Blood Cells Separation and Sorting Techniques of Passive Microfluidic Devices: From Fabrication to Applications
Abstract
:1. Introduction
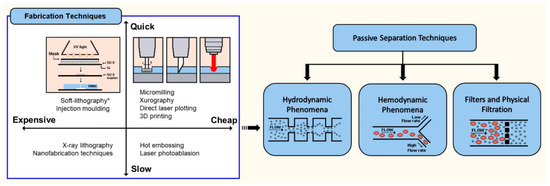
2. Fabrication of Polymeric Microfluidic Devices
3. Design of Microfluidic Devices for Biomedical Applications
4. Microfluidic Cell Separation and Sorting Techniques
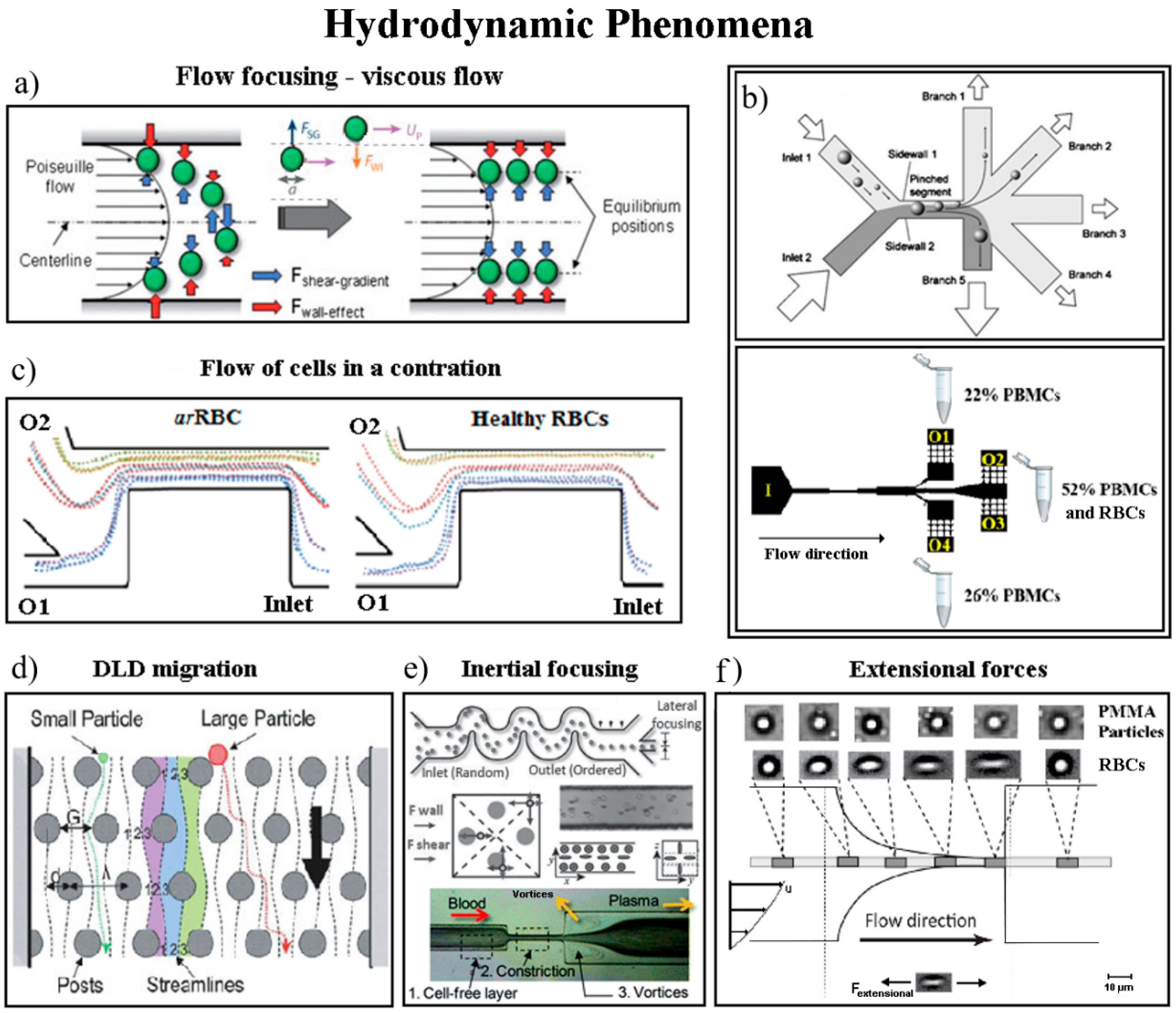
4.1. Hydrodynamic Separation and Sorting Techniques
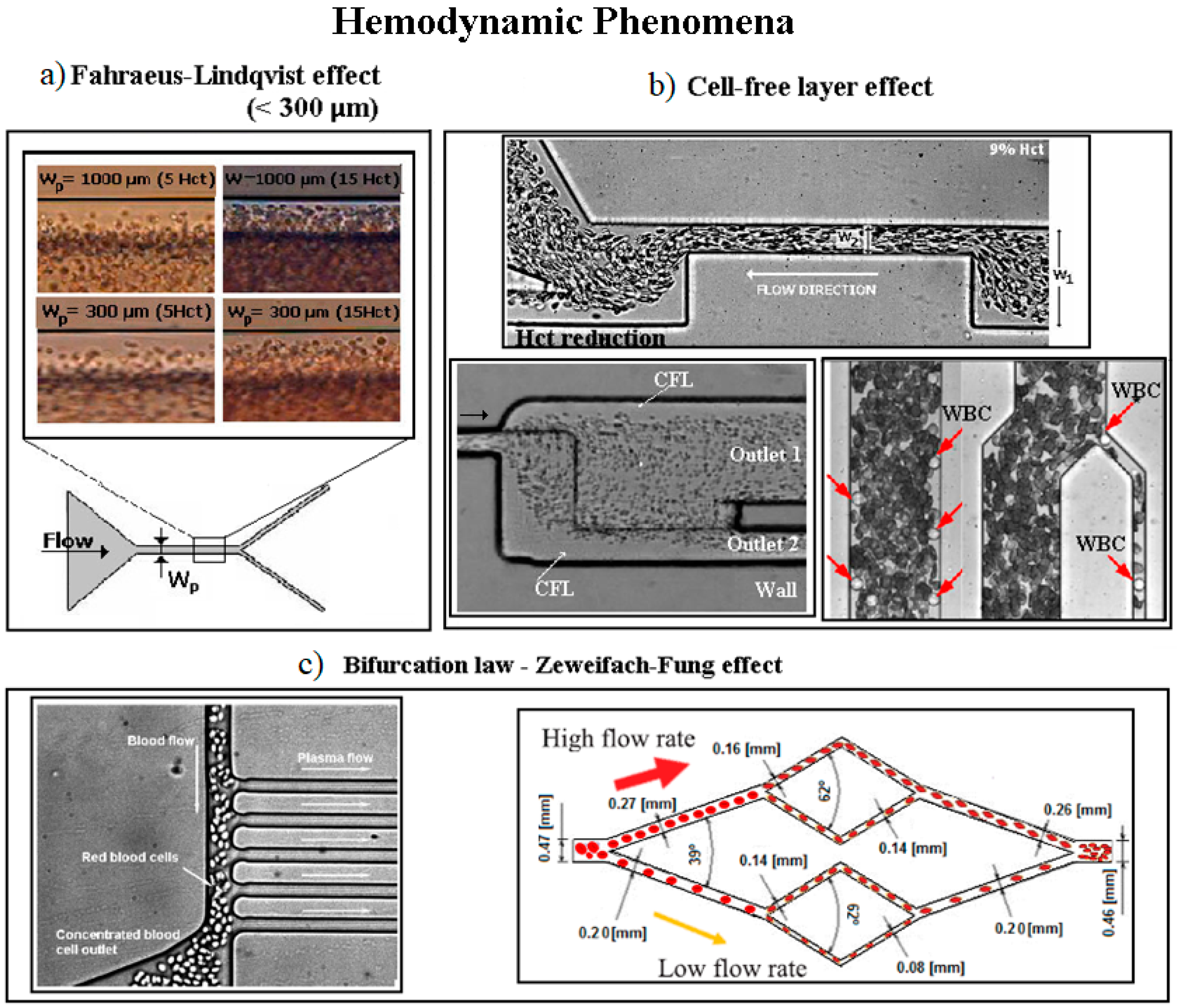
4.2. Hemodynamic Phenomena on Cell Separation Techniques
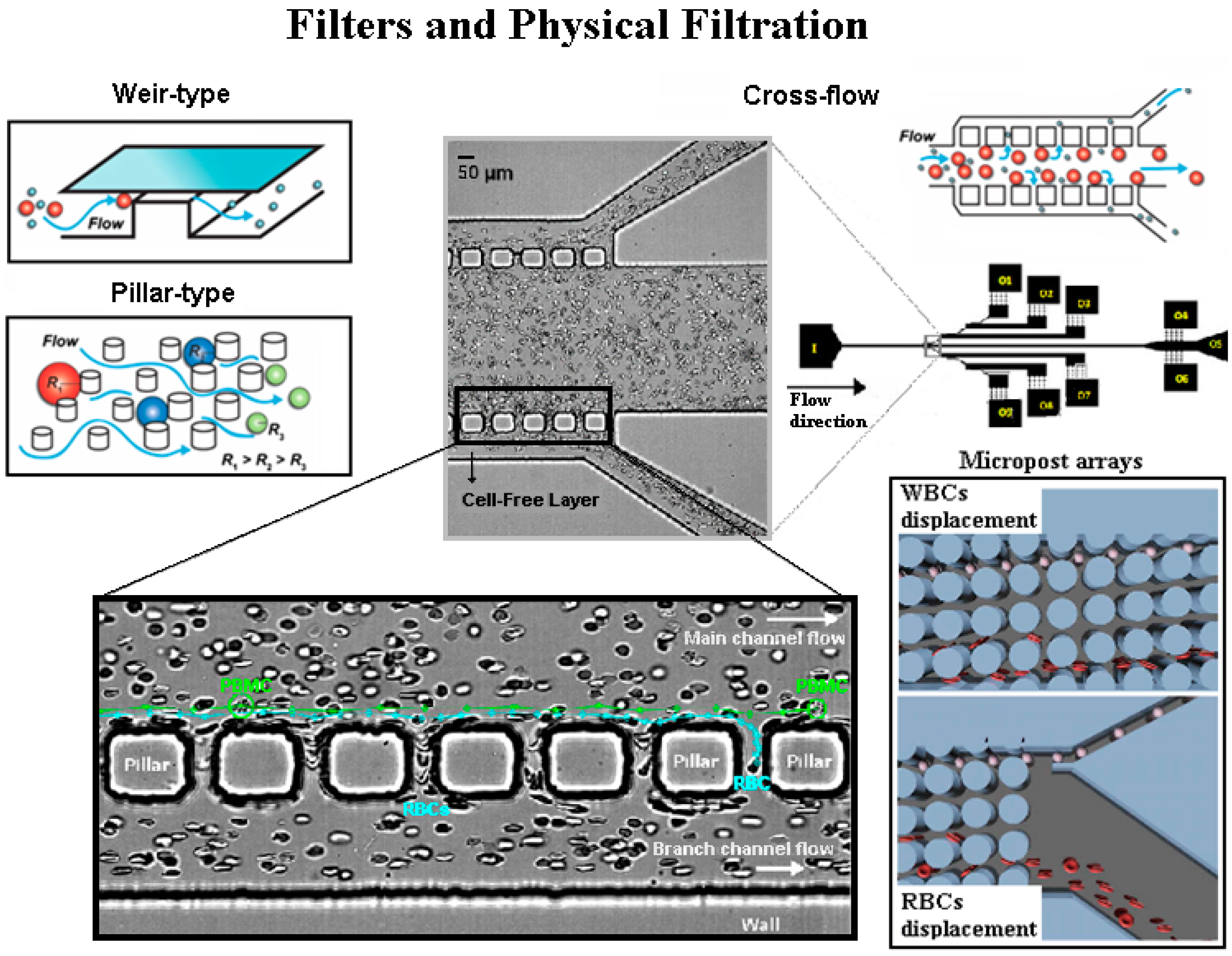
4.3. Microfluidic Filters-Physical Filtration Techniques
4.4. Comparison between the Separation Methods
5. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Convery, N.; Gadegaard, N. 30 years of microfluidics. Micro Nano Eng. 2019, 2, 76–91. [Google Scholar] [CrossRef]
- Mchedlishvili, G.; Maeda, N. Blood flow structure related to red cell flow: A determinant of blood fluidity in narrow microvessels. Jpn. J. Physiol. 2001, 51, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, D.M.; Derzsi, L.; Tamborski, S.; Szkulmowski, M.; Garstecki, P.; Wojtkowski, M. Assessment of the flow velocity of blood cells in a microfluidic device using joint spectral and time domain optical coherence tomography. Opt. Express 2013, 21, 24025–24038. [Google Scholar] [CrossRef] [PubMed]
- Abkarian, M.; Faivre, M.; Stone, H.A. High-speed microfluidic differential manometer for cellular-scale hydrodynamics. Proc. Natl. Acad. Sci. USA 2006, 103, 538–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, S.; DasGupta, S.; Chakraborty, S. Collective dynamics of red blood cells on an in vitro microfluidic platform. Lab Chip 2018, 18, 3939–3948. [Google Scholar]
- Zhao, R.; Antaki, J.F.; Naik, T.; Bachman, T.N.; Kameneva, M.V.; Wu, Z.J. Microscopic investigation of erythrocyte deformation dynamics. Biorheology 2006, 43, 747–765. [Google Scholar] [PubMed]
- Fujiwara, H.; Ishikawa, T.; Lima, R.; Matsuki, N.; Imai, Y.; Kaji, H.; Nishizawa, M.; Yamaguchi, T. Red blood cell motions in high-hematocrit blood flowing through a stenosed microchannel. J. Biomech. 2009, 42, 838–843. [Google Scholar] [CrossRef] [Green Version]
- Leble, V.; Lima, R.; Dias, R.; Fernandes, C.; Ishikawa, T.; Imai, Y.; Yamaguchi, T. Asymmetry of red blood cell motions in a microchannel with a diverging and converging bifurcation. Biomicrofluidics 2011, 5, 044120. [Google Scholar] [CrossRef]
- Manouk, A.; Magalie, F.; Renita, H.; Kristian, S.; Catherine, A.B.-P.; Howard, A.S. Cellular-scale hydrodynamics. Biomed. Mater. 2008, 3, 034011. [Google Scholar]
- Lima, R.; Ishikawa, T.; Imai, Y.; Yamaguchi, T. Blood Flow Behavior in Microchannels: Past, Current and Future Trends. In Single and Two-Phase Flows on Chemical and Biomedical Engineering; Dias, R., Martins, A.A., Lima, R., Mata, T.M., Eds.; Bentham Science: Sharjah, UAE, 2012; pp. 513–547. [Google Scholar] [Green Version]
- Tomaiuolo, G.; Barra, M.; Preziosi, V.; Cassinese, A.; Rotoli, B.; Guido, S. Microfluidics analysis of red blood cell membrane viscoelasticity. Lab Chip 2011, 11, 449–454. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Schueller, O.J.; Whitesides, G.M. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Rodrigues, R.O.; Lima, R.; Gomes, H.T.; Silva, A.M.T. Polymer microfluidic devices: An overview of fabrication methods. U. Porto J. Eng. 2015, 1, 67–79. [Google Scholar] [CrossRef]
- Van Toan, N.; Toda, M.; Ono, T. An Investigation of Processes for Glass Micromachining. Micromachines 2016, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Halldorsson, S.; Lucumi, E.; Gómez-Sjöberg, R.; Fleming, R.M.T. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron. 2015, 63, 218–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gale, B.; Jafek, A.; Lambert, C.; Goenner, B.; Moghimifam, H.; Nze, U.; Kamarapu, S. A Review of current methods in microfluidic device fabrication and future commercialization prospects. Inventions 2018, 3, 60. [Google Scholar] [CrossRef]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 2010, 5, 491. [Google Scholar] [CrossRef] [PubMed]
- Folch, A. Introduction to BioMEMS; CRC Press: Boca Raton, FL, USA, 2013; p. 528. [Google Scholar]
- Walsh, D.I.; Kong, D.S.; Murthy, S.K.; Carr, P.A. Enabling microfluidics: From clean rooms to makerspaces. Trends Biotechnol. 2017, 35, 383–392. [Google Scholar] [CrossRef]
- Pinto, V.C.; Sousa, P.J.; Cardoso, V.F.; Minas, G. Optimized SU-8 processing for low-cost microstructures fabrication without cleanroom facilities. Micromachines 2014, 5, 738–755. [Google Scholar] [CrossRef]
- Faustino, V.; Catarino, S.O.; Lima, R.; Minas, G. Biomedical microfluidic devices by using low-cost fabrication techniques: A review. J. Biomech. 2016, 49, 2280–2292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, E.; Faustino, V.; Rodrigues, R.; Pinho, D.; Garcia, V.; Miranda, J.; Lima, R. A rapid and low-cost nonlithographic method to fabricate biomedical microdevices for blood flow analysis. Micromachines 2015, 6, 121–135. [Google Scholar] [CrossRef]
- Bento, D.; Sousa, L.; Yaginuma, T.; Garcia, V.; Lima, R.; Miranda, J.M. Microbubble moving in blood flow in microchannels: Effect on the cell-free layer and cell local concentration. Biomed. Microdevices 2017, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Guckenberger, D.J.; de Groot, T.E.; Wan, A.M.D.; Beebe, D.J.; Young, E.W.K. Micromilling: A method for ultra-rapid prototyping of plastic microfluidic devices. Lab Chip 2015, 15, 2364–2378. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.; Rodrigues, R.O.; Pinho, D.; Garcia, V.; Schütte, H.; Lima, R.; Gassmann, S. Low cost microfluidic device for partial cell separation: Micromilling approach. In Proceedings of the 2015 IEEE International Conference on Industrial Technology (ICIT), Seville, Spain, 17–19 March 2015; pp. 3347–3350. [Google Scholar]
- Ren, Y.; Ray, S.; Liu, Y. Reconfigurable Acrylic-tape Hybrid Microfluidics. Sci. Rep. 2019, 9, 4824. [Google Scholar] [CrossRef] [PubMed]
- Gaal, G.; Mendes, M.; de Almeida, T.P.; Piazzetta, M.H.; Gobbi, Â.L.; Riul, A., Jr.; Rodrigues, V. Simplified fabrication of integrated microfluidic devices using fused deposition modeling 3D printing. Sens. Actuators B Chem. 2017, 242, 35–40. [Google Scholar] [CrossRef]
- Li, Z.A.; Yang, J.; Li, K.; Zhu, L.; Tang, W. Fabrication of PDMS microfluidic devices with 3D wax jetting. RSC Adv. 2017, 7, 3313–3320. [Google Scholar] [CrossRef] [Green Version]
- Faria, C.L.; Pinho, D.; Santos, J.; Gonçalves, L.M.; Lima, R. Low cost 3D printed biomodels for biofluid mechanics applications. J. Mech. Eng. Biomech. 2018, 3, 1–7. [Google Scholar] [CrossRef]
- Rodrigues, R.O.; Pinho, D.; Bento, D.; Lima, R.; Ribeiro, J. Wall expansion assessment of an intracranial aneurysm model by a 3D Digital Image Correlation System. Measurement 2016, 88, 262–270. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.; Glinsner, T.; Kreindl, G.; Lindner, P.; Wimplinger, M. Industrial Applications Demanding Low and High Resolution Features Realized by Soft UV-NIL and Hot Embossing; SPIE: Bellingham, WA, USA, 2009; 72712J. [Google Scholar]
- He, Y.; Fu, J.-Z.; Chen, Z.-C. Research on optimization of the hot embossing process. J. Micromech. Microeng. 2007, 17, 2420–2425. [Google Scholar] [CrossRef]
- Stormonth-Darling, J.M.; Pedersen, R.H.; How, C.; Gadegaard, N. Injection molding of ultra high aspect ratio nanostructures using coated polymer tooling. J. Micromech. Microeng. 2014, 24, 075019. [Google Scholar] [CrossRef]
- Sarig-Nadir, O.; Livnat, N.; Zajdman, R.; Shoham, S.; Seliktar, D. laser photoablation of guidance microchannels into hydrogels directs cell growth in three dimensions. Biophys. J. 2009, 96, 4743–4752. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-R.; Hsieh, Y.-S.; Hwang, G.-Y.; Lee, Y.-D. Photoablation characteristics of novel polyimides synthesized for high-aspect-ratio excimer laser LIGA process. J. Micromech. Microeng. 2004, 14, 480–489. [Google Scholar] [CrossRef]
- Hartley, F.T.; Malek, C.G.K. Nanometer X-ray Lithography. In Proceedings of the Asia Pacific Symposium on Microelectronics and MEMS, Gold Coast, Australia, 8 October 1999. [Google Scholar]
- Hizawa, T.; Takano, A.; Parthiban, P.; Doyle, P.S.; Iwase, E.; Hashimoto, M. Rapid prototyping of fluoropolymer microchannels by xurography for improved solvent resistance. Biomicrofluidics 2018, 12, 064105. [Google Scholar] [CrossRef] [PubMed]
- Bartholomeusz, D.A.; Boutte, R.W.; Andrade, J.D. Xurography: Rapid prototyping of microstructures using a cutting plotter. J. Microelectromech. Syst. 2005, 14, 1364–1374. [Google Scholar] [CrossRef]
- Lamont, A.C.; Alsharhan, A.T.; Sochol, R.D. Geometric Determinants of In-Situ Direct Laser Writing. Sci. Rep. 2019, 9, 394. [Google Scholar] [CrossRef] [PubMed]
- Do, M.T.; Li, Q.; Nguyen, T.T.N.; Benisty, H.; Ledoux-Rak, I.; Lai, N.D. High aspect ratio submicrometer two-dimensional structures fabricated by one-photon absorption direct laser writing. Microsyst. Technol. 2014, 20, 2097–2102. [Google Scholar] [CrossRef]
- Friedrich, C.R.; Vasile, M.J. The micromilling process for high aspect ratio microstructures. Microsyst. Technol. 1996, 2, 144–148. [Google Scholar] [CrossRef]
- Kitson, P.J.; Rosnes, M.H.; Sans, V.; Dragone, V.; Cronin, L. Configurable 3D-Printed millifluidic and microfluidic ‘lab on a chip’ reactionware devices. Lab Chip 2012, 12, 3267–3271. [Google Scholar] [CrossRef]
- Au, A.K.; Huynh, W.; Horowitz, L.F.; Folch, A.V. 3D-Printed microfluidics. Angew. Chem. Int. Ed. 2016, 55, 3862–3881. [Google Scholar] [CrossRef]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D printed microfluidic devices: Enablers and barriers. Lab Chip 2016, 16, 1993–2013. [Google Scholar] [CrossRef]
- Chang, C.; Sakdinawat, A. Ultra-high aspect ratio high-resolution nanofabrication for hard X-ray diffractive optics. Nat. Commun. 2014, 5, 4243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isobe, G.; Kanno, I.; Kotera, H.; Yokokawa, R. Perfusable multi-scale channels fabricated by integration of nanoimprint lighography (NIL) and UV lithography (UVL). Microelectron. Eng. 2012, 98, 58–63. [Google Scholar] [CrossRef]
- Kim, J.; Gale, B.K. Quantitative and qualitative analysis of a microfluidic DNA extraction system using a nanoporous AlOx membrane. Lab Chip 2008, 8, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Larsen, N.B. Stereolithographic hydrogel printing of 3D culture chips with biofunctionalized complex 3D perfusion networks. Lab Chip 2017, 17, 4273–4282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, L.Y.; Chang, H.C.; Chan, P.P.; Friend, J.R. Microfluidic devices for bioapplications. Small 2011, 7, 12–48. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Zhou, J.; Wu, H. Materials for Microfluidic Chip Fabrication. Acc. Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef] [PubMed]
- Friend, J.; Yeo, L. Fabrication of microfluidic devices using polydimethylsiloxane. Biomicrofluidics 2010, 4, 026502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, V.F.; Minas, G. Micro Total Analysis Systems. In Microfluidics and Nanofluid. Handbook: Fabrication, Implementation and Applications; CPTF Group, Ed.; LLC Publishers: Boca Raton, FL, USA, 2011; Volume 5, pp. 319–366. [Google Scholar]
- Haeberle, S.; Zengerle, R. Microfluidic platforms for lab-on-a-chip applications. Lab Chip 2007, 7, 1094–1110. [Google Scholar] [CrossRef]
- Rife, J.C.; Bell, M.I.; Horwitz, J.S.; Kabler, M.N.; Auyeung, R.C.Y.; Kim, W.J. Miniature valveless ultrasonic pumps and mixers. Sens. Actuators A Phys. 2000, 86, 135–140. [Google Scholar] [CrossRef]
- Fung, Y.C. Biomechanics-Circulation; Springer: New York, NY, USA, 1997. [Google Scholar]
- Roselli, R.J.; Diller, K.R. Biotransport: Principles and Applications; Springer: New York, NY, USA, 2011. [Google Scholar]
- Mohamed, M. Use of Microfluidic Technology for Cell Separation. In Blood Cell-An Overview of Studies in Hematology; InTech: London, UK, 2012. [Google Scholar]
- Shields, C.W., 4th; Reyes, C.D.; Lopez, G.P. Microfluidic cell sorting: A review of the advances in the separation of cells from debulking to rare cell isolation. Lab Chip 2015, 15, 1230–1249. [Google Scholar] [CrossRef]
- Songjaroen, T.; Dungchai, W.; Chailapakul, O.; Henry, C.S.; Laiwattanapaisal, W. Blood separation on microfluidic paper-based analytical devices. Lab Chip 2012, 12, 3392–3398. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Woenker, T.; Adamec, J.; Regnier, F.E. Simple, Miniaturized blood plasma extraction method. Anal. Chem. 2013, 85, 11501–11508. [Google Scholar] [CrossRef] [PubMed]
- Haeberle, S.; Brenner, T.; Zengerle, R.; Ducrée, J. Centrifugal extraction of plasma from whole blood on a rotating disk. Lab Chip 2006, 6, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Amasia, M.; Madou, M. Large-volume centrifugal microfluidic device for blood plasma separation. Bioanalysis 2010, 2, 1701–1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kersaudy-Kerhoas, M.; Sollier, E. Micro-scale blood plasma separation: From acoustophoresis to egg-beaters. Lab Chip 2013, 13, 3323–3346. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Undar, A.; Zahn, J.D. A microfluidic device for continuous, real time blood plasma separation. Lab Chip 2006, 6, 871–880. [Google Scholar] [CrossRef]
- Shevkoplyas, S.S.; Yoshida, T.; Munn, L.L.; Bitensky, M.W. Biomimetic autoseparation of leukocytes from whole blood in a microfluidic device. Anal. Chem. 2005, 77, 933–937. [Google Scholar] [CrossRef]
- Ishikawa, T.; Fujiwara, H.; Matsuki, N.; Yoshimoto, T.; Imai, Y.; Ueno, H.; Yamaguchi, T. Asymmetry of blood flow and cancer cell adhesion in a microchannel with symmetric bifurcation and confluence. Biomed. Microdevices 2011, 13, 159–167. [Google Scholar] [CrossRef]
- Karimi, A.; Yazdi, S.; Ardekani, A.M. Hydrodynamic mechanisms of cell and particle trapping in microfluidics. Biomicrofluidics 2013, 7, 21501. [Google Scholar] [CrossRef] [Green Version]
- Martel, J.M.; Toner, M. Inertial focusing in microfluidics. Annu. Rev. Biomed. Eng. 2014, 16, 371–396. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Yuan, D.; Alici, G.; Nguyen, N.T.; Warkiani, M.E.; Li, W. Fundamentals and applications of inertial microfluidics: A review. Lab Chip 2016, 16, 10–34. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-Y.; Chang, C.-L.; Wang, Y.-N.; Fu, L.-M. Microfluidic mixing: A review. Int. J. Mol. Sci. 2011, 12, 3263–3287. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.K.; Kang, S. A Review on mixing in microfluidics. Micromachines 2010, 1, 82–111. [Google Scholar] [CrossRef]
- Pamme, N.; Manz, A. On-chip free-flow magnetophoresis: Continuous flow separation of magnetic particles and agglomerates. Anal. Chem. 2004, 76, 7250–7256. [Google Scholar] [CrossRef] [PubMed]
- Pamme, N. Continuous flow separations in microfluidic devices. Lab Chip 2007, 7, 1644–1659. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-H.; Kim, S.-H.; Ahn, K.; Lee, S.-H.; Park, J.Y. Separation and sorting of cells in microsystems using physical principles. J. Micromech. Microeng. 2015, 26, 013003. [Google Scholar] [CrossRef] [Green Version]
- Kang, T.G.; Yoon, Y.-J.; Ji, H.; Lim, P.Y.; Chen, Y. A continuous flow micro filtration device for plasma/blood separation using submicron vertical pillar gap structures. J. Micromech. Microeng. 2014, 24, 087001. [Google Scholar] [CrossRef]
- Pinho, D.; Rodrigues, R.O.; Faustino, V.; Yaginuma, T.; Exposto, J.; Lima, R. Red blood cells radial dispersion in blood flowing through microchannels: The role of temperature. J. Biomech. 2016, 49, 2293–2298. [Google Scholar] [CrossRef]
- Hou, H.W.; Bhagat, A.A.; Chong, A.G.; Mao, P.; Tan, K.S.; Han, J.; Lim, C.T. Deformability based cell margination--a simple microfluidic design for malaria-infected erythrocyte separation. Lab Chip 2010, 10, 2605–2613. [Google Scholar] [CrossRef]
- Chung, Y.C.; Hsu, Y.L.; Jen, C.P.; Lu, M.C.; Lin, Y.C. Design of passive mixers utilizing microfluidic self-circulation in the mixing chamber. Lab Chip 2004, 4, 70–77. [Google Scholar] [CrossRef]
- Khosravi Parsa, M.; Hormozi, F.; Jafari, D. Mixing enhancement in a passive micromixer with convergent–divergent sinusoidal microchannels and different ratio of amplitude to wave length. Comput. Fluids 2014, 105, 82–90. [Google Scholar] [CrossRef]
- Rodrigues, R.O.; Pinho, D.; Faustino, V.; Lima, R. A simple microfluidic device for the deformability assessment of blood cells in a continuous flow. Biomed. Microdevices 2015, 17, 108. [Google Scholar] [CrossRef] [PubMed]
- Squires, T.M.; Quake, S.R. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005, 77, 977–1026. [Google Scholar] [CrossRef] [Green Version]
- Tsutsui, H.; Ho, C.-M. Cell separation by non-inertial force fields in microfluidic systems. Mech. Res. Commun. 2009, 36, 92–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gossett, D.R.; Weaver, W.M.; Mach, A.J.; Hur, S.C.; Tse, H.T.; Lee, W.; Amini, H.; Di Carlo, D. Label-free cell separation and sorting in microfluidic systems. Anal. Bioanal. Chem. 2010, 397, 3249–3267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Carlo, D. Inertial microfluidics. Lab Chip 2009, 9, 3038–3046. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, A.A.; Bow, H.; Hou, H.W.; Tan, S.J.; Han, J.; Lim, C.T. Microfluidics for cell separation. Med. Biol. Eng. Comput. 2010, 48, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Pinho, D.; Rodrigues, R.O.; Yaginuma, T.; Faustino, V.; Bento, D.; Fernandes, C.S.; Garcia, V.; Pereira, A.I.; Lima, R. Motion of rigid particles flowing in a microfluidic device with a pronounced stenosis: Trajectories and deformation index. In Proceedings of the 1th World Congress on Computational Mechanics, Barcelona, Spain, 20–25 July 2014. [Google Scholar]
- Yu, Z.T.F.; Aw Yong, K.M.; Fu, J. Microfluidic blood cell sorting: Now and beyond. Small 2014, 10, 1687–1703. [Google Scholar] [CrossRef] [PubMed]
- Calejo, J.; Pinho, D.; Galindo-Rosales, F.J.; Lima, R.; Campo-Deaño, L. Particulate blood analogues reproducing the erythrocytes cell-free layer in a microfluidic device containing a hyperbolic contraction. Micromachines 2015, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, J.; Papautsky, I. Vortex-aided inertial microfluidic device for continuous particle separation with high size-selectivity, efficiency, and purity. Biomicrofluidics 2013, 7, 44119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Huang, F.; Du, J.; Shu, W.; Feng, H.; Xu, X.; Chen, Y. Rapid isolation of cancer cells using microfluidic deterministic lateral displacement structure. Biomicrofluidics 2013, 7, 11801. [Google Scholar] [CrossRef] [PubMed]
- Rosenbluth, M.J.; Lam, W.A.; Fletcher, D.A. Analyzing cell mechanics in hematologic diseases with microfluidic biophysical flow cytometry. Lab Chip 2008, 8, 1062–1070. [Google Scholar] [CrossRef]
- Pinho, D.; Campo-Deaño, L.; Lima, R.; Pinho, F.T. In vitro particulate analogue fluids for experimental studies of rheological and hemorheological behavior of glucose-rich RBC suspensions. Biomicrofluidics 2017, 11, 054105. [Google Scholar] [CrossRef]
- Jäggi, R.D.; Sandoz, R.; Effenhauser, C.S. Microfluidic depletion of red blood cells from whole blood in high-aspect-ratio microchannels. Microfluid. Nanofluid. 2007, 3, 47–53. [Google Scholar] [CrossRef]
- Singhal, J.; Pinho, D.; Lopes, R.; C Sousa, P.; Garcia, V.; Schütte, H.; Lima, R.; Gassmann, S. Blood Flow Visualization and Measurements in Microfluidic Devices Fabricated by a Micromilling Technique. Micro Nanosyst. 2015, 7, 148–153. [Google Scholar] [CrossRef]
- Pinho, D.; Yaginuma, T.; Lima, R. A microfluidic device for partial cell separation and deformability assessment. Biochip J. 2013, 7, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Cidre, D.; Rodrigues, R.O.; Faustino, V.; Pinto, E.; Pinho, D.; Bento, D.; Correia, T.; Fernandes, C.S.; Dias, R.P.; Lima, R. Flow of red blood cells in microchannel networks: In vitro studies. In Perspectives in Fundamental and Applied Rheology; Rubio-Hernández, F.J., Gómez-Merino, A.I., Pino, C., Parras, L., Campo-Deaño, L., Galindo-Rosales, F.J., Velázquez-Navarro, J.F., Eds.; In Iberian Meeting on Rheology: Málaga, Spain, 2013; pp. 271–275. [Google Scholar]
- Faivre, M.; Abkarian, M.; Bickraj, K.; Stone, H.A. Geometrical focusing of cells in a microfluidic device: An approach to separate blood plasma. Biorheology 2006, 43, 147–159. [Google Scholar] [PubMed]
- Lima, R.; Oliveira, M.S.; Ishikawa, T.; Kaji, H.; Tanaka, S.; Nishizawa, M.; Yamaguchi, T. Axisymmetric polydimethysiloxane microchannels for in vitro hemodynamic studies. Biofabrication 2009, 1, 035005. [Google Scholar] [CrossRef] [Green Version]
- Bento, D.; Pereira, A.I.; Lima, J.; Miranda, J.M.; Lima, R. Cell-free layer measurements of in vitro blood flow in a microfluidic network: An automatic and manual approach. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2018, 6, 629–637. [Google Scholar] [CrossRef]
- Bento, D.; Fernandes, C.S.; Miranda, J.M.; Lima, R. In vitro blood flow visualizations and cell-free layer (CFL) measurements in a microchannel network. Exp. Therm. Fluid Sci. 2019, 109, 109847. [Google Scholar] [CrossRef]
- Tripathi, S.; Varun Kumar, Y.V.B.; Prabhakar, A.; Joshi, S.S.; Agrawal, A. Passive blood plasma separation at the microscale: A review of design principles and microdevices. J. Micromech. Microeng. 2015, 25, 083001. [Google Scholar] [CrossRef]
- Bacchin, P.; Meireles, M.; Aimar, P. Modelling of filtration: From the polarised layer to deposit formation and compaction. Desalination 2002, 145, 139–146. [Google Scholar] [CrossRef]
- Keskinler, B.; Yildiz, E.; Erhan, E.; Dogru, M.; Bayhan, Y.K.; Akay, G. Crossflow microfiltration of low concentration-nonliving yeast suspensions. J. Membr. Sci. 2004, 233, 59–69. [Google Scholar] [CrossRef]
- Chen, X.; Cui, D.F.; Liu, C.C.; Li, H. Microfluidic chip for blood cell separation and collection based on crossflow filtration. Sens. Actuators B Chem. 2008, 130, 216–221. [Google Scholar] [CrossRef]
- Lee, Y.; Clark, M.M. Modeling of flux decline during crossflow ultrafiltration of colloidal suspensions. J. Membr. Sci. 1998, 149, 181–202. [Google Scholar] [CrossRef]
- Crowley, T.A.; Pizziconi, V. Isolation of plasma from whole blood using planar microfilters for lab-on-a-chip applications. Lab Chip 2005, 5, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Kai, K.; Choi, D.S.; Iwamoto, T.; Nguyen, Y.H.; Wong, H.; Landis, M.D.; Ueno, N.T.; Chang, J.; Qin, L. Microfluidics separation reveals the stem-cell-like deformability of tumor-initiating cells. Proc. Natl. Acad. Sci. USA 2012, 109, 18707–18712. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Hyun, J.-C.; Yang, S. On-chip Extraction of Intracellular Molecules in White Blood Cells from Whole Blood. Sci. Rep. 2015, 5, 15167. [Google Scholar] [CrossRef] [PubMed]
- Kuntaegowdanahalli, S.S.; Bhagat, A.A.; Kumar, G.; Papautsky, I. Inertial microfluidics for continuous particle separation in spiral microchannels. Lab Chip 2009, 9, 2973–2980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.G.; Choi, S.; Kim, H.J.; Lim, H.K.; Kim, J.H.; Huh, N.; Park, J.K. Inertial blood plasma separation in a contraction–expansion array microchannel. Appl. Phys. Lett. 2011, 98, 253702. [Google Scholar] [CrossRef]
- Van Delinder, V.; Groisman, A. Perfusion in microfluidic cross-flow: Separation of white blood cells from whole blood and exchange of medium in a continuous flow. Anal. Chem. 2007, 79, 2023–2030. [Google Scholar] [CrossRef] [PubMed]
- Van Delinder, V.; Groisman, A. Separation of plasma from whole human blood in a continuous cross-flow in a molded microfluidic device. Anal. Chem. 2006, 78, 3765–3771. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, J.M.; Tai, Y.-C.; Ho, C.-M. Micromachined membrane particle filters. Sens. Actuators A Phys. 1999, 73, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Streets, A.M.; Huang, Y. Chip in a lab: Microfluidics for next generation life science research. Biomicrofluidics 2013, 7, 11302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shevkoplyas, S.S.; Yoshida, T.; Gifford, S.C.; Bitensky, M.W. Direct measurement of the impact of impaired erythrocyte deformability on microvascular network perfusion in a microfluidic device. Lab Chip 2006, 6, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Faustino, V.; Catarino, S.O.; Pinho, D.; Lima, R.A.; Minas, G. A passive microfluidic device based on crossflow filtration for cell separation measurements: A spectrophotometric characterization. Biosensors 2018, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Catarino, S.O.; Lima, R.; Minas, G. Smart devices: Lab-on-a-chip. In Bioinspired Materials for Drug Delivery and Analysis; Rodrigues, L., Mota, M., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 331–369. [Google Scholar]
- Minas, G.; Catarino, S.O. Lab-on-a-chip devices for chemical analysis. In Encyclopedia of Microfluidics and Nanofluidics; Li, D., Ed.; Springer: New York NY, USA, 2015; pp. 1511–1531. [Google Scholar]
- Clausell-Tormos, J.; Lieber, D.; Baret, J.C.; El-Harrak, A.; Miller, O.J.; Frenz, L.; Blouwolff, J.; Humphry, K.J.; Köster, S.; Duan, H.; et al. Droplet-based microfluidic platforms for the encapsulation and screening of mammalian cells and multicellular organisms. Chem. Biol. 2008, 15, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Orwar, O.; Olofsson, J.; Weber, S.G. Single-cell electroporation. Anal. Bioanal. Chem. 2010, 397, 3235–3248. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.K.; Soriano, A.; Mahadevan, L.; Higgins, J.M.; Bhatia, S.N. A biophysical indicator of vaso-occlusive risk in sickle cell disease. Sci. Transl. Med. 2012, 4, 123ra26. [Google Scholar] [CrossRef]
- Di Caprio, G.; Stokes, C.; Higgins, J.M.; Schonbrun, E. Single-cell measurement of red blood cell oxygen affinity. Proc. Natl. Acad. Sci. USA 2015, 112, 9984–9989. [Google Scholar] [CrossRef] [Green Version]
- Bhise, N.S.; Ribas, J.; Manoharan, V.; Zhang, Y.S.; Polini, A.; Massa, S.; Dokmeci, M.E.R.; Khademhosseini, A. Organ-on-a-chip platforms for studying drug delivery systems. J. Control. Release 2014, 190, 82–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Radisic, M. Organ-on-a-chip devices advance to market. Lab Chip 2017, 17, 2395. [Google Scholar] [CrossRef] [PubMed]



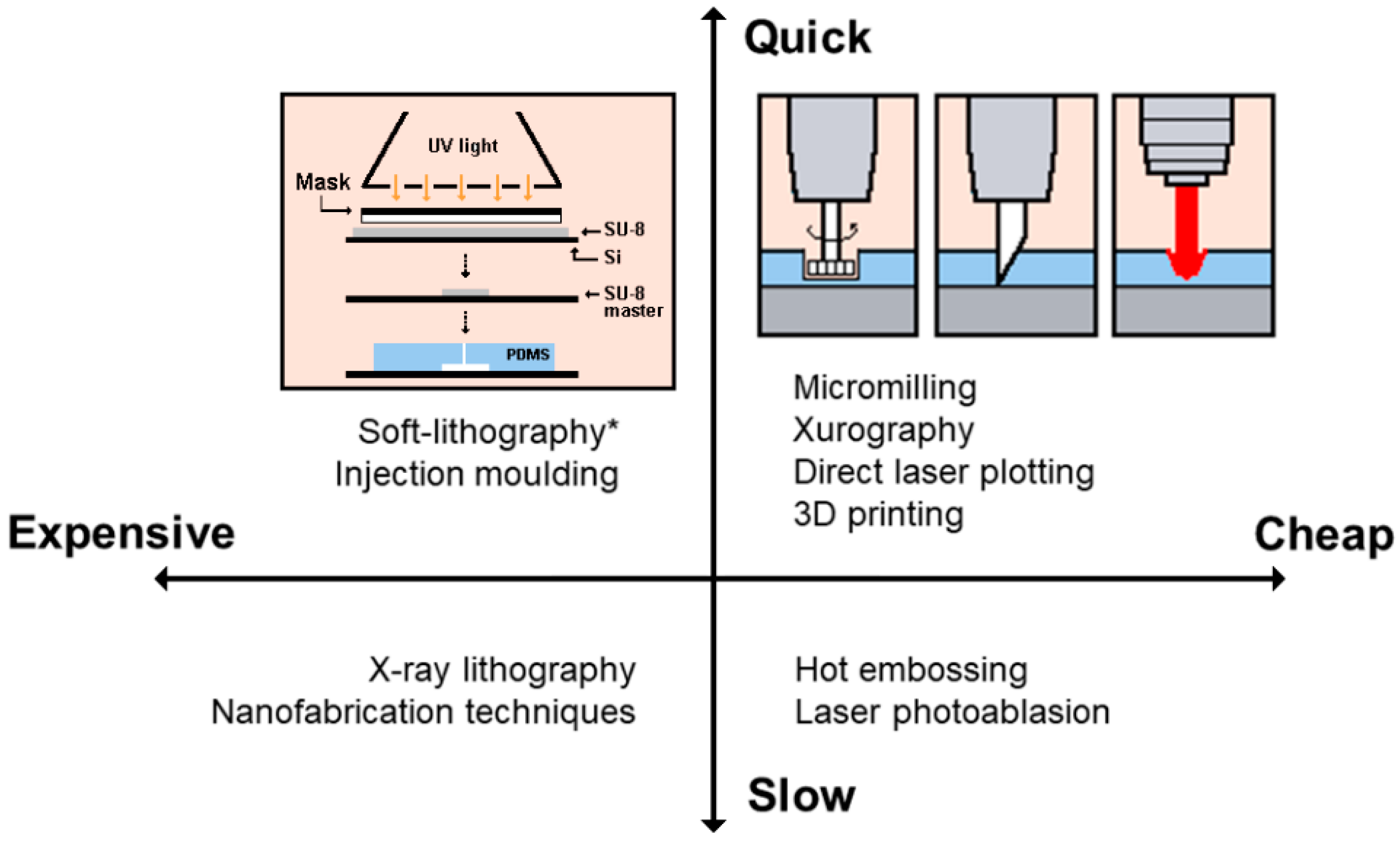

| Fabrication Technique | Advantages | Disadvantages | Resolution Range and Aspect Ratio |
|---|---|---|---|
| Hot embossing | Precise and rapid in the replication of microstructures. Mass production. | Restricted to thermoplastics. Time-consuming. Complex 3D structures are difficult to be fabricated. | Resolution between sub-100 nm and millimetre. Moderate aspect ratio (5:1) [32,33] |
| Injection molding | Mass production. Fine features. Low cycle time. Highly automated. | Restricted to thermoplastics. High cost mold. Nano-size precision is limited. | Resolution between sub-100 nm and millimetre. High aspect ratio (20:1) [34] |
| Laser photoablation | Rapid. Large format production. | Limited materials. Multiple treatment session. Difficulties for mass production. Micro-size precision is limited. | Resolution between micrometre and millimetre. High aspect ratio (30:1) [35,36] |
| X-ray lithography | High-resolution. Straight and smooth walls. | Complex and difficult master fabrication. Time consuming and high cost process. | Resolution between few nanometres and micrometres. Ultra-high aspect ratio (350:1) [37] |
| Soft-lithography | High-resolution and 3D geometries. Cost-effective. Excellent micro-size precision. | Pattern deformation and vulnerability to defects. Difficult to fabricate circular 3D geometries. | Resolution between 30 nm and 500 m. High aspect ratio (20:1) [18] |
| Xurography | Low-cost and rapid technique. | Complex 3D structures are difficult to be fabricated. Micro-size precision is limited. | Resolution between 150 m and millimetre. Moderate aspect ratio (8:1) [21,23,38,39] |
| Direct laser plotting | Low-cost and rapid technique. Free-mask technique. Good micro-size precision. | Complex 3D structures are difficult to be fabricated. Micro-size precision is limited. Reproducibility of the microdevices. | Resolution between 10–500 m. Moderate aspect ratio (7:1) [40,41] |
| Micromilling | Low-cost and rapid technique. Free-mask technique. | Complex 3D structures are difficult to be fabricated. Micro-size precision is limited. Reproducibility of the microdevices. Roughness. | Resolution between 30 m and millimetre. Moderate aspect ratio (8:1) [26,42] |
| Desktop fused deposition modeling (FDM), 3D-printing | Low-cost and rapid technique to fabricate prototypes. | Micro-size precision is limited. High roughness and complex to perform flow visualizations. Not suitable for mass production. | Resolution between 100 m and millimetre. Moderate aspect ratio (10:1) [43,44,45] |
| Nanofabrication | High-resolution of 2D and 3D geometries. Excellent nano-size precision. Highly repeatable, periodical structures. | High cost. Multiple process steps. Limited for microfluidic applications. | Resolution between 1–800 nm. Ultra-high aspect ratio (100:1) [17,46] |
| Characteristics | Silicon | Glass | Thermoplastics | Elastomers (PDMS) |
|---|---|---|---|---|
| Protein crystallization | Poor | Poor | Good | Moderate |
| Droplet formation | Excellent | Excellent | Good | Moderate |
| Porosity | Poor | Poor | Moderate | Moderate |
| Permeability | Poor | Poor | Moderate | Good |
| Bio-culture | Moderate | Moderate | Moderate | Good |
| Reusability | Yes | Yes | Yes | No |
| Disposable device use | Expensive | Expensive | Good | Good |
| Method | Hydrodynamic Separation | Hemodynamic Separation | Physical Filtration |
|---|---|---|---|
| Separation criteria | Size | Size, deformability, cells concentration (hematocrit), cell aggregation [102] | Size, shape, deformability |
| Target sample | Cells, microparticles | RBCs, WBCs, plasma | Cells, particles |
| Separation Efficiency | Above 90% [90,110]; 80–99% [91]; 62.2% [111] | 100% separation efficiency with 15–25% plasma separation volume [65]; 92% separation efficiency with diluted blood (Hct 4.5%) and 37% with whole blood (Hct 45%) [94] | More than 95% of the RBCs and 27% of the WBCs removed from whole blood [105]; 65–100% [102]; 98%, 8% (plasma from whole blood) [112,113] |
| Throughput | 2 mL/min [91]; 106 cells/min [110]; 1.2 mL/h (1010 cells/min) [111] | 3–4 µL/min [112]; 5 mL/min [94] | 2 × l03 cells/s [112,113] |
| Potential effects on cells | Shear stress | Shear stress | Clogging, fouling, shear stress |
| Required instrumentation | Fluidic pumps | Fluidic pumps | Fluidic pumps |
| Processing layout | Continuous flow | Continuous flow | Batch; Continuous flow |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catarino, S.O.; Rodrigues, R.O.; Pinho, D.; Miranda, J.M.; Minas, G.; Lima, R. Blood Cells Separation and Sorting Techniques of Passive Microfluidic Devices: From Fabrication to Applications. Micromachines 2019, 10, 593. https://doi.org/10.3390/mi10090593
Catarino SO, Rodrigues RO, Pinho D, Miranda JM, Minas G, Lima R. Blood Cells Separation and Sorting Techniques of Passive Microfluidic Devices: From Fabrication to Applications. Micromachines. 2019; 10(9):593. https://doi.org/10.3390/mi10090593
Chicago/Turabian StyleCatarino, Susana O., Raquel O. Rodrigues, Diana Pinho, João M. Miranda, Graça Minas, and Rui Lima. 2019. "Blood Cells Separation and Sorting Techniques of Passive Microfluidic Devices: From Fabrication to Applications" Micromachines 10, no. 9: 593. https://doi.org/10.3390/mi10090593
APA StyleCatarino, S. O., Rodrigues, R. O., Pinho, D., Miranda, J. M., Minas, G., & Lima, R. (2019). Blood Cells Separation and Sorting Techniques of Passive Microfluidic Devices: From Fabrication to Applications. Micromachines, 10(9), 593. https://doi.org/10.3390/mi10090593