcium carbonate templating. Angew. Chem. Int. Ed Engl. 2010, 49, 9258–9261. [Google Scholar] [CrossRef]
- Schmidt, S.; Uhlig, K.; Duschl, C.; Volodkin, D. Stability and cell uptake of calcium carbonate templated insulin microparticles. Acta Biomater. 2014, 10, 1423–1430. [Google Scholar] [CrossRef]
- Volodkin, D.V.; Petrov, A.I.; Prevot, M.; Sukhorukov, G.B. Matrix Polyelectrolyte Microcapsules: New System for Macromolecule Encapsulation. Langmuir 2004, 20, 3398–3406. [Google Scholar] [CrossRef]
- Yashchenok, A.M.; Borisova, D.; Parakhonskiy, B.V.; Masic, A.; Pinchasik, B.; Möhwald, H.; Skirtach, A.G. Nanoplasmonic smooth silica versus porous calcium carbonate bead biosensors for detection of biomarkers. Ann. Phys. 2012, 524, 723–732. [Google Scholar] [CrossRef]
- Kamyshinsky, R.; Marchenko, I.; Parakhonskiy, B.; Yashchenok, A.; Chesnokov, Y.; Mikhutkin, A.; Gorin, D.; Vasiliev, A.; Bukreeva, T. Composite materials based on Ag nanoparticles in situ synthesized on the vaterite porous matrices. Nanotechnology 2019, 30, 35603. [Google Scholar] [CrossRef] [PubMed]
- Bukreeva, T.V.; Orlova, O.A.; Sulyanov, S.N.; Grigoriev, Y.V.; Dorovatovskiy, P.V. A new approach to modification of polyelectrolyte capsule shells by magnetite nanoparticles. Crystallogr. Rep. 2011, 56, 880–883. [Google Scholar] [CrossRef]
- Gorin, D.A.; Portnov, S.A.; Inozemtseva, O.A.; Luklinska, Z.; Yashchenok, A.M.; Pavlov, A.M.; Skirtach, A.G.; Möhwald, H.; Sukhorukov, G.B. Magnetic/gold nanoparticle functionalized biocompatible microcapsules with sensitivity to laser irradiation. Phys. Chem. Chem. Phys. 2008, 10, 6899–6905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, R.; Venkatraman, S.S.; Neu, B. Layer-by-layer polyelectrolyte-polyester hybrid microcapsules for encapsulation and delivery of hydrophobic drugs. Biomacromolecules 2013, 14, 2262–2271. [Google Scholar] [CrossRef]
- Skirtach, A.G.; Dejugnat, C.; Braun, D.; Susha, A.S.; Rogach, A.L.; Parak, W.J.; Möhwald, H.; Sukhorukov, G.B. The Role of Metal Nanoparticles in Remote Release of Encapsulated Materials. Nano Lett. 2005, 5, 1371–1377. [Google Scholar] [CrossRef]
- Volodkin, D. Colloids of pure proteins by hard templating. Colloid Polym Sci. 2014, 292, 1249–1259. [Google Scholar] [CrossRef]
- Schmidt, S.; Behra, M.; Uhlig, K.; Madaboosi, N.; Hartmann, L.; Duschl, C.; Volodkin, D. Mesoporous Protein Particles Through Colloidal CaCO3 Templates. Adv. Funct. Mater. 2013, 23, 116–123. [Google Scholar] [CrossRef]
- Feoktistova, N.; Stoychev, G.; Puretskiy, N.; Ionov, L.; Volodkin, D. Porous thermo-responsive pNIPAM microgels. Eur. Polym. J. 2015, 68, 650–656. [Google Scholar] [CrossRef]
- Behra, M.; Schmidt, S.; Hartmann, J.; Volodkin, D.V.; Hartmann, L. Synthesis of porous PEG microgels using CaCO3 microspheres as hard templates. Macromol. Rapid Commun. 2012, 33, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Behra, M.; Azzouz, N.; Schmidt, S.; Volodkin, D.V.; Mosca, S.; Chanana, M.; Seeberger, P.H.; Hartmann, L. Magnetic porous sugar-functionalized PEG microgels for efficient isolation and removal of bacteria from solution. Biomacromolecules 2013, 14, 1927–1935. [Google Scholar] [CrossRef]
- Goss, S.L.; Lemons, K.A.; Kerstetter, J.E.; Bogner, R.H. Determination of calcium salt solubility with changes in pH and PCO2, simulating varying gastrointestinal environments. J. Pharm. Pharmacol. 2007, 59, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Paulraj, T.; Feoktistova, N.; Velk, N.; Uhlig, K.; Duschl, C.; Volodkin, D. Microporous polymeric 3D scaffolds templated by the layer-by-layer self-assembly. Macromol. Rapid Commun. 2014, 35, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Vikulina, A.S.; Skirtach, A.G.; Volodkin, D. Hybrids of Polymer Multilayers, Lipids, and Nanoparticles: Mimicking the Cellular Microenvironment. Langmuir 2019. [Google Scholar] [CrossRef]
- Balabushevich, N.G.; Lopez de Guerenu, A.V.; Feoktistova, N.A.; Skirtach, A.G.; Volodkin, D. Protein-Containing Multilayer Capsules by Templating on Mesoporous CaCO3 Particles: POST- and PRE-Loading Approaches. Macromol. Biosci. 2016, 16, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Balabushevich, N.G.; Lopez de Guerenu, A.V.; Feoktistova, N.A.; Volodkin, D. Protein loading into porous CaCO3 microspheres: Adsorption equilibrium and bioactivity retention. Phys. Chem. Chem. Phys. 2015, 17, 2523–2530. [Google Scholar] [CrossRef]
- Vikulina, A.S.; Feoktistova, N.A.; Balabushevich, N.G.; Skirtach, A.G.; Volodkin, D. The mechanism of catalase loading into porous vaterite CaCO3 crystals by co-synthesis. Phys. Chem. Chem. Phys. 2018, 20, 8822–8831. [Google Scholar] [CrossRef]
- Volodkin, D.; von Klitzing, R.; Moehwald, H. Polyelectrolyte Multilayers: Towards Single Cell Studies. Polymers 2014, 6, 1502–1527. [Google Scholar] [CrossRef]
- Saveleva, M.S.; Eftekhari, K.; Abalymov, A.; Douglas, T.E.L.; Volodkin, D.; Parakhonskiy, B.V.; Skirtach, A.G. Hierarchy of Hybrid Materials—The Place of Inorganics-in-Organics in it, Their Composition and Applications. Front. Chem. 2019, 7, 1129. [Google Scholar] [CrossRef] [PubMed]
- Balabushevich, N.G.; Pechenkin, M.A.; Shibanova, E.D.; Volodkin, D.V.; Mikhalchik, E.V. Multifunctional polyelectrolyte microparticles for oral insulin delivery. Macromol. Biosci. 2013, 13, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Jeannot, L.; Bell, M.; Ashwell, R.; Volodkin, D.; Vikulina, A.S. Internal Structure of Matrix-Type Multilayer Capsules Templated on Porous Vaterite CaCO3 Crystals as Probed by Staining with a Fluorescence Dye. Micromachines 2018, 9, 547. [Google Scholar] [CrossRef] [PubMed]
- Volodkin, D.V.; Balabushevitch, N.G.; Sukhorukov, G.B.; Larionova, N.I. Model system for controlled protein release: pH-sensitive polyelectrolyte microparticles. STP Pharma Sci. 2003, 13, 163–170. [Google Scholar]
- Volodkin, D.; Skirtach, A.; Madaboosi, N.; Blacklock, J.; von Klitzing, R.; Lankenau, A.; Duschl, C.; Möhwald, H. IR-light triggered drug delivery from micron-sized polymer biocoatings. J. Control. Release 2010, 148, e70–e71. [Google Scholar] [CrossRef] [PubMed]
- Stetciura, I.Y.; Yashchenok, A.; Masic, A.; Lyubin, E.V.; Inozemtseva, O.A.; Drozdova, M.G.; Markvichova, E.A.; Khlebtsov, B.N.; Fedyanin, A.A.; Sukhorukov, G.B.; et al. Composite SERS-based satellites navigated by optical tweezers for single cell analysis. Analyst 2015, 140, 4981–4986. [Google Scholar] [CrossRef] [Green Version]
- Parakhonskiy, B.V.; Yashchenok, A.M.; Möhwald, H.; Volodkin, D.; Skirtach, A.G. Release from Polyelectrolyte Multilayer Capsules in Solution and on Polymeric Surfaces. Adv. Mater. Interfaces 2017, 4, 1600273. [Google Scholar] [CrossRef]
- Hernández-Hernández, A.; Rodríguez-Navarro, A.B.; Gómez-Morales, J.; Jiménez-Lopez, C.; Nys, Y.; García-Ruiz, J.M. Influence of Model Globular Proteins with Different Isoelectric Points on the Precipitation of Calcium Carbonate. Cryst. Growth Des. 2008, 8, 1495–1502. [Google Scholar] [CrossRef]
- Parakhonskiy, B.V.; Yashchenok, A.M.; Donatan, S.; Volodkin, D.V.; Tessarolo, F.; Antolini, R.; Möhwald, H.; Skirtach, A.G. Macromolecule loading into spherical, elliptical, star-like and cubic calcium carbonate carriers. ChemPhysChem 2014, 15, 2817–2822. [Google Scholar] [CrossRef]
- Andreassen, J.-P.; Beck, R.; Nergaard, M. Biomimetic type morphologies of calcium carbonate grown in absence of additives. Faraday Discuss. 2012, 159, 247. [Google Scholar] [CrossRef]
- Guo, X.; Liu, L.; Wang, W.; Zhang, J.; Wang, Y.; Yu, S.-H. Controlled crystallization of hierarchical and porous calcium carbonate crystals using polypeptide type block copolymer as crystal growth modifier in a mixed solution. CrystEngComm 2011, 13, 2054. [Google Scholar] [CrossRef]
- Parakhonskiy, B.V.; Haase, A.; Antolini, R. Sub-Micrometer Vaterite Containers: Synthesis, Substance Loading, and Release. Angew. Chem. Int. Ed. 2012, 51, 1195–1197. [Google Scholar] [CrossRef]
- Lopez-Heredia, M.A.; Łapa, A.; Mendes, A.C.; Balcaen, L.; Samal, S.K.; Chai, F.; van der Voort, P.; Stevens, C.V.; Parakhonskiy, B.V.; Chronakis, I.S.; et al. Bioinspired, biomimetic, double-enzymatic mineralization of hydrogels for bone regeneration with calcium carbonate. Mater. Lett. 2017, 190, 13–16. [Google Scholar] [CrossRef]
- Savelyeva, M.S.; Abalymov, A.A.; Lyubun, G.P.; Vidyasheva, I.V.; Yashchenok, A.M.; Douglas, T.E.L.; Gorin, D.A.; Parakhonskiy, B.V. Vaterite coatings on electrospun polymeric fibers for biomedical applications. J. Biomed. Mater. Res. A 2017, 105, 94–103. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Syromotina, D.S.; Shkarina, S.N.; Shkarin, R.; Cecilia, A.; Weinhardt, V.; Baumbach, T.; Saveleva, M.S.; Gorin, D.A.; Douglas, T.E.L.; et al. Effect of low-temperature plasma treatment of electrospun polycaprolactone fibrous scaffolds on calcium carbonate mineralisation. RSC Adv. 2018, 8, 39106–39114. [Google Scholar] [CrossRef] [Green Version]
- Butler, M.F.; Glaser, N.; Weaver, A.C.; Kirkland, M.; Heppenstall-Butler, M. Calcium Carbonate Crystallization in the Presence of Biopolymers. Cryst. Growth Des. 2006, 6, 781–794. [Google Scholar] [CrossRef]
- Kosanović, C.; Fermani, S.; Falini, G.; Kralj, D. Crystallization of Calcium Carbonate in Alginate and Xanthan Hydrogels. Crystals 2017, 7, 355. [Google Scholar] [CrossRef]
- Olderøy, M.Ø.; Xie, M.; Strand, B.L.; Flaten, E.M.; Sikorski, P.; Andreassen, J.-P. Growth and Nucleation of Calcium Carbonate Vaterite Crystals in Presence of Alginate. Cryst. Growth Des. 2009, 9, 5176–5183. [Google Scholar] [CrossRef]
- Xie, M.; Olderøy, M.Ø.; Andreassen, J.-P.; Selbach, S.M.; Strand, B.L.; Sikorski, P. Alginate-controlled formation of nanoscale calcium carbonate and hydroxyapatite mineral phase within hydrogel networks. Acta Biomater. 2010, 6, 3665–3675. [Google Scholar] [CrossRef]
- Douglas, T.E.L.; Sobczyk, K.; Łapa, A.; Włodarczyk, K.; Brackman, G.; Vidiasheva, I.; Reczyńska, K.; Pietryga, K.; Schaubroeck, D.; Bliznuk, V.; et al. Ca:Mg:Zn:CO3 and Ca:Mg:CO3-tri- and bi-elemental carbonate microparticles for novel injectable self-gelling hydrogel-microparticle composites for tissue regeneration. Biomed. Mater. 2017, 12, 25015. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; He, X.; Zhou, M.; Meng, F. Chemically induced alterations in the characteristics of fouling-causing bio-macromolecules—Implications for the chemical cleaning of fouled membranes. Water Res. 2017, 108, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, H.; Poh, P.S.P.; Machens, H.-G.; Schilling, A.F. Hydrogels for Engineering of Perfusable Vascular Networks. Int. J. Mol. Sci. 2015, 16, 15997–16016. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, A.S. Porous Alginate Scaffolds: Design and Loading/Release Opportunities. Ph.D. Thesis, Technical University Berlin, Berlin, Germany, 2017. [Google Scholar]
- Sergeeva, A.S.; Volkova, E.K.; Bratashov, D.N.; Shishkin, M.I.; Atkin, V.S.; Markin, A.V.; Skaptsov, A.A.; Volodkin, D.V.; Gorin, D.A. Layer-by-layer assembled highly absorbing hundred-layer films containing a phthalocyanine dye: Fabrication and photosensibilization by thermal treatment. Thin Solid Film. 2015, 583, 60–69. [Google Scholar] [CrossRef]
- Bosio, V.E.; Cacicedo, M.L.; Calvignac, B.; León, I.; Beuvier, T.; Boury, F.; Castro, G.R. Synthesis and characterization of CaCO3-biopolymer hybrid nanoporous microparticles for controlled release of doxorubicin. Colloids Surf. B Biointerfaces 2014, 123, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.; El-Kemary, M.; Leporatti, S. Optimizing CaCO3 Matrix Might Allow To Raise Their Potential Use In Biomedical Application. J. Nanosci. Curr. Res. 2018, 3, 124. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergeeva, A.; Vikulina, A.S.; Volodkin, D. Porous Alginate Scaffolds Assembled Using Vaterite CaCO3 Crystals. Micromachines 2019, 10, 357. https://doi.org/10.3390/mi10060357
Sergeeva A, Vikulina AS, Volodkin D. Porous Alginate Scaffolds Assembled Using Vaterite CaCO3 Crystals. Micromachines. 2019; 10(6):357. https://doi.org/10.3390/mi10060357
Chicago/Turabian StyleSergeeva, Alena, Anna S. Vikulina, and Dmitry Volodkin. 2019. "Porous Alginate Scaffolds Assembled Using Vaterite CaCO3 Crystals" Micromachines 10, no. 6: 357. https://doi.org/10.3390/mi10060357
APA StyleSergeeva, A., Vikulina, A. S., & Volodkin, D. (2019). Porous Alginate Scaffolds Assembled Using Vaterite CaCO3 Crystals. Micromachines, 10(6), 357. https://doi.org/10.3390/mi10060357






