An Easy Method for Pressure Measurement in Microchannels Using Trapped Air Compression in a One-End-Sealed Capillary
Abstract
:1. Introduction
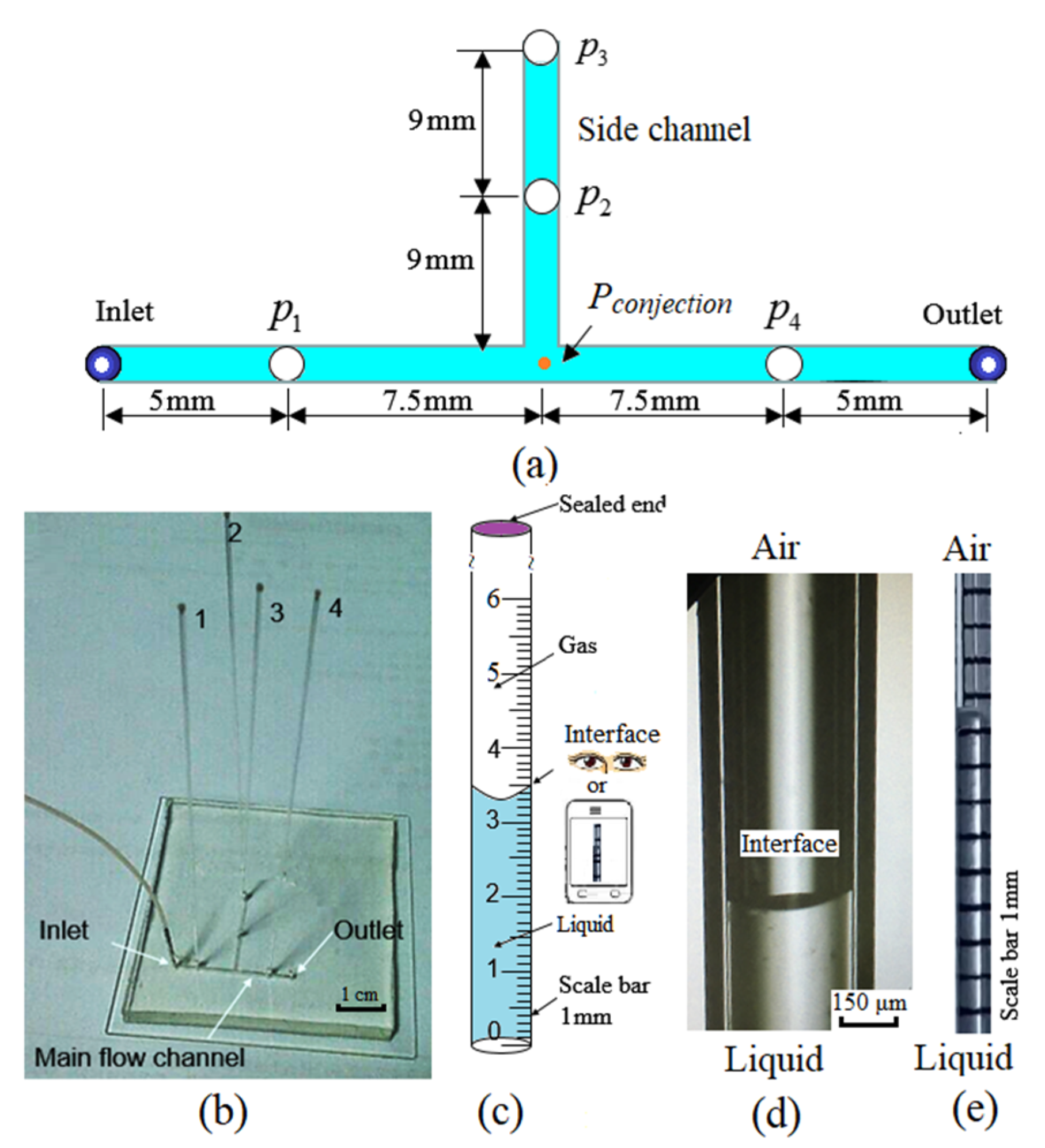
2. Materials and Methods
3. Theory and Factors Analysis
3.1. Liquid Gravity
3.2. The Capillary Pressure Drop
3.3. Air Dissolution in Water
3.4. Temperature Changes
3.5. Theory of Pressure Drop
4. Simulation Method
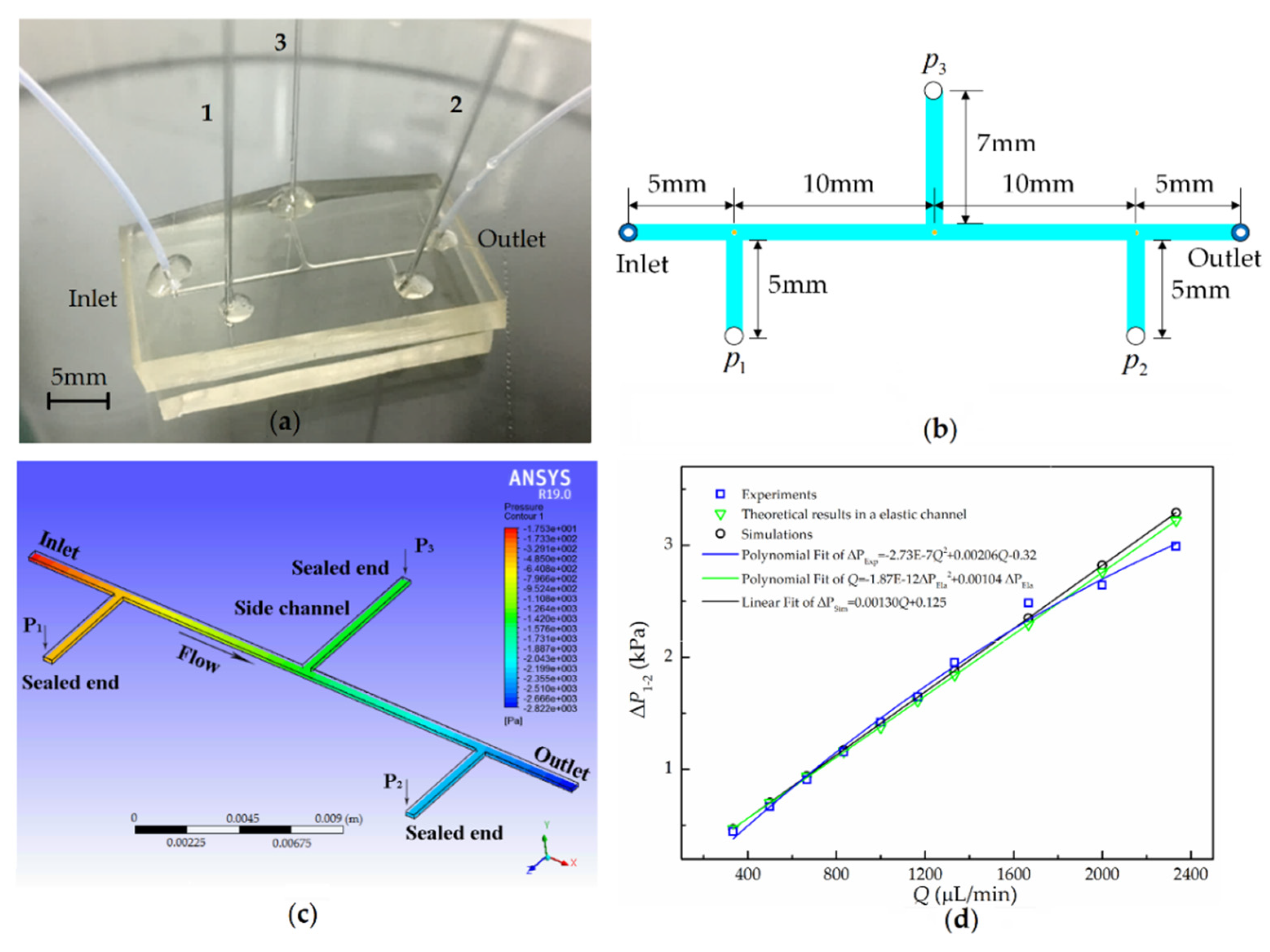
4.1. Geometry
4.2. Governing Equations
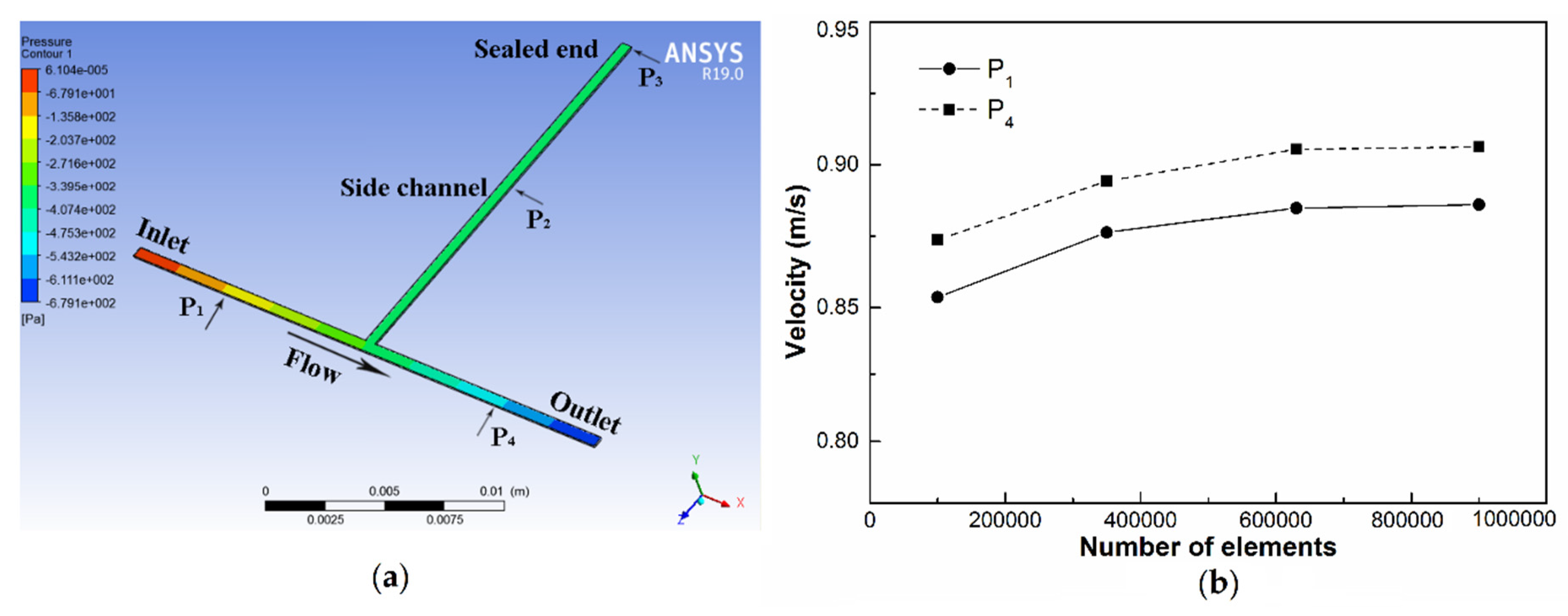
4.3. Grid Independency Study and Validation
5. Results and Discussion
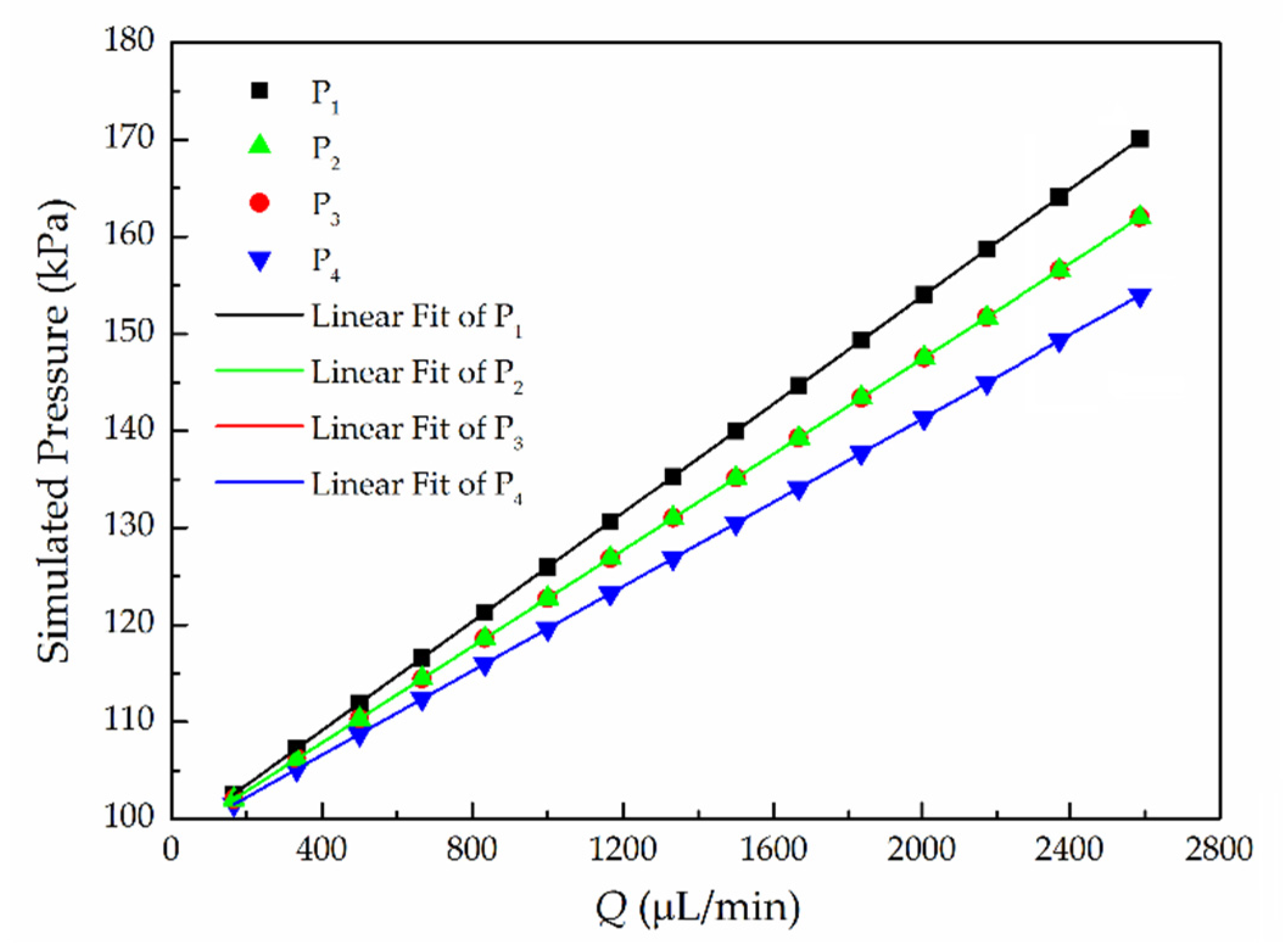
5.1. Simulation Analysis
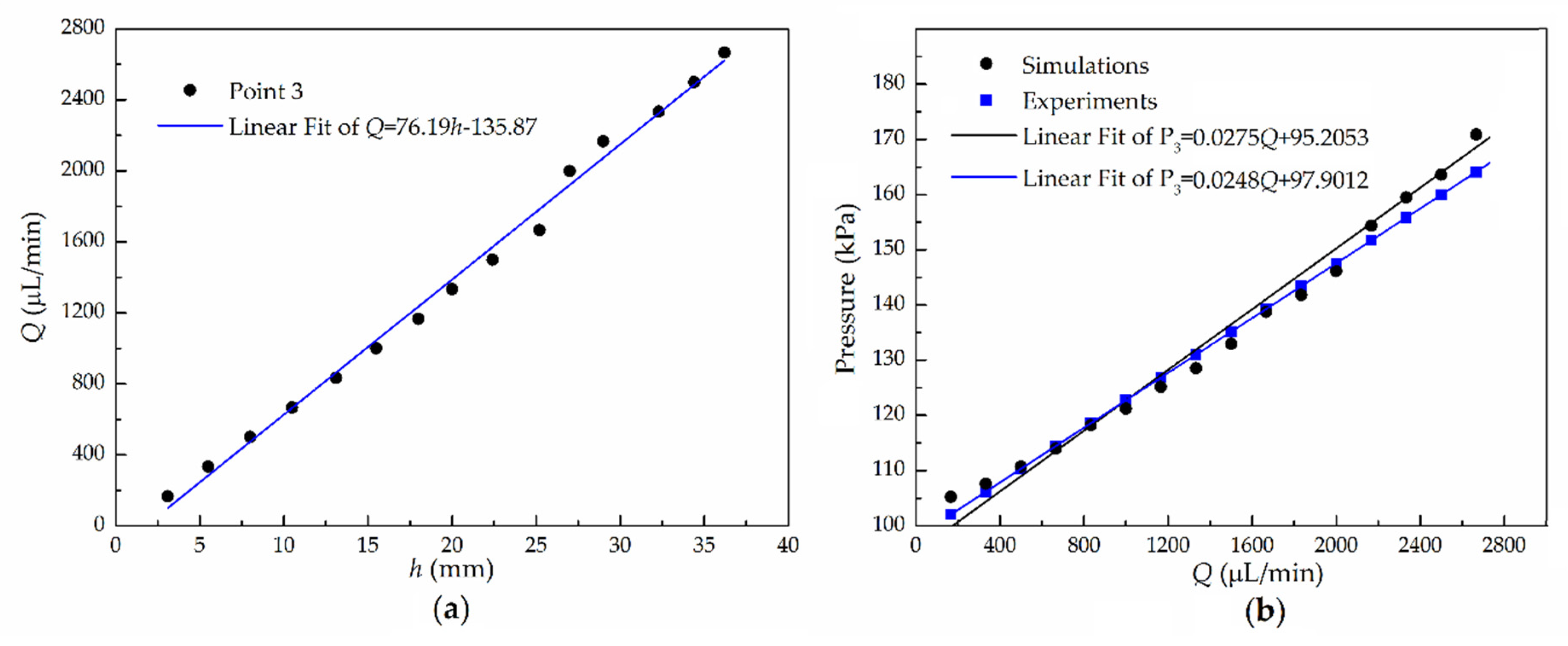
5.2. One-Point Pressure Measurement
5.3. Multiple-Points Pressure Measurement
5.4. Improvement and Application
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Wei, Y.; Nguyen, J.; Zheng, Y. Recent advances in microfluidic techniques for single-cell biophysical characterization. Lab Chip 2013, 13, 2464–2483. [Google Scholar]
- Monzawa, T.; Kaneko, M.; Tsai, C.H.D.; Sakuma, S.; Arai, F. On-chip actuation transmitter for enhancing the dynamic response of cell manipulation using a macro-scale pump. Biomicrofluidics 2015, 9, 12584–13412. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.D.; Sakuma, S.; Arai, F.; Taniguchi, T.; Ohtani, T.; Sakata, Y.; Kaneko, M. Geometrical alignment for improving cell evaluation in a microchannel with application on multiple myeloma red blood cells. RSC Adv. 2014, 4, 45050–45058. [Google Scholar] [CrossRef]
- Fultz, D.W.; Allen, J.S. Nonintrusive pressure measurement in microfluidic systems via backscattering interferometry. Exp. Fluids 2014, 55, 1754. [Google Scholar] [CrossRef]
- Sousa, P.C.; Pinho, F.T.; Oliveira, M.S.N.; Alves, M.A. Extensional flow of blood analog solutions in microfluidic devices. Biomicrofluidics 2011, 5, 14108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abkarian, M.; Stone, H.A.; Faivre, M. High-speed microfluidic differential manometer for cellular-scale hydrodynamics. Proc. Natl. Acad. Sci. USA 2006, 103, 538–542. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.J. Microfluidic quantification of blood pressure and compliance properties using velocity fields under periodic on–off blood flows. Appl. Sci. 2020, 10, 5273. [Google Scholar] [CrossRef]
- Suteria, N.S.; Nekouei, M.; Vanapalli, S.A. Microfluidic bypass manometry: Highly parallelized measurement of flow resistance of complex channel geometries and trapped droplets. Lab Chip 2018, 18, 343–355. [Google Scholar] [CrossRef]
- Vanapalli, S.A.; Nekouei, M.; Bithi, S.S. Bistability in the hydrodynamic resistance of a drop trapped at a microcavity junction. Microfluid. Nanofluid. 2017, 21, 1–10. [Google Scholar]
- Dosso, F.D.; Tripodi, L.; Spasic, D.; Kokalj, T.; Lammertyn, J. Innovative hydrophobic valve allows complex liquid manipulations in a self-powered channel-based microfluidic device. ACS Sens. 2019, 4, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, S.R. Immune-cell function under pressure. Nature 2019, 573, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Nakajima, M.; Takeuchi, M. On-chip self-assembly of cell embedded microstructures to vascular-like microtubes. Lab Chip 2014, 14, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Garstecki, P.; Gitlin, I.; DiLuzio, W.; Whitesides, G.M.; Kumacheva, E.; Stone, H.A. Formation of monodisperse bubbles in a microfluidic flow-focusing device. Appl. Phys. Lett. 2004, 85, 2649–2651. [Google Scholar] [CrossRef] [Green Version]
- Shen, F.; Xue, S.; Xu, M.; Pang, Y.; Liu, Z.M. Experimental study of single-particle trapping mechanisms into microcavities using microfluidics. Phys. Fluids 2019, 31, 42002. [Google Scholar]
- Liu, M.C.; Shih, H.C.; Wu, J.G.; Weng, T.W.; Wu, C.Y.; Lu, J.C.; Tung, Y.C. Electrofluidic pressure sensor embedded microfluidic device: A study of endothelial cells under hydrostatic pressure and shear stress combinations. Lab Chip 2013, 13, 1743–1753. [Google Scholar] [CrossRef]
- Christov, I.C.; Vincent, C.; Shidhore, T.C.; Stone, H.A. Flow rate-pressure drop relation for deformable shallow microfluidic channels. J. Fluid Mech. 2018, 841, 267–286. [Google Scholar] [CrossRef] [Green Version]
- Solomon, M.J.; Younger, J.G.; Hohne, D.N. Flexible microfluidic device for mechanical property characterization of soft viscoelastic solids such as bacterial biofilms. Langmuir 2009, 25, 7743–7751. [Google Scholar]
- Zheng, Y.; Tan, Q.; Chen, J. Classification of cell types using a microfluidic device for mechanical and electrical measurement on single cells. Lab Chip 2011, 11, 3174–3181. [Google Scholar]
- Tsai, C.D.; Sakuma, S.; Arai, F.; Kaneko, M. A new dimensionless index for evaluating cell Stiffness-Based deformability in microchannel. IEEE Trans. Biomed. Eng. 2014, 61, 1187–1195. [Google Scholar] [CrossRef]
- Abkarian, M.; Faivre, M.; Horton, R.; Smistrup, K.; Best-Popescu, C.A.; Stone, H.A. Cellular-scale hydrodynamics. Biomed. Mater. 2008, 3, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Yan, X.; Li, Y.; Liu, B. Static pressure-driven microfluidic gradient generator for long-term cell culture and adaptive cytoprotection analysis. Microfluid. Nanofluid. 2019, 23, 61–62. [Google Scholar] [CrossRef]
- Araci, I.E.; Su, B.; Quake, S.R.; Mandel, Y. An implantable microfluidic device for self-monitoring of intraocular pressure. Nat. Med. 2014, 20, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Chen, L.; Liu, X.; Zhao, B.; Zhang, H.; Wu, Z. Microfluidic contact lenses for unpowered, continuous and non-invasive intraocular pressure monitoring. Sens. Actuators A Phys. 2019, 295, 177–187. [Google Scholar] [CrossRef]
- Tsai, C.H.D.; Kaneko, M. On-chip pressure sensor using single-layer concentric chambers. Biomicrofluidics 2016, 10, 2464–2483. [Google Scholar] [CrossRef] [Green Version]
- Cheung, P.; Toda-Peters, K.; Shen, A.Q. In situ pressure measurement within deformable rectangular polydimethylsiloxane microfluidic devices. Biomicrofluidics 2012, 6, 26501. [Google Scholar] [CrossRef] [Green Version]
- Kamruzzaman, A.; Koksal, Y.A.; Yin, X.; Kazemi, H.; Ozkan, E.; Kaya, N.U. Non-invasive pressure sensing in microfluidic chips using laser interferometry. In Proceedings of the SPIE, Denver, CO, USA, 18 March 2019; Volume 10973, p. 109730M-1. [Google Scholar]
- Rodrigues, T.; Galindo-Rosales, F.J.; Campo-Deaño, L. Towards an optimal pressure tap design for Fluid-Flow characterisation at microscales. Materials 2019, 12, 1086. [Google Scholar] [CrossRef] [Green Version]
- Chaudhury, A.R.; Pantazis, A.K.; Chronis, N. An image contrast-based pressure sensor. Sens. Actuators A Phys. 2016, 245, 63–67. [Google Scholar] [CrossRef]
- Hoera, C.; Kiontke, A.; Pahl, M.; Belder, D. A chip-integrated optical microfluidic pressure sensor. Sens. Actuators B Chem. 2018, 255, 2407–2415. [Google Scholar] [CrossRef]
- Escudero, P.; Yeste, J.; Pascual-Izarra, C.; Villa, R.; Alvarez, M. Color tunable pressure sensors based on polymer nanostructured membranes for optofluidic applications. Sci. Rep. 2019, 9, 3259. [Google Scholar] [CrossRef]
- Dai, C.; Lu, P.; Chang, C.; Liu, C. Capacitive micro pressure sensor integrated with a ring oscillator circuit on chip. Sensors 2009, 9, 10158–10170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zhang, M.; Yang, M.; Zhu, W.; Wu, J.; Gong, X.; Wen, W. Polydimethylsiloxane-integratable micropressure sensor for microfluidic chips. Biomicrofluidics 2009, 3, 34105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Zhu, Y.; Nomani, M.W.; Wen, X.; Hsia, T.; Koley, G. A highly sensitive pressure sensor using a Au-patterned polydimethylsiloxane membrane for biosensing applications. J. Micromech. Microeng. 2013, 23, 25021–25022. [Google Scholar] [CrossRef]
- Eaton, W.P.; Smith, J.H. Micromachined pressure sensors: Review and recent developments. Smart Mater. Struct. 1997, 6, 530–539. [Google Scholar] [CrossRef]
- Kuoni, A.; Holzherr, R.L.; Boillat, M.; Rooij, N.F.D. Polyimide membrane with ZnO piezoelectric thin film pressure transducers as a differential pressure liquid flow sensor. J. Micromech. Microeng. 2003, 13, S103–S107. [Google Scholar] [CrossRef]
- Chang, Y.W.; Chu, H.C.; Lin, C.Y. A flexible piezoelectric sensor for microfluidic applications using polyvinylidene fluoride. IEEE Sens. J. 2008, 8, 495–500. [Google Scholar] [CrossRef]
- Lee, H.; Chung, K.; Lu, H. Multiplex pressure measurement in microsystems using volume displacement of particle suspensions. Lab Chip 2009, 9, 3345–3353. [Google Scholar]
- Gaber, N.; Altayyeb, A.; Soliman, S.; Sabry, Y.; Marty, F.; Bourouina, T. On-Channel integrated optofluidic pressure sensor with optically boosted sensitivity. Sensors 2019, 19, 944. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Psaltis, D. Optofluidic membrane interferometer: An imaging method for measuring microfluidic pressure and flow rate simultaneously on a chip. Biomicrofluidics 2011, 5, 44110. [Google Scholar] [CrossRef] [Green Version]
- Kohl, M.; Abdel-Khalik, S.; Jeter, S.; Sadowski, D. A microfluidic experimental platform with internal pressure measurements. Sens. Actuators A Phys. 2005, 118, 212–221. [Google Scholar] [CrossRef]
- Abdel-Khalik, S.I.; Kohl, M.J.; Jeter, S.M.; Sadowski, D.L. An experimental investigation of microchannel flow with internal pressure measurements. Int. J. Heat Mass Transf. 2005, 48, 1518–1533. [Google Scholar]
- Crozier, K.B.; Orth, A.; Schonbrun, E. Multiplexed pressure sensing with elastomer membranes. Lab Chip 2011, 11, 3810–3815. [Google Scholar]
- Ozsun, O.; Yakhot, V.; Ekinci, K.L. Noninvasive measurement of the pressure distribution in a deformable Micro-Channel. J. Fluid Mech. 2013, 734, R1. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.H.; Shim, T.S. Real-time pressure monitoring system for microfluidic devices using deformable colloidal crystal membrane. Lab Chip 2019, 19, 3954–3961. [Google Scholar] [CrossRef]
- Jung, T.; Yang, S. Highly stable liquid Metal-Based pressure sensor integrated with a microfluidic channel. Sensors 2015, 15, 11823–11835. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, Y.; Mori, H.; Niimi, T.; Uenishi, H.; Hirako, M. Development of pressure sensitive molecular film applicable to pressure measurement for high Knudsen number flows. Exp. Fluids 2007, 42, 543–550. [Google Scholar] [CrossRef]
- Matsuda, Y.; Misaki, R.; Yamaguchi, H.; Suzuki, S.; Uchida, T.; Niimi, T. Pressure-sensitive molecular film for investigation of micro gas flows. Microfluid. Nanofluid. 2011, 10, 165–171. [Google Scholar] [CrossRef]
- Huang, C.; Matsuda, Y.; Gregory, J.W.; Nagai, H.; Asai, K. The applications of pressure-sensitive paint in microfluidic systems. Microfluid. Nanofluid. 2015, 18, 739–753. [Google Scholar] [CrossRef]
- Huang, C.; Lai, C. Pressure measurements with molecule-based pressure sensors in straight and constricted PDMS microchannels. J. Micromech. Microeng. 2012, 22, 65021. [Google Scholar] [CrossRef]
- Banerjee, N.; Mastrangelo, C.H. Microballoon pressure sensors for particle imaging manometry in liquid and gaseous media. Analyst 2016, 141, 1413–1420. [Google Scholar] [CrossRef]
- Zhou, X.; Gui, L.; Zhang, R.; Li, L.; Liu, B.; Wang, L.; Deng, Z.; Zhang, L. A liquid metal based capacitive soft pressure microsensor. Lab Chip 2019, 19, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Suter, J.D.; Hohimer, C.J.; Fricke, J.M.; Christ, J. Principles of meniscus-based MEMS gas or liquid pressure sensors. J. Microelectromech. Syst. 2013, 22, 670–677. [Google Scholar] [CrossRef]
- Srivastava, N.; Burns, M.A. Microfluidic pressure sensing using trapped air compression. Lab Chip 2007, 7, 633–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Evans, A.T.; Kim, H. A rapid-prototyped on-chip vacuum gauge utilizing the volumetric expansion of trapped air in a sealed microchamber. In Proceedings of the International Conference on Miniaturized Systems for Chemistry and Life Sciences, Seattle, WA, USA, 2–6 October 2011; pp. 1119–1121. [Google Scholar]
- Tang, L.; Hong, W.; Wang, X.; Sun, W.; Yang, B.; Wei, M.; Pan, J.; Liu, J. Ultraminiature and flexible sensor based on interior corner flow for direct pressure sensing in biofluids. Small 2019, 15, 1900950. [Google Scholar] [CrossRef]
- Yu, N.; Liu, Y.; Ji, B.; Wang, S.; Chen, Y.; Sun, T.; Zhang, J.; Bai, Y. High-sensitivity microliter blood pressure sensors based on patterned micro-nanostructure arrays. Lab Chip 2020, 20, 1554–1561. [Google Scholar] [CrossRef]
- Holmes, R.I. Molar mass version of the ideal gas law points to a very low climate sensitivity. Earth Sci. 2017, 6, 157–163. [Google Scholar] [CrossRef]
- Yu, N.; Zhang, J.; Ye, S.; Wang, S.; Yang, B.; Nan, J.; Liu, H.; Ge, P. Integrated obstacle microstructures for gas-liquid separation and flow switching in microfluidic networks. Sens. Actuators B Chem. 2018, 256, 735–743. [Google Scholar] [CrossRef]
- Wang, S.; Yu, N.; Wang, T.; Ge, P.; Ye, S.; Xue, P.; Liu, W.; Shen, H.; Zhang, J.; Yang, B. Morphology-Patterned anisotropic wetting surface for fluid control and Gas-Liquid separation in microfluidics. ACS Appl. Mater. Interface 2016, 8, 13094–13103. [Google Scholar] [CrossRef]
- Zumdahl, S.S. Chemistry, 5th ed.; Houghton Mifflin Company: Boston, MA, USA, 2000. [Google Scholar]
- Shen, F.; Xiao, P.; Liu, Z. Microparticle image velocimetry (μPIV) study of microcavity flow at low Reynolds number. Microfluid. Nanofluid. 2015, 19, 403–417. [Google Scholar] [CrossRef]


| T (°C) | 0 | 10 | 20 | 30 | 40 | 50 |
|---|---|---|---|---|---|---|
| KH (L/kPa·m3) | 0.285 | 0.218 | 0.180 | 0.158 | 0.135 | 0.120 |
| T (°C) | 0 | 5 | 10 | 15 | 20 | 25 | 30 |
|---|---|---|---|---|---|---|---|
| σ (×10−2 N/m) | 7.56 | 7.49 | 7.42 | 7.35 | 7.28 | 7.21 | 7.12 |
| ρ (kg/m3) | 999.9 | 1000 | 999.7 | 999.1 | 998.2 | 997.1 | 995.7 |
| μ (×10−3 Pa·s) | 1.787 | 1.519 | 1.307 | 1.140 | 1.002 | 0.890 | 0.798 |
| Q (μL/min) | h1 (mm) | h2 (mm) | h3 (mm) | h4 (mm) |
|---|---|---|---|---|
| 167 | 1 | 1 | 1 | 0.8 |
| 333 | 4 | 3.6 | 3.4 | 3 |
| 500 | 7 | 6.3 | 6 | 5.5 |
| 667 | 10 | 9 | 8.8 | 8 |
| 833 | 12.5 | 11.5 | 11 | 10.5 |
| 1000 | 15.5 | 14 | 14 | 12.8 |
| 1167 | 18 | 16.5 | 16.5 | 15.5 |
| 1333 | 21.5 | 19.3 | 19.3 | 18 |
| 1667 | 27.5 | 25 | 25 | 23.5 |
| 2000 | 31.5 | 29 | 29 | 28 |
| 2333 | 37.5 | 34.5 | 34.5 | 34 |
| 2667 | 41 | 38 | 38 | 37 |
| h (mm) | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 20 | 30 | 40 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| papplied (kPa) | 101 | 102 | 103 | 104 | 105 | 106 | 107 | 108 | 110 | 111 | 112 | 127 | 147 | 174 |
| prelative (kPa) | 0 | 1.07 | 2.16 | 3.27 | 4.41 | 5.58 | 6.77 | 7.99 | 9.23 | 10.51 | 11.8 | 26.8 | 46.4 | 74.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, F.; Ai, M.; Ma, J.; Li, Z.; Xue, S. An Easy Method for Pressure Measurement in Microchannels Using Trapped Air Compression in a One-End-Sealed Capillary. Micromachines 2020, 11, 914. https://doi.org/10.3390/mi11100914
Shen F, Ai M, Ma J, Li Z, Xue S. An Easy Method for Pressure Measurement in Microchannels Using Trapped Air Compression in a One-End-Sealed Capillary. Micromachines. 2020; 11(10):914. https://doi.org/10.3390/mi11100914
Chicago/Turabian StyleShen, Feng, Mingzhu Ai, Jianfeng Ma, Zonghe Li, and Sen Xue. 2020. "An Easy Method for Pressure Measurement in Microchannels Using Trapped Air Compression in a One-End-Sealed Capillary" Micromachines 11, no. 10: 914. https://doi.org/10.3390/mi11100914





