High-Throughput Platform for Efficient Chemical Transfection, Virus Packaging, and Transduction
Abstract
:1. Introduction
2. Materials and Methods
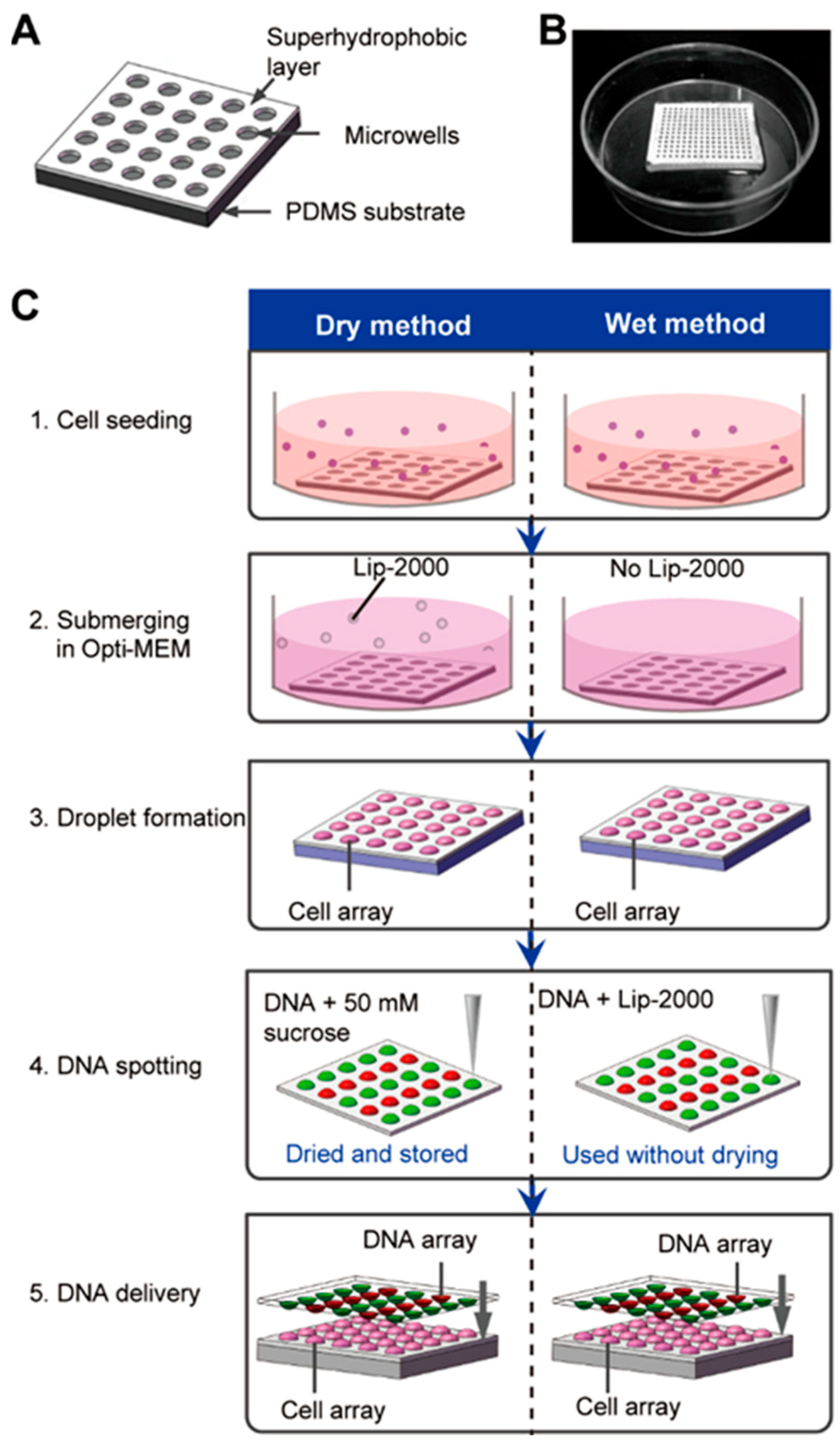
2.1. Superhydrophobic Microwell Array Chip Fabrication
2.2. Macroscale Cell Culture
2.3. On Chip Cell Culture
2.4. Macroscale Cell Transfection
2.5. On Chip Transfection
2.6. Macroscale Virus Packaging and Transduction
2.7. On Chip Virus Packaging and Transduction
2.8. Image Acquisition and Analysis
3. Results
3.1. High Throughput Chemical Transfection on the SMAR-Chip
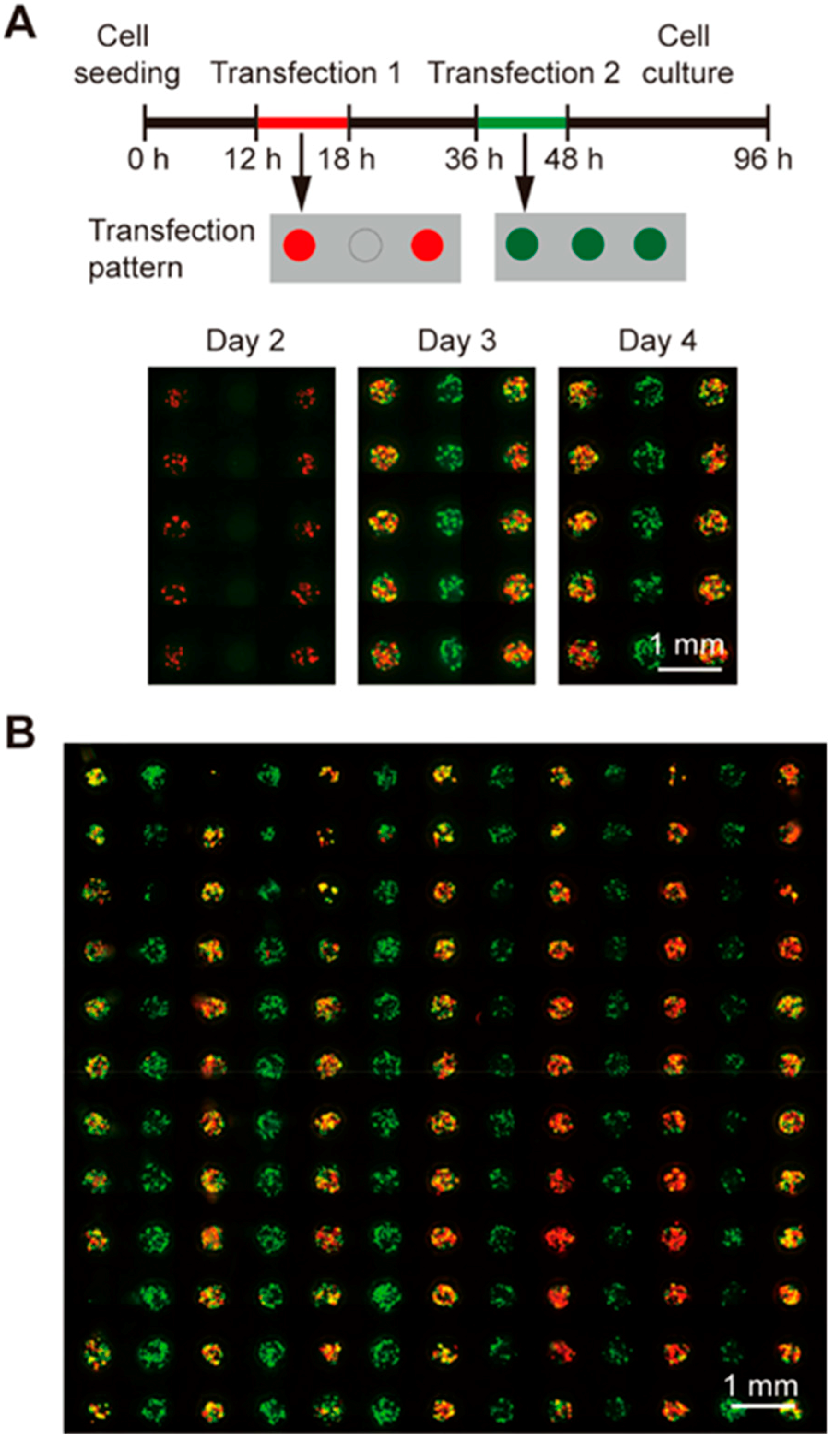
3.2. Sequential Gene Transfection
3.3. Virus Packaging and Transduction
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Sakurai, K.; Talukdar, I.; Patil, V.S.; Dang, J.; Li, Z.H.; Chang, K.Y.; Lu, C.C.; Delorme-Walker, V.; DerMardirossian, C.; Anderson, K.; et al. Kinome-wide Functional Analysis Highlights the Role of Cytoskeletal Remodeling in Somatic Cell Reprogramming. Cell Stem Cell 2014, 14, 523–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.J.; Horlbeck, M.A.; Cho, S.W.; Birk, H.S.; Malatesta, M.; He, D.; Attenello, F.J.; Villalta, J.E.; Cho, M.Y.; Chen, Y.W.; et al. CRISPRi-based genome-scale identification of functional long noncoding RNA loci in human cells. Science 2017, 355, 6320. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.A.; Tanenbaum, M.E.; Horlbeck, M.A.; Adamson, B.; Villalta, J.E.; Vale, R.D.; Kampmann, M.; Weissman, J.S. Using genome-scale CRISPR-mediated loss-of-function and gain-of-function screens to analyze gene-drug interactions in cancer. Cancer Res. 2015, 75, 22. [Google Scholar]
- Dean, D.A.; Strong, D.D.; Zimmer, W.E. Nuclear entry of nonviral vectors. Gene Ther. 2015, 12, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Kang, J.; Sopher, B.; Gillespie, J.; Aloi, M.S.; Odom, G.L.; Hopkins, S.; Case, A.; Wang, D.B.; Chamberlain, J.S.; et al. Recombinant adeno-associated viral (rAAV) vectors mediate efficient gene transduction in cultured neonatal and adult microglia. J. Neurochem. 2016, 136, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Erfle, H.; Neumann, B.; Liebel, U.; Rogers, P.; Held, M.; Walter, T.; Ellenberg, J.; Pepperkok, R. Reverse transfection on cell arrays for high content screening microscopy. Nat. Protoc. 2007, 2, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Sturzl, M.; Konrad, A.; Sander, G.; Wies, E.; Neipel, F.; Naschberger, E.; Reipschlager, S.; Gonin-Laurent, N.; Horch, R.E.; Kneser, U.; et al. High throughput screening of gene functions in mammalian cells using reversely transfected cell arrays: Review and protocol. Comb. Chem. High Throughput Scr. 2008, 11, 159–192. [Google Scholar] [CrossRef]
- Ziauddin, J.; Sabatini, D.M. Microarrays of cells expressing defined cDNAs. Nature 2001, 411, 107–110. [Google Scholar] [CrossRef]
- Gobaa, S.; Hoehnel, S.; Roccio, M.; Negro, A.; Kobel, S.; Lutolf, M.P. Artificial niche microarrays for probing single stem cell fate in high throughput. Nat. Methods 2011, 8, 949–955. [Google Scholar] [CrossRef]
- Luni, C.; Giulitti, S.; Serena, E.; Ferrari, L.; Zambon, A.; Gagliano, O.; Giobbe, G.G.; Michielin, F.; Knobel, S.; Bosio, A.; et al. High-efficiency cellular reprogramming with microfluidics. Nat. Methods 2016, 13, 446. [Google Scholar] [CrossRef]
- Bailey, S.N.; Ali, S.M.; Carpenter, A.E.; Higgins, C.O.; Sabatini, D.M. Microarrays of lentiviruses for gene function screens in immortalized and primary cells. Nat. Methods 2006, 3, 117–122. [Google Scholar] [CrossRef] [PubMed]
- McConnell, K.I.; Schweller, R.M.; Diehl, M.R.; Suh, J. Live-cell microarray surface coatings supporting reverse transduction by adeno-associated viruses. Biotechniques 2011, 51, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, K.; Maerkl, S.J. A High-Throughput Microfluidic Platform for Mammalian Cell Transfection and Culturing. Sci. Rep. 2016, 6, 23937. [Google Scholar] [CrossRef] [PubMed]
- Giupponi, E.; Visone, R.; Occhetta, P.; Colombo, F.; Rasponi, M.; Candiani, G. Development of a microfluidic platform for high-throughput screening of non-viral gene delivery vectors. Biotechnol. Bioeng. 2018, 115, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.T.; Zhou, Y.C.; Hu, Y.W.; Cheng, J.; Chen, X.F.; Xu, Y.C.; Liu, P. High-throughput in situ cell electroporation microsystem for parallel delivery of single guide RNAs into mammalian cells. Sci. Rep. 2017, 7, 42512. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Nathamgari, S.S.P.; Kessler, J.A.; Espinosa, H.D. Combined Numerical and Experimental Investigation of Localized Electroporation-Based Cell Transfection and Sampling. ACS Nano 2018, 12, 12118–12128. [Google Scholar] [CrossRef] [PubMed]
- Luni, C.; Michielin, F.; Barzon, L.; Calabro, V.; Elvassore, N. Stochastic Model-Assisted Development of Efficient Low-Dose Viral Transduction in Microfluidics. Biophys. J. 2013, 104, 934–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, P.N.; Atto, Z.; Regeenes, R.; Tufa, U.; Chen, Y.Y.; Chan, W.C.W.; Volchuk, A.; Kilkenny, D.M.; Rocheleau, J.V. Highly efficient adenoviral transduction of pancreatic islets using a microfluidic device. Lab Chip 2016, 16, 2921–2934. [Google Scholar] [CrossRef] [PubMed]
- Tran, R.; Myers, D.R.; Denning, G.; Shields, J.E.; Lytle, A.M.; Alrowais, H.; Qiu, Y.Z.; Sakurai, Y.; Li, W.C.; Brand, O.; et al. Microfluidic Transduction Harnesses Mass Transport Principles to Enhance Gene Transfer Efficiency. Mol. Ther. 2017, 25, 2372–2382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.F.; Zhang, J.X.; Bian, S.T.; Chen, Z.Y.; Hu, Y.W.; Hu, R.W.; Li, J.Q.; Cheng, Y.C.; Zhang, X.C.; Zhou, Y.M.; et al. High-throughput superhydrophobic microwell arrays for investigating multifactorial stem cell niches. Lab Chip 2016, 16, 2996–3006. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zeng, X.; Liu, M.; Deng, Y.; He, N.Y. Current Progress in Gene Delivery Technology Based on Chemical Methods and Nano-carriers. Theranostics 2014, 4, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.P.; Sharei, A.; Ding, X.Y.; Sahay, G.; Langer, R.; Jensen, K.F. In vitro and ex vivo strategies for intracellular delivery. Nature 2016, 538, 183–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fouriki, A.; Dobson, J. Nanomagnetic Gene Transfection for Non-Viral Gene Delivery in NIH 3T3 Mouse Embryonic Fibroblasts. Materials 2013, 6, 255–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.; Chea, K.; Pyda, R.; Chua, M.; Dominguez, I. Comparative Analysis of Non-viral Transfection Methods in Mouse Embryonic Fibroblast Cells. J. Biomol. Tech. 2017, 28, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.Y.; Wu, Y.S.; Chen, Y.; Zhang, J.X.; Chen, X.F.; Liu, P. Nanoliter Centrifugal Liquid Dispenser Coupled with Superhydrophobic Microwell Array Chips for High-Throughput Cell Assays. Micromachines 2018, 9, 286. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Hu, Y.; Wang, X.; Liu, P.; Chen, X. High-Throughput Platform for Efficient Chemical Transfection, Virus Packaging, and Transduction. Micromachines 2019, 10, 387. https://doi.org/10.3390/mi10060387
Zhang J, Hu Y, Wang X, Liu P, Chen X. High-Throughput Platform for Efficient Chemical Transfection, Virus Packaging, and Transduction. Micromachines. 2019; 10(6):387. https://doi.org/10.3390/mi10060387
Chicago/Turabian StyleZhang, Jianxiong, Yawei Hu, Xiaoqing Wang, Peng Liu, and Xiaofang Chen. 2019. "High-Throughput Platform for Efficient Chemical Transfection, Virus Packaging, and Transduction" Micromachines 10, no. 6: 387. https://doi.org/10.3390/mi10060387
APA StyleZhang, J., Hu, Y., Wang, X., Liu, P., & Chen, X. (2019). High-Throughput Platform for Efficient Chemical Transfection, Virus Packaging, and Transduction. Micromachines, 10(6), 387. https://doi.org/10.3390/mi10060387





