A Review on Optoelectrokinetics-Based Manipulation and Fabrication of Micro/Nanomaterials
Abstract
:1. Introduction
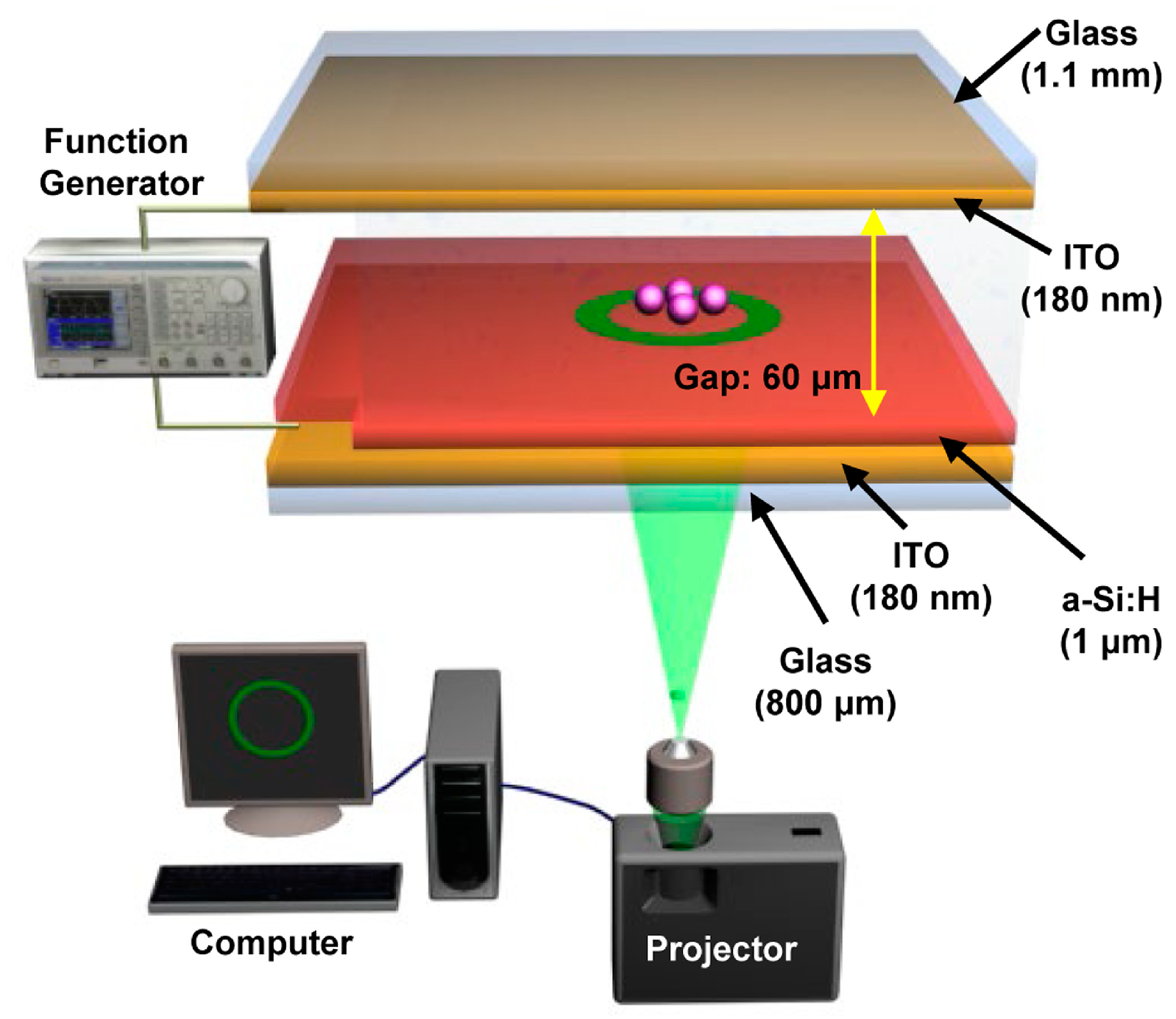
2. Optoelectrokinetics (OEK) Chips and Their Working Principles
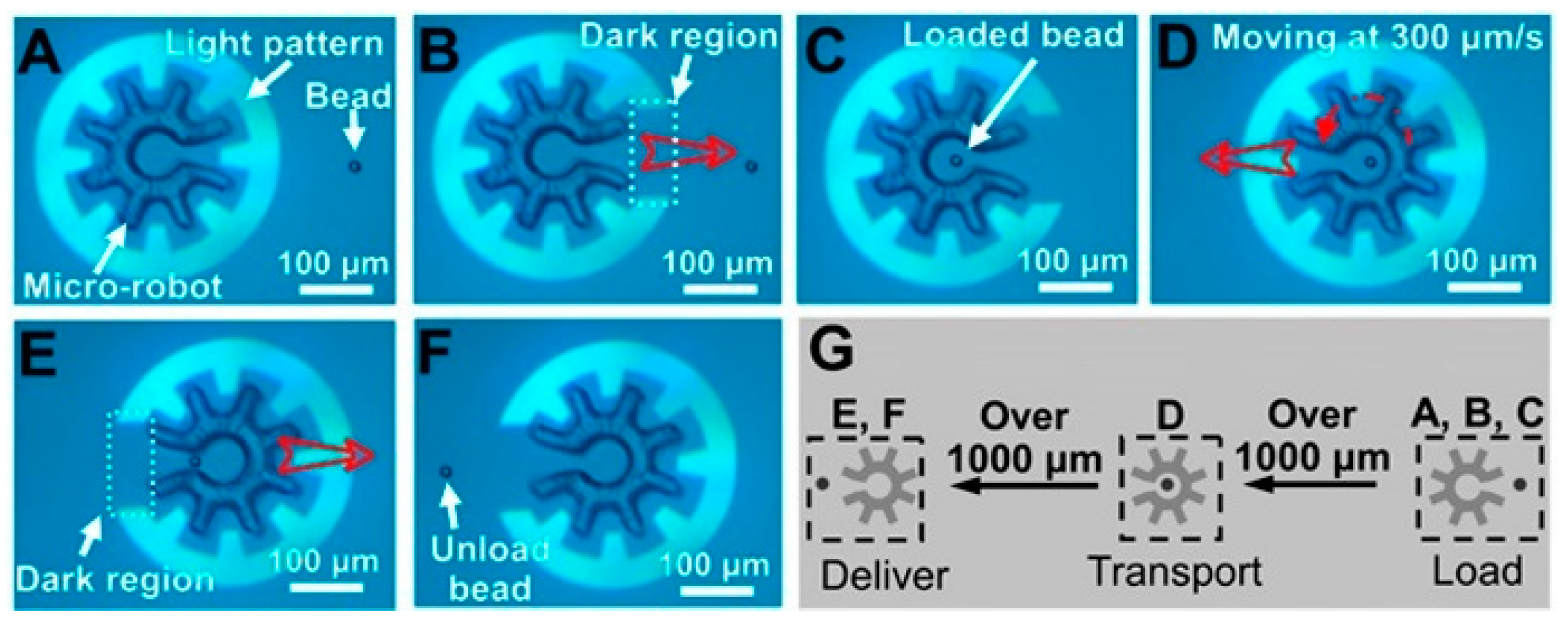
3. OEK-Based Manipulation of Micro/Nanomaterials
3.1. Separation and Assembly of Micro-Scaled Particles
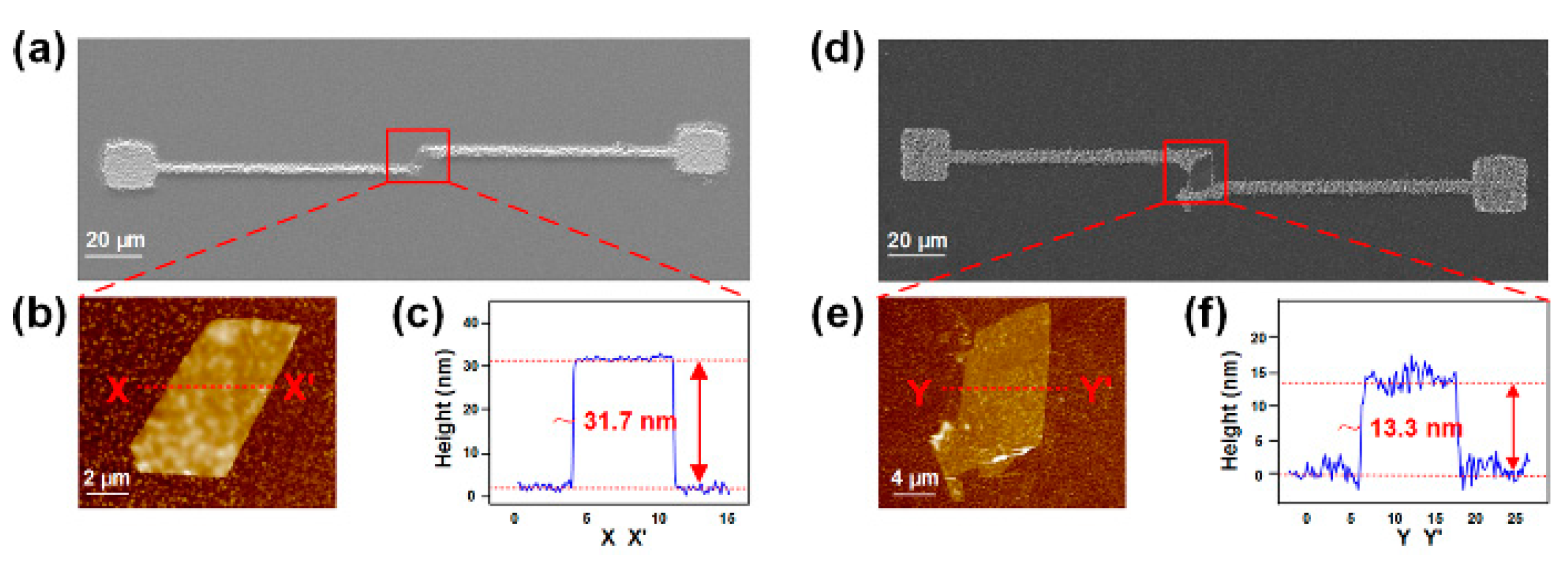
3.2. Manipulation of Nano-Scaled Particles
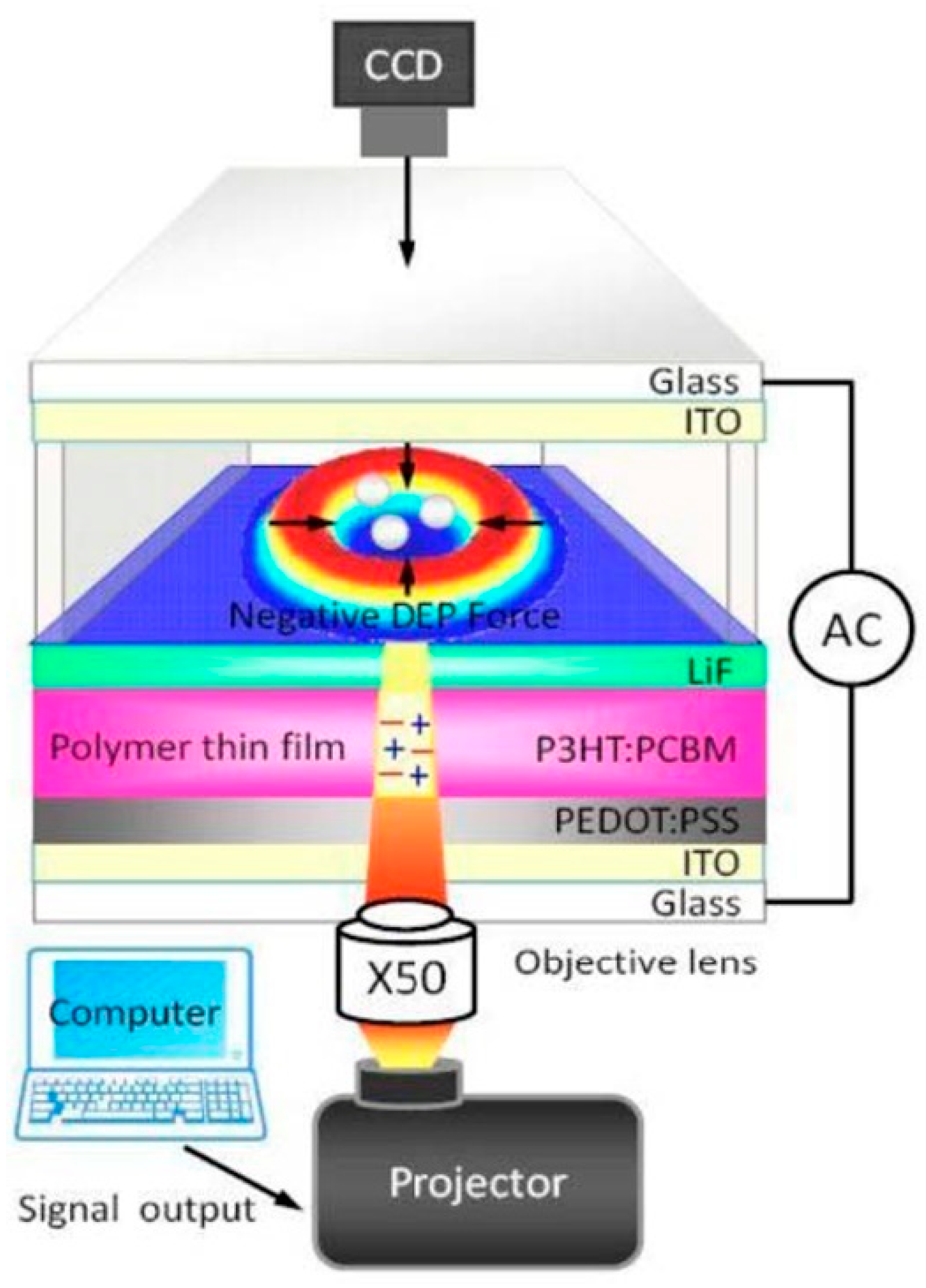
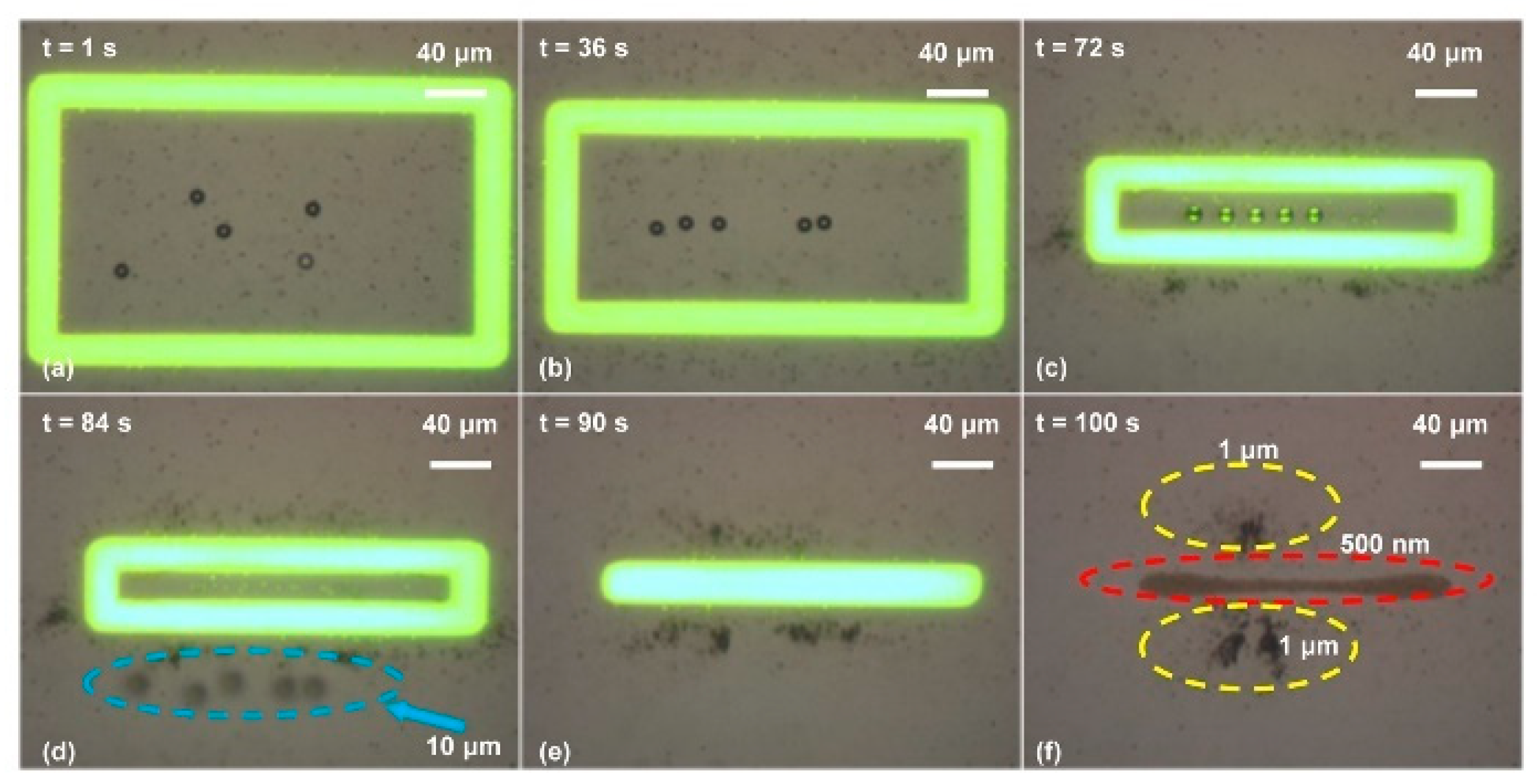
4. Mask-Free Fabrication of Electrodes and Devices
5. Fabrication and Assembly of Hydrogel-based Micro/Nanostructures
6. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Slota, M.; Keerthi, A.; Myers, W.K.; Tretyakov, E.; Baumgarten, M.; Ardavan, A.; Sadeghi, H.; Lambert, C.J.; Narita, A.; Müllen, K.; et al. Magnetic edge states and coherent manipulation of graphene nanoribbons. Nature 2018, 557, 691–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.; Qin, C.; Qiao, Z.; Gong, Y.; Zhang, X.; Zhang, G.; Chen, R.; Gao, Y.; Xiao, L.; Jia, S. In situ manipulation of fluorescence resonance energy transfer between quantum dots and monolayer graphene oxide by laser irradiation. Nanoscale 2019, 11, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Mu, L.; Duan, X.; Pang, W.; Reed, M.A. Trapping of sub-100 nm nanoparticles using gigahertz acoustofluidic tweezers for biosensing applications. Nanoscale 2019, 11, 14625–14634. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Yehye, W.A.; Julkapli, N.M.; Rahmati, S.; Ibn Sina, A.A.; Basirun, W.J.; Johan, M.R. Graphene oxide and gold nanoparticle based dual platform with short DNA probe for the PCR free DNA biosensing using surface-enhanced Raman scattering. Biosens. Bioelectron. 2019, 131, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Kyriazi, M.-E.; Giust, D.; El-Sagheer, A.H.; Lackie, P.M.; Muskens, O.L.; Brown, T.; Kanaras, A.G. Multiplexed mRNA Sensing and Combinatorial-Targeted Drug Delivery Using DNA-Gold Nanoparticle Dimers. ACS Nano 2018, 12, 3333–3340. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Ge, H.; Long, S.; Sun, W.; Fan, J.; Peng, X. Gold nanoparticle-based plasmonic probe for selective recognition of adenosine. Sens. Actuators B Chem. 2019, 296, 126591. [Google Scholar] [CrossRef]
- Sailor, M.J.; Wu, E.C. Photoluminescence-based sensing with porous silicon films, microparticles, and nanoparticles. Adv. Funct. Mater. 2009, 19, 3195–3208. [Google Scholar] [CrossRef]
- Li, N.; Zheng, J.; Li, C.; Wang, X.; Ji, X.; He, Z. An enzyme-free DNA walker that moves on the surface of functionalized magnetic microparticles and its biosensing analysis. Chem. Commun. 2017, 53, 8486–8488. [Google Scholar] [CrossRef]
- Cauda, V.; Stassi, S.; Lamberti, A.; Morello, M.; Pirri, C.F.; Canavese, G. Leveraging ZnO morphologies in piezoelectric composites for mechanical energy harvesting. Nano Energy 2015, 18, 212–221. [Google Scholar] [CrossRef]
- Bhattacharjee, M.; Pasumarthi, V.; Chaudhuri, J.; Singh, A.K.; Nemade, H.B.; Bandyopadhyay, D. Self-spinning nanoparticle laden microdroplets for sensing with energy harvesting. Nanoscale 2016, 8, 6118–6128. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Wang, M.; Li, H.; Saqib, M.; Ge, C.; Zhang, X.; Jin, Y. Enhancing luminol electrochemiluminescence by combined use of cobalt-based metal organic frameworks and silver nanoparticles and its application in ultrasensitive detection of cardiac troponin i. Anal. Chem. 2019, 91, 3048–3054. [Google Scholar] [CrossRef] [PubMed]
- Goossens, S.; Navickaitė, G.; Monasterio, C.; Gupta, S.; Piqueras, J.J.; Perez, R.; Burwell, G.; Nikitskiy, I.; Lasanta, T.; Galán, T.; et al. Broadband image sensor array based on graphene–CMOS integration. Nat. Photon. 2017, 11, 366–371. [Google Scholar] [CrossRef]
- Li, S.-X.; Xia, H.; Xu, Y.-S.; Lv, C.; Wang, G.; Dai, Y.-Z.; Sun, H.-B. Gold nanoparticle densely packed micro/nanowire-based pressure sensors for human motion monitoring and physiological signal detection. Nanoscale 2019, 11, 4925–4932. [Google Scholar] [CrossRef] [PubMed]
- Xiang, N.; Wang, J.; Li, Q.; Han, Y.; Huang, D.; Ni, Z. Precise size-based cell separation via the coupling of inertial microfluidics and deterministic lateral displacement. Anal. Chem. 2019, 91, 10328–10334. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Feng, Q.; Chen, Q.; Liu, C.; Li, T.; Sun, J. Manipulation of bio-micro/nanoparticles in non-newtonian microflows. Microfluid. Nanofluidics 2019, 23, 68. [Google Scholar] [CrossRef]
- Mao, Z.; Li, P.; Wu, M.; Bachman, H.; Mesyngier, N.; Guo, X.; Liu, S.; Costanzo, F.; Huang, T.J. Enriching NanoparticlesviaAcoustofluidics. ACS Nano 2017, 11, 603–612. [Google Scholar] [CrossRef] [Green Version]
- Zheng, T.; Wang, C.; Xu, C.; Hu, Q.; Wei, S. Patterning microparticles into a two-dimensional pattern using one column standing surface acoustic waves. Sens. Actuators A Phys. 2018, 284, 168–171. [Google Scholar] [CrossRef]
- Park, S.; Yossifon, G. Combining dielectrophoresis and concentration polarization-based preconcentration to enhance bead-based immunoassay sensitivity. Nanoscale 2019, 11, 9436–9443. [Google Scholar] [CrossRef]
- Liu, L.; Chen, K.; Xiang, N.; Ni, Z. Dielectrophoretic manipulation of nanomaterials: A review. Electrophoresis 2019, 40, 873–889. [Google Scholar] [CrossRef]
- Zhang, Y.; DaSilva, M.; Ashall, B.; Doyle, G.; Zerulla, D.; Sands, T.D.; Lee, G.U. Magnetic manipulation and optical imaging of an active plasmonic single-particle Fe–Au nanorod. Langmuir 2011, 27, 15292–15298. [Google Scholar] [CrossRef]
- Ebrahimian, H.; Giesguth, M.; Dietz, K.-J.; Reiss, G.; Herth, S. Magnetic tweezers for manipulation of magnetic particles in single cells. Appl. Phys. Lett. 2014, 104, 63701. [Google Scholar] [CrossRef]
- Nan, F.; Yan, Z. Silver-nanowire-based interferometric optical tweezers for enhanced optical trapping and binding of nanoparticles. Adv. Funct. Mater. 2019, 29, 1808258. [Google Scholar] [CrossRef]
- Gould, O.E.C.; Box, S.J.; Boott, C.E.; Ward, A.D.; Winnik, M.A.; Miles, M.J.; Manners, I. Manipulation and deposition of complex, functional block copolymer nanostructures using optical tweezers. ACS Nano 2019, 13, 3858–3866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manna, R.K.; Shklyaev, O.E.; Kauffman, J.; Tansi, B.; Sen, A.; Balazs, A.C. Light-induced convective segregation of different sized microparticles. ACS Appl. Mater. Interfaces 2019, 11, 18004–18012. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Matsumoto, Y.; Kugimiya, R.; Doi, K.; Kawano, S. Separation of nano—and microparticle flows using thermophoresis in branched microfluidic channels. Micromachines 2019, 10, 321. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Han, Y.; Duan, T.; Leifer, K. Size-dependent elasticity of gold nanoparticle measured by atomic force microscope based nanoindentation. Appl. Phys. Lett. 2019, 115, 053104. [Google Scholar] [CrossRef]
- Moreno, M.M.; Ares, P.; Moreno, C.; Zamora, F.; Gomez-Navarro, C.; Herrero, J.G. AFM manipulation of gold nanowires to build electrical circuits. Nano Lett. 2019, 19, 5459–5468. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, D.; Baasch, T.; Blondel, N.; Läubli, N.; Dual, J.; Nelson, B.J. Neutrophil-inspired propulsion in a combined acoustic and magnetic field. Nat. Commun. 2017, 8, 770. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Ouyang, Y.; Wang, Z.; Zhang, R.; Huang, P.-H.; Chen, C.; Li, H.; Li, P.; Quinn, D.; Dao, M.; et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc. Natl. Acad. Sci. USA 2017, 114, 10584–10589. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Yu, H.; Wen, Y.; Wang, F.; Yang, Y.; Liu, Z.; Liu, L.; Li, W.J. Detection of micro/nano-particle concentration using modulated light-emitting diode white light source. Sens. Actuators A Phys. 2019, 285, 89–97. [Google Scholar] [CrossRef]
- Shkolnikov, V.; Xin, D.; Chen, C. Continuous dielectrophoretic particle separation via isomotive dielectrophoresis with bifurcating stagnation flow. Electrophoresis 2019, 40, 2988–2995. [Google Scholar] [CrossRef]
- Chiou, P.Y.; Ohta, A.T.; Wu, M.C. Massively parallel manipulation of single cells and microparticles using optical images. Nature 2005, 436, 370–372. [Google Scholar] [CrossRef]
- Yang, X.; Niu, X.; Liu, Z.; Zhao, Y.; Zhang, G.; Liang, W.; Li, W.J. Accurate extraction of the self-rotational speed for cells in an electrokinetics force field by an image matching algorithm. Micromachines 2017, 8, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, W.; Wang, Y.; Zhang, H.; Liu, L. Characterization of the self-rotational motion of stored red blood cells by using optically-induced electrokinetics. Opt. Lett. 2016, 41, 2763. [Google Scholar] [CrossRef]
- Liang, W.; Zhang, K.; Yang, X.; Liu, L.; Yu, H.; Zhang, W. Distinctive translational and self-rotational motion of lymphoma cells in an optically induced non-rotational alternating current electric field. Biomicrofluidics 2015, 9, 014121. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.; Zhao, Y.; Liu, L.; Wang, Y.; Dong, Z.; Li, W.J.; Lee, G.-B.; Xiao, X.; Zhang, W. Rapid and Label-Free Separation of Burkitt’s Lymphoma Cells from Red Blood Cells by Optically-Induced Electrokinetics. PLoS ONE 2014, 9, e90827. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.-Y.; Kuo, Z.-K.; Chen, Y.-S.; Yeh, T.-Y.; Dong, M.; Tseng, H.-W.; Liu, C.-H. Cancer immunotherapy μ-environment LabChip: taking advantage of optoelectronic tweezers. Lab Chip 2018, 18, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Kamata, M.; Taguchi, Y.; Nagasaka, Y. Design of an optofluidic diffusion sensor by transient grating using dielectrophoresis. Opt. Express 2018, 26, 16970–16983. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.C. Optoelectronic tweezers. Nat. Photon. 2011, 5, 322–324. [Google Scholar] [CrossRef]
- Hwang, H.; Park, J.-K. Optoelectrofluidic platforms for chemistry and biology. Lab Chip 2011, 11, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Liu, L.; Zhang, H.; Wang, Y.; Li, W.J. Optoelectrokinetics-based microfluidic platform for bioapplications: A review of recent advances. Biomicrofluidics 2019, 13, 051502. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Liu, N.; Dong, Z.; Liu, L.; Mai, J.D.; Lee, G.-B.; Li, W.J. Simultaneous separation and concentration of micro- and nano-particles by optically induced electrokinetics. Sens. Actuators A Phys. 2013, 193, 103–111. [Google Scholar] [CrossRef]
- Castellanos, A.; Ramos, A.; González, A.; Green, N.G.; Morgan, H. Electrohydrodynamics and dielectrophoresis in microsystems: scaling laws. J. Phys. D Appl. Phys. 2003, 36, 2584–2597. [Google Scholar] [CrossRef]
- Valley, J.K.; Jamshidi, A.; Ohta, A.T.; Hsu, H.-Y.; Wu, M.C. Operational regimes and physics present in optoelectronic tweezers. J. Microelectrom. Syst. 2008, 17, 342–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiou, P.-Y.; Ohta, A.; Jamshidi, A.; Hsu, H.-Y.; Wu, M. Light-actuated AC electroosmosis for nanoparticle manipulation. J. Microelectrom. Syst. 2008, 17, 525–531. [Google Scholar] [CrossRef]
- Hwang, H.; Park, J.-K. Rapid and selective concentration of microparticles in an optoelectrofluidic platform. Lab Chip 2009, 9, 199–206. [Google Scholar] [CrossRef]
- Wang, W.; Lin, Y.-H.; Guan, R.-S.; Wen, T.-C.; Guo, T.-F.; Lee, G.-B. Bulk-heterojunction polymers in optically-induced dielectrophoretic devices for the manipulation of microparticles. Opt. Express 2009, 17, 17603–17613. [Google Scholar] [CrossRef]
- Wang, W.; Lin, Y.-H.; Wen, T.-C.; Guo, T.-F.; Lee, G.-B. Selective manipulation of microparticles using polymer-based optically induced dielectrophoretic devices. Appl. Phys. Lett. 2010, 96, 113302. [Google Scholar] [CrossRef]
- Lin, S.-J.; Hung, S.-H.; Jeng, J.-Y.; Guo, T.-F.; Lee, G.-B. Manipulation of micro-particles by flexible polymer-based optically-induced dielectrophoretic devices. Opt. Express 2012, 20, 583–592. [Google Scholar] [CrossRef]
- Yang, S.-M.; Yu, T.-M.; Huang, H.-P.; Ku, M.-Y.; Hsu, L.; Liu, C.-H. Dynamic manipulation and patterning of microparticles and cells by using TiOPc-based optoelectronic dielectrophoresis. Opt. Lett. 2010, 35, 1959–1961. [Google Scholar] [CrossRef]
- Yang, S.-M.; Yu, T.-M.; Huang, H.-P.; Ku, M.-Y.; Tseng, S.-Y.; Tsai, C.-L.; Chen, H.-P.; Hsu, L.; Liu, C.-H. Light-driven manipulation of picobubbles on a titanium oxide phthalocyanine-based optoelectronic chip. Appl. Phys. Lett. 2011, 98, 153512. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.-M.; Yang, S.-M.; Fu, C.-Y.; Liu, M.-H.; Hsu, L.; Chang, H.-Y.; Liu, C.-H. Integration of organic opto-electrowetting and poly(ethylene) glycol diacrylate (PEGDA) microfluidics for droplets manipulation. Sens. Actuators B Chem. 2013, 180, 35–42. [Google Scholar] [CrossRef]
- Yang, S.-M.; Tseng, S.-Y.; Chen, H.-P.; Hsu, L.; Liu, C.-H. Cell patterning via diffraction-induced optoelectronic dielectrophoresis force on an organic photoconductive chip. Lab Chip 2013, 13, 3893. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Park, Y.-H.; Park, J.-K. Optoelectrofluidic control of colloidal assembly in an optically induced electric field. Langmuir 2009, 25, 6010–6014. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-Y.; Lu, Y.-S.; Yeh, J.A. Self-assembled high NA microlens arrays using global dielectricphoretic energy wells. Opt. Express 2006, 14, 10779. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-Y.; Lin, Y.-H.; Lee, G.-B. Separation of micro-particles utilizing spatial difference of optically induced dielectrophoretic forces. Microfluid. Nanofluidics 2010, 8, 217–229. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kalim, S.; Callahan, C.; Teitell, M.A.; Chiou, E.P.Y. A light-induced dielectrophoretic droplet manipulation platform. Lab Chip 2009, 9, 3228. [Google Scholar] [CrossRef]
- Lee, H.; Hwang, H.; Park, J.-K. Generation and manipulation of droplets in an optoelectrofluidic device integrated with microfluidic channels. Appl. Phys. Lett. 2009, 95, 164102. [Google Scholar] [CrossRef]
- Hung, S.; Lin, Y.; Lee, G. A microfluidic platform for manipulation and separation of oil-in-water emulsion dielectrophoresis. J Micromechan. Microeng. 2010, 20, 045026. [Google Scholar] [CrossRef]
- Zhang, S.; Juvert, J.; Cooper, J.M.; Neale, S.L. Manipulating and assembling metallic beads with optoelectronic tweezers. Sci. Rep. 2016, 6, 32840. [Google Scholar] [CrossRef]
- Liang, W.; Wang, S.; Dong, Z.; Lee, G.-B.; Li, W.J. Optical spectrum and electric field waveform dependent optically-induced dielectrophoretic (ODEP) micro-manipulation. Micromachines 2012, 3, 492–508. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Hwang, H.; Park, J.-K. Optoelectrofluidic behavior of metal–polymer hybrid colloidal particles. Appl. Phys. Lett. 2013, 102, 054105. [Google Scholar] [CrossRef]
- Zhang, S.; Scott, E.Y.; Singh, J.; Chen, Y.; Zhang, Y.; Elsayed, M.; Chamberlain, M.D.; Shakiba, N.; Adams, K.; Yu, S.; et al. The optoelectronic microrobot: A versatile toolbox for micromanipulation. Proc. Natl. Acad. Sci. USA 2019, 116, 14823–14828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamshidi, A.; Pauzauskie, P.J.; Schuck, P.J.; Ohta, A.T.; Chiou, P.-Y.; Chou, J.; Yang, P.; Wu, M.C. Dynamic manipulation and separation of individual semiconducting and metallic nanowires. Nat. Photon. 2008, 2, 86–89. [Google Scholar] [CrossRef]
- Pauzauskie, P.J.; Jamshidi, A.; Valley, J.K.; Satcher, J.H.; Wu, M.C. Parallel trapping of multiwalled carbon nanotubes with optoelectronic tweezers. Appl. Phys. Lett. 2009, 95, 113104. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.-W.; Lin, Y.-H.; Lee, G.-B. Manipulation and patterning of carbon nanotubes utilizing optically induced dielectrophoretic forces. Microfluid. Nanofluidics 2010, 8, 609–617. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Ho, K.-S.; Yang, C.-T.; Wang, J.-H.; Lai, C.-S. A highly flexible platform for nanowire sensor assembly using a combination of optically induced and conventional dielectrophoresis. Opt. Express 2014, 22, 13811–13824. [Google Scholar] [CrossRef]
- Lim, M.B.; Felsted, R.G.; Zhou, X.; Smith, B.E.; Pauzauskie, P.J. Patterning of graphene oxide with optoelectronic tweezers. Appl. Phys. Lett. 2018, 113, 031106. [Google Scholar] [CrossRef]
- Jamshidi, A.; Neale, S.L.; Yu, K.; Pauzauskie, P.J.; Schuck, P.J.; Valley, J.K.; Hsu, H.-Y.; Ohta, A.T.; Wu, M.C. NanoPen: Dynamic, Low-power, and light-actuated patterning of nanoparticles. Nano Lett. 2009, 9, 2921–2925. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.-M.; Harishchandra, P.T.; Yu, T.-M.; Liu, M.-H.; Hsu, L.; Liu, C.-H. Concentration of magnetic beads utilizing light-induced electro-osmosis flow. IEEE Trans. Magn. 2011, 47, 2418–2421. [Google Scholar] [CrossRef]
- Ota, S.; Wang, S.; Wang, Y.; Yin, X.; Zhang, X. Lipid bilayer-integrated optoelectronic tweezers for nanoparticle manipulations. Nano Lett. 2013, 13, 2766–2770. [Google Scholar] [CrossRef]
- Liang, W.; Liu, L.; Lai, S.H.-S.; Wang, Y.; Lee, G.-B.; Li, W.J. Rapid assembly of gold nanoparticle-based microstructures using optically-induced electrokinetics. Opt. Mater. Express 2014, 4, 2368. [Google Scholar] [CrossRef]
- Liu, N.; Liang, W.; Mai, J.D.; Liu, L.; Lee, G.-B.; Li, W.J. Rapid fabrication of nanomaterial electrodes using digitally controlled electrokinetics. IEEE Trans. Nanotechnol. 2014, 13, 245–253. [Google Scholar] [CrossRef]
- Liang, W.; Liu, L.; Wang, Y.; Lee, G.-B.; Li, W.J. Rapid assembly of CARBON Nanoparticles into electrical elements by optically-induced electroosmotic flow. IEEE Trans. Nanotechnol. 2018, 17, 1045–1052. [Google Scholar] [CrossRef]
- Liu, N.; Wei, F.; Liu, L.; Lai, H.S.S.; Yu, H.; Wang, Y.; Lee, G.-B.; Li, W.J. Optically-controlled digital electrodeposition of thin-film metals for fabrication of nano-devices. Opt. Mater. Express 2015, 5, 838. [Google Scholar] [CrossRef]
- Liu, N.; Wang, F.; Liu, L.; Yu, H.; Xie, S.; Wang, J.; Wang, Y.; Lee, G.-B.; Li, W.J. Rapidly patterning micro/nano devices by directly assembling ions and nanomaterials. Sci. Rep. 2016, 6, 32106. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Liu, N.; Yu, H.; Wang, F.; Liu, L.; Lee, G.-B.; Wang, Y.; Li, W.J. Silver nanostructures synthesis via optically induced electrochemical deposition. Sci. Rep. 2016, 6, 28035. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Liu, N.; Li, P.; Shi, J.; Li, G.; Xi, N.; Wang, Y.; Liu, L. Performance investigation of multilayer MoS2 thin-film transistors fabricated via mask-free optically induced electrodeposition. ACS Appl. Mater. Interfaces 2017, 9, 8361–8370. [Google Scholar] [CrossRef]
- Liu, N.; Li, M.; Liu, L.; Yang, Y.; Mai, J.; Pu, H.; Sun, Y.; Li, W.J. Single-step fabrication of electrodes with controlled nanostructured surface roughness using optically-induced electrodeposition. J. Micromechan. Microeng. 2018, 28, 025011. [Google Scholar] [CrossRef]
- Wang, S.; Liang, W.; Dong, Z.; Lee, V.G.B.; Li, W.J. Fabrication of micrometer—and nanometer-scale polymer structures by visible light induced dielectrophoresis (DEP) force. Micromachines 2011, 2, 431–442. [Google Scholar] [CrossRef]
- Liu, N.; Li, P.; Liu, L.; Yu, H.; Wang, Y.; Lee, G.-B.; Li, W.J. 3-D Non-UV digital printing of hydrogel microstructures by optically controlled digital electropolymerization. J. Microelectrom. Syst. 2015, 24, 2128–2135. [Google Scholar] [CrossRef]
- Li, Y.; Lai, S.H.S.; Liu, N.; Zhang, G.; Liu, L.; Lee, G.-B.; Li, W.J. Fabrication of high-aspect-ratio 3D Hydrogel microstructures using optically induced electrokinetics. Micromachines 2016, 7, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Yu, H.; Li, G.; Wang, Y.; Liu, L. Tissue engineering: High-throughput fabrication and modular assembly of 3D heterogeneous microscale tissues (small 5/2017). Small 2017, 13, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Yu, H.; Liu, N.; Wang, F.; Lee, G.-B.; Wang, Y.; Liu, L.; Li, W.J. Visible light induced electropolymerization of suspended hydrogel bioscaffolds in a microfluidic chip. Biomater. Sci. 2018, 6, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Liang, W.; Liu, L.; Wang, Y.; Mai, J.D.; Lee, G.-B.; Li, W.J. Extracellular-controlled breast cancer cell formation and growth using non-UV patterned hydrogels via optically-induced electrokinetics. Lab Chip 2014, 14, 1367. [Google Scholar] [CrossRef] [PubMed]
- Chan-Park, M.B.; Yan, Y.; Neo, W.K.; Zhou, W.; Zhang, J.; Yue, C.Y. Fabrication of high aspect ratio poly(ethylene glycol)-containing microstructures by UV embossing. Langmuir 2003, 19, 4371–4380. [Google Scholar] [CrossRef]
- Terakawa, M.; Torres-Mapa, M.L.; Takami, A.; Heinemann, D.; Nedyalkov, N.N.; Nakajima, Y.; Hördt, A.; Ripken, T.; Heisterkamp, A. Femtosecond laser direct writing of metal microstructure in a stretchable poly(ethylene glycol) diacrylate (PEGDA) hydrogel. Opt. Lett. 2016, 41, 1392–1395. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, W.; Zhang, G.; Mai, J.D.; Liu, L.; Lee, G.-B.; Li, W.J. Distinguishing cells by their first-order transient motion response under an optically induced dielectrophoretic force field. Appl. Phys. Lett. 2013, 103, 183702. [Google Scholar] [CrossRef]
- Liang, W.; Zhao, Y.; Liu, L.; Wang, Y.; Li, W.J.; Lee, G.-B. Determination of cell membrane capacitance and conductance via optically induced electrokinetics. Biophys. J. 2017, 113, 1531–1539. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Lai, H.S.S.; Zhang, G.; Lee, G.-B.; Li, W.J. Measurement of single leukemia cell’s density and mass using optically induced electric field in a microfluidics chip. Biomicrofluidics 2015, 9, 022406. [Google Scholar] [CrossRef] [Green Version]
- Chiu, T.-K.; Chao, A.-C.; Chou, W.-P.; Liao, C.-J.; Wang, H.-M.; Chang, J.-H.; Chen, P.-H.; Wu, M.-H. Optically-induced-dielectrophoresis (ODEP)-based cell manipulation in a microfluidic system for high-purity isolation of integral circulating tumor cell (CTC) clusters based on their size characteristics. Sens. Actuators B Chem. 2018, 258, 1161–1173. [Google Scholar] [CrossRef]
- Chu, P.-Y.; Liao, C.-J.; Hsieh, C.-H.; Wang, H.-M.; Chou, W.-P.; Chen, P.-H.; Wu, M.-H. Utilization of optically induced dielectrophoresis in a microfluidic system for sorting and isolation of cells with varied degree of viability: Demonstration of the sorting and isolation of drug-treated cancer cells with various degrees of anti-cancer drug resistance gene expression. Sens. Actuators B Chem. 2019, 283, 621–631. [Google Scholar] [CrossRef]
- Huang, S.-H.; Hung, L.-Y.; Lee, G.-B. Continuous nucleus extraction by optically-induced cell lysis on a batch-type microfluidic platform. Lab Chip 2016, 16, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.-C.; Wang, C.-H.; Lee, W.-B.; Lee, G.-B. Automatic cell fusion via optically-induced dielectrophoresis and optically-induced locally-enhanced electric field on a microfluidic chip. Biomicrofluidics 2018, 12, 034108. [Google Scholar] [CrossRef]
- Hwang, H.; Oh, Y.; Kim, J.-J.; Choi, W.; Park, J.-K.; Kim, S.-H.; Jang, J. Reduction of nonspecific surface-particle interactions in optoelectronic tweezers. Appl. Phys. Lett. 2008, 92, 024108. [Google Scholar] [CrossRef]
- Valley, J.K.; Ohta, A.T.; Neale, S.L.; Jamshidi, A.; Wu, M.C.; Hsu, H.-Y. Optoelectronic tweezers as a tool for parallel single-cell manipulation and stimulation. IEEE Trans. Biomed. Circuits Syst. 2009, 3, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-H.; Lee, G.-B. Optically induced flow cytometry for continuous microparticle counting and sorting. Biosens. Bioelectron. 2008, 24, 572–578. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Lee, G.-B. An integrated cell counting and continuous cell lysis device using an optically induced electric field. Sens. Actuators B Chem. 2010, 145, 854–860. [Google Scholar] [CrossRef]
- Huang, K.-W.; Su, T.-W.; Ozcan, A.; Chiou, P.-Y. Optoelectronic tweezers integrated with lensfree holographic microscopy for wide-field interactive cell and particle manipulation on a chip. Lab Chip 2013, 13, 2278–2284. [Google Scholar] [CrossRef]
- Lee, G.-B.; Chang, C.-J.; Wang, C.-H.; Lu, M.-Y.; Luo, Y.-Y. Continuous medium exchange and optically induced electroporation of cells in an integrated microfluidic system. Microsyst. Nanoeng. 2015, 1, 15007. [Google Scholar] [CrossRef]
- Witte, C.; Kremer, C.; Cooper, J.M.; Neale, S.L. Continuous cell lysis in microfluidics through acoustic and optoelectronic tweezers. SPIE MOEMS-MEMS 2013, 8615, 86150. [Google Scholar] [CrossRef] [Green Version]
- Witte, C.; Wilson, R.; Cooper, J.M.; Neale, S.L. OET Meets Acoustic Tweezing. In Proceedings of the Optical Trapping and Optical Micromanipulation IX, San Diego, CA, USA, 10 October 2012; pp. 411–418. [Google Scholar]












© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, W.; Liu, L.; Wang, J.; Yang, X.; Wang, Y.; Li, W.J.; Yang, W. A Review on Optoelectrokinetics-Based Manipulation and Fabrication of Micro/Nanomaterials. Micromachines 2020, 11, 78. https://doi.org/10.3390/mi11010078
Liang W, Liu L, Wang J, Yang X, Wang Y, Li WJ, Yang W. A Review on Optoelectrokinetics-Based Manipulation and Fabrication of Micro/Nanomaterials. Micromachines. 2020; 11(1):78. https://doi.org/10.3390/mi11010078
Chicago/Turabian StyleLiang, Wenfeng, Lianqing Liu, Junhai Wang, Xieliu Yang, Yuechao Wang, Wen Jung Li, and Wenguang Yang. 2020. "A Review on Optoelectrokinetics-Based Manipulation and Fabrication of Micro/Nanomaterials" Micromachines 11, no. 1: 78. https://doi.org/10.3390/mi11010078
APA StyleLiang, W., Liu, L., Wang, J., Yang, X., Wang, Y., Li, W. J., & Yang, W. (2020). A Review on Optoelectrokinetics-Based Manipulation and Fabrication of Micro/Nanomaterials. Micromachines, 11(1), 78. https://doi.org/10.3390/mi11010078





