The Effect of Diclofenac Sodium-Loaded Poly(Lactide-co-Glycolide) Rods on Bone Formation and Inflammation: A Histological and Histomorphometric Study in the Femora of Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Implant Material
2.2. Surgery
2.3. Evaluation Methods
2.3.1. Histology
2.3.2. Histomorphometry
2.4. Statistics
3. Results
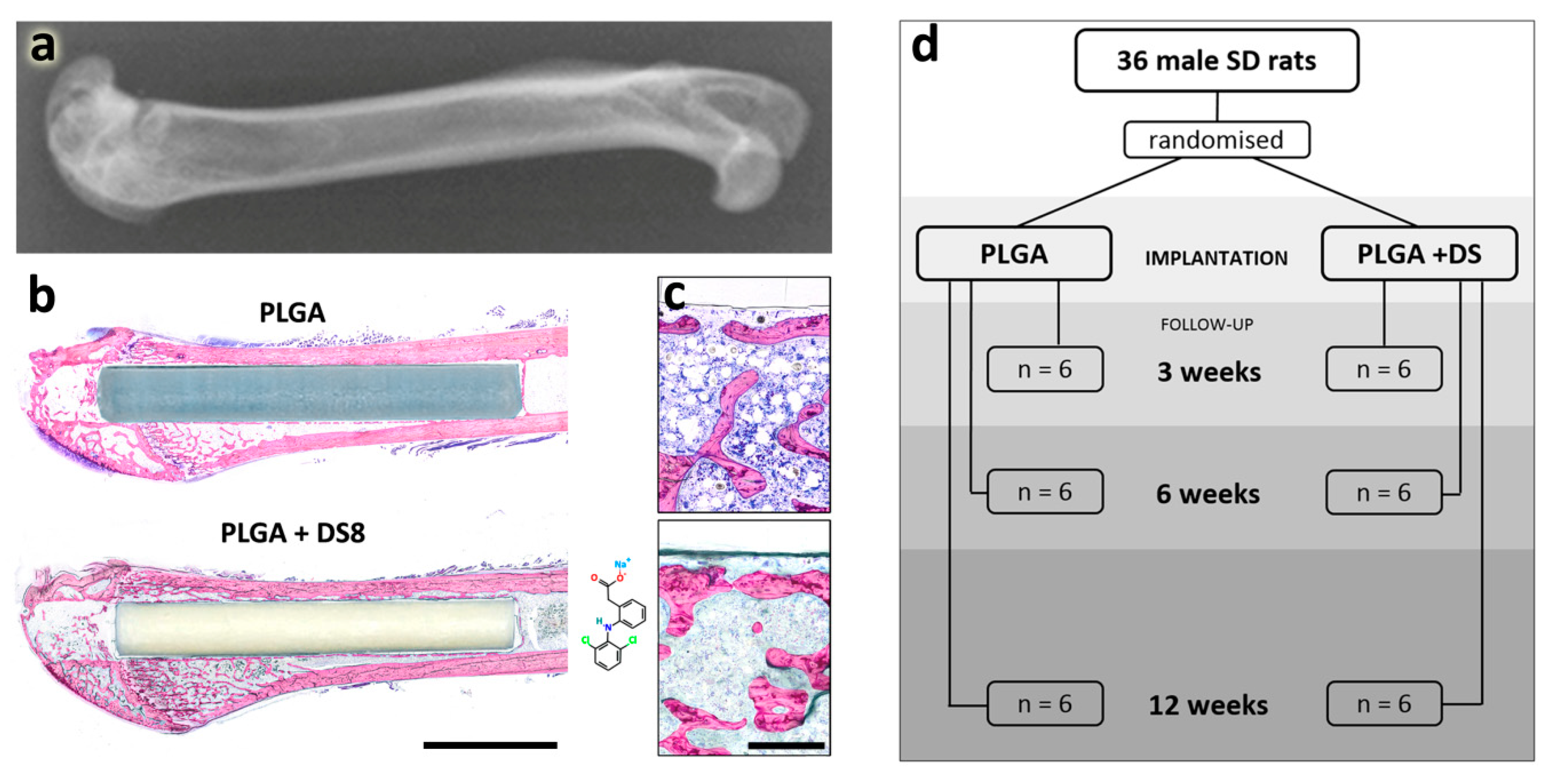
3.1. Macroscopic Results
3.2. Histological Findings
3.2.1. PLGA and PLGA+DS Implants
3.2.2. New Bone Formation
3.2.3. Cells at the Implant Surface
3.2.4. Inflammation
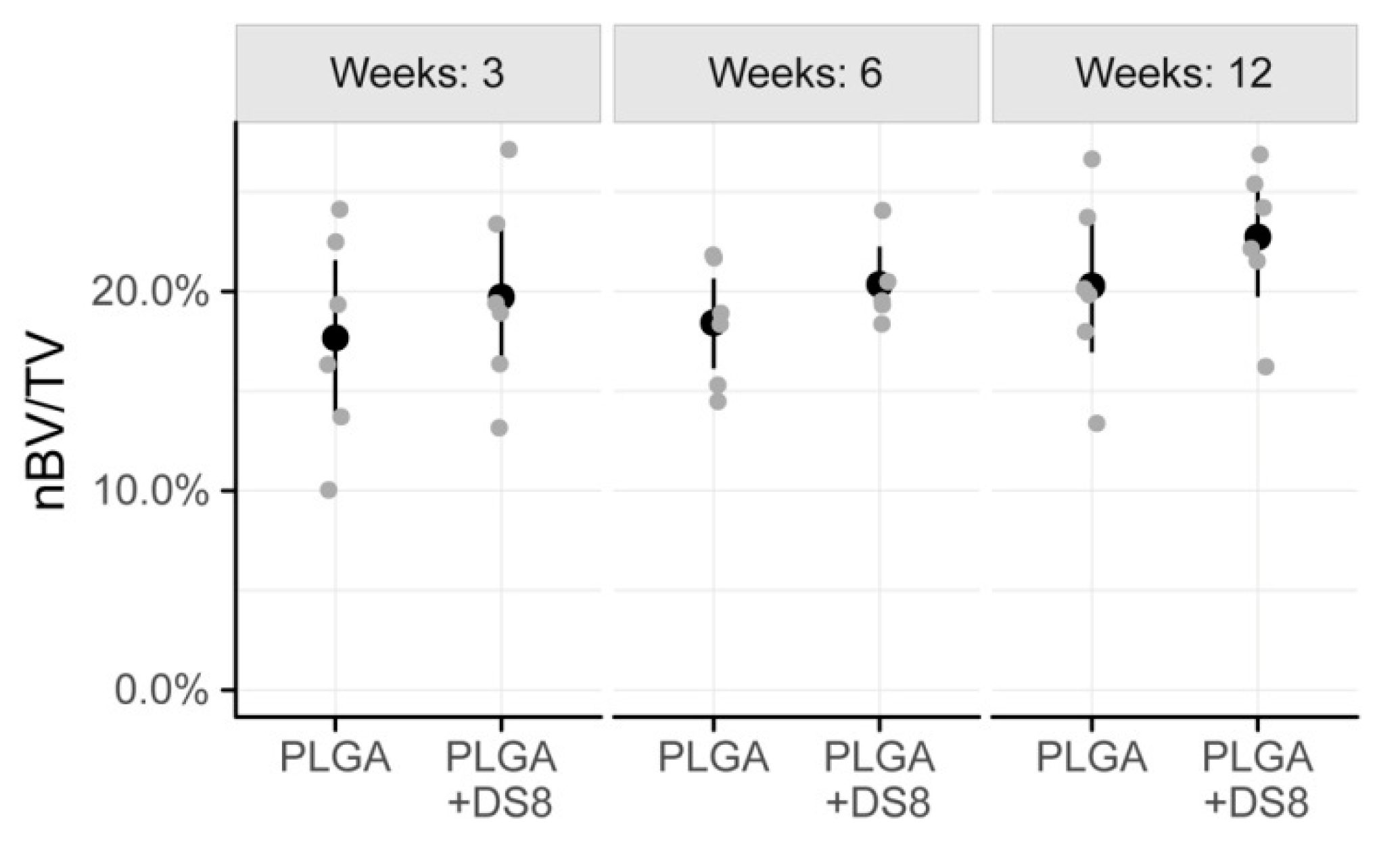
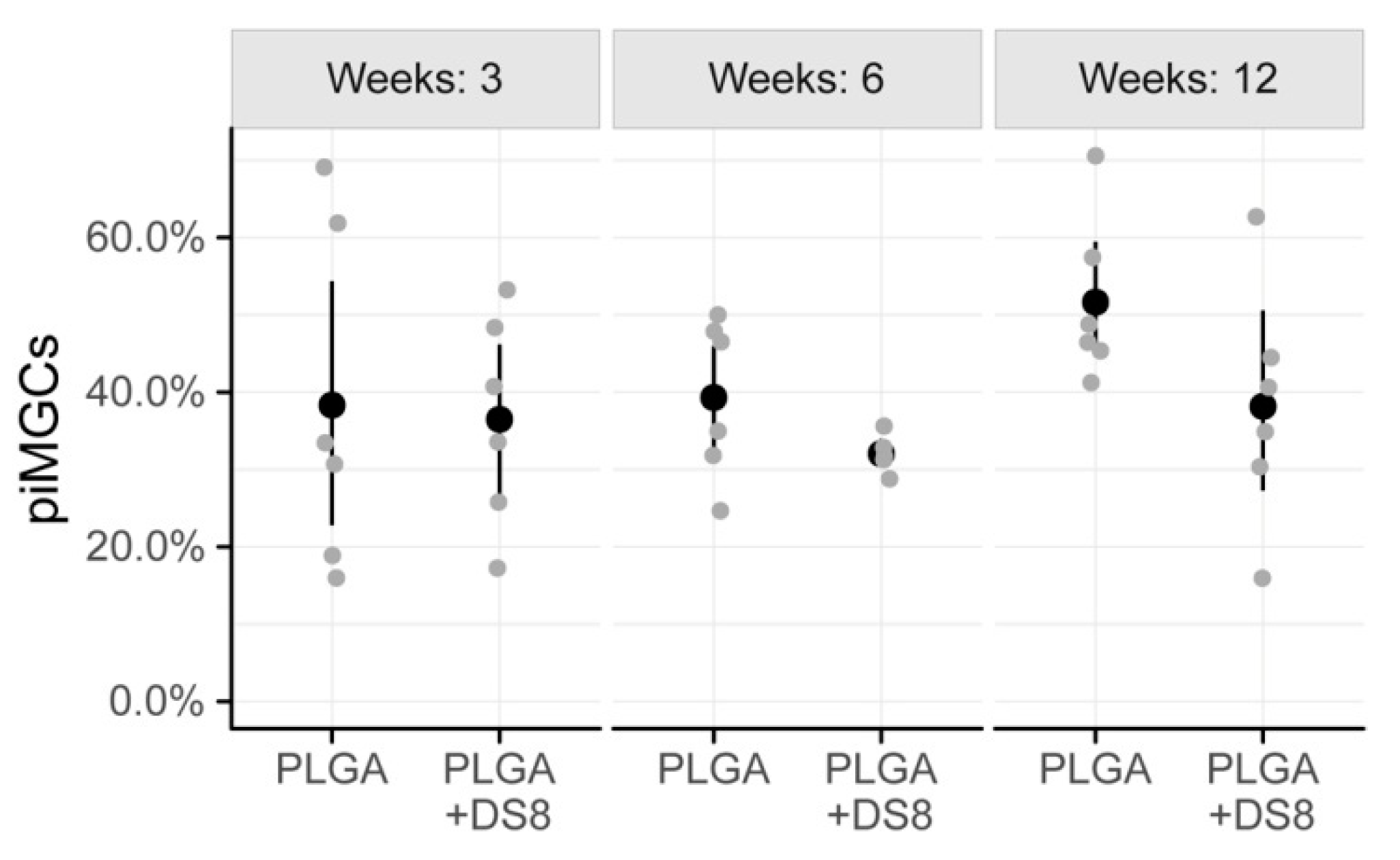
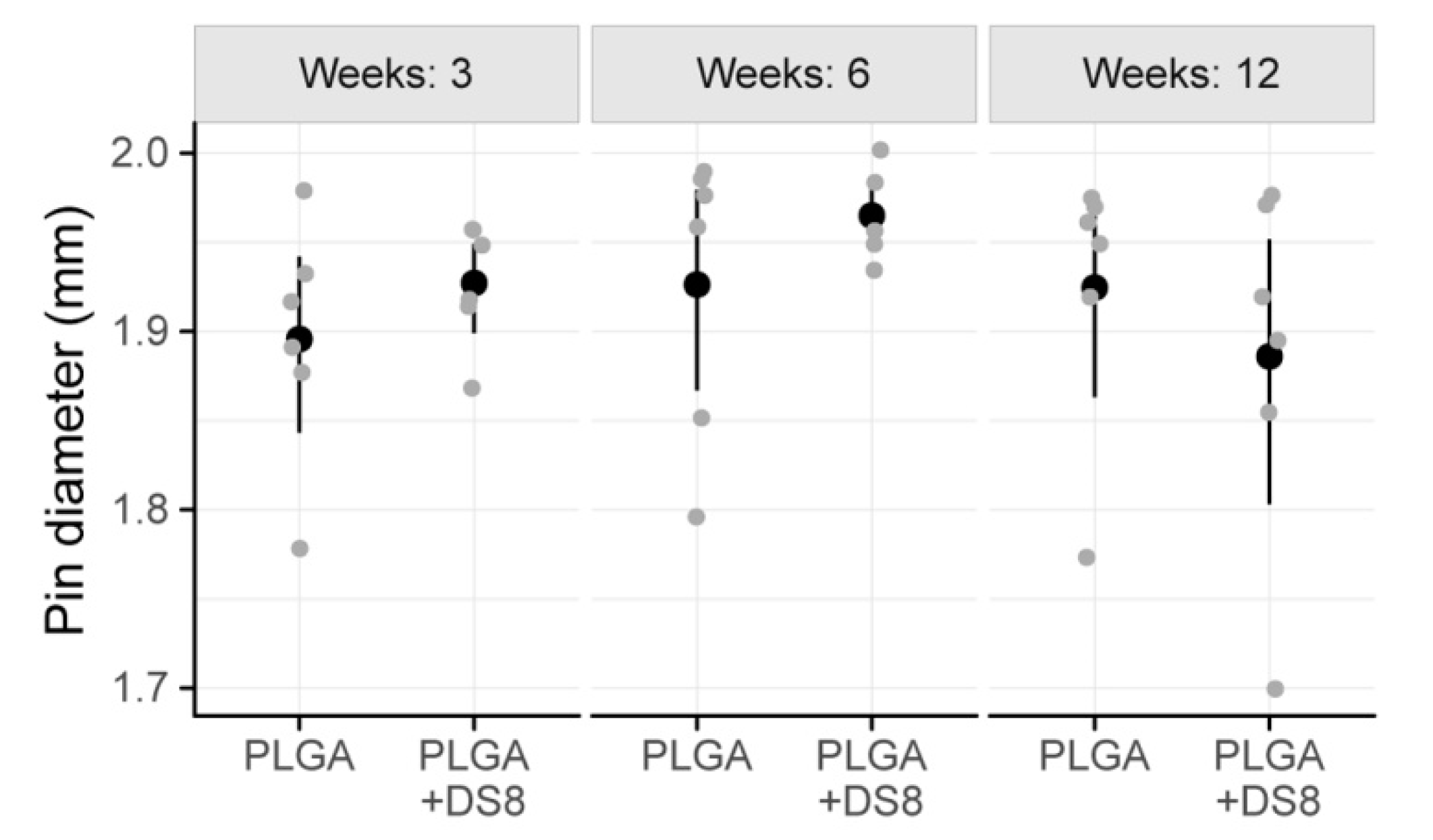
3.3. Histomorphometry
3.3.1. New Bone Volume per Tissue Volume (nBV/TV)
3.3.2. Peri-Implant Bone Capsule (piBC)
3.3.3. Peri-Implant Multinucleated Giant Cells (piMGCs)
3.3.4. Implant Diameter (I.DM)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Navarro, M.; Michiardi, A.; Castaño, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [Green Version]
- Jago, R.D.; Hindley, C.J. The removal of metalwork in children. Injury 1998, 29, 439–441. [Google Scholar] [CrossRef]
- Peterson, H.A. Metallic Implant Removal in Children. J. Pediatric Orthop. 2005, 25, 107–115. [Google Scholar]
- Loder, R.T.; Feinberg, J.R. Orthopaedic Implants in Children: Survey Results Regarding Routine Removal by the Pediatric and Nonpediatric Specialists. J. Pediatric Orthop. 2006, 26, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Grün, N.G.; Holweg, P.; Tangl, S.; Eichler, J.; Berger, L.; van den Beucken, J.J.J.P.; Löffler, J.F.; Klestil, T.; Weinberg, A.M. Comparison of a resorbable magnesium implant in small and large growing-animal models. Acta Biomater. 2018, 78, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Eppley, B.L.; Morales, L.; Wood, R.; Pensler, J.; Goldstein, J.; Havlik, R.J.; Habal, M.; Losken, A.; Williams, J.K.; Burstein, F.; et al. Resorbable PLLA-PGA plate and screw fixation in pediatric craniofacial surgery: Clinical experience in 1883 patients. Plast. Reconstr. Surg. 2004, 114, 850–856, discussion 857. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Peltoniemi, H.; Waris, E.; Suuronen, R.; Serlo, W.; Kellomäki, M.; Törmälä, P.; Waris, T. Developments in craniomaxillofacial surgery: Use of self-reinforced bioabsorbable osteofixation devices. Plast. Reconstr. Surg. 2001, 108, 167–180. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Renier, D.; Arnaud, E.; Marchac, D.; Ninkovic, M.; Donaway, D.; Jones, B.; Serlo, W.; Laurikainen, K.; Törmälä, P.; et al. Successful Use of Biosorb Osteofixation Devices in 165 Cranial and Maxillofacial Cases: A Multicenter Report. J. Craniofacial Surg. 2004, 15, 692–701. [Google Scholar] [CrossRef]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials (Basel) 2015, 8, 5744–5794. [Google Scholar] [CrossRef]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Félix Lanao, R.P.; Jonker, A.M.; Wolke, J.G.C.; Jansen, J.A.; van Hest, J.C.M.; Leeuwenburgh, S.C.G. Physicochemical properties and applications of poly(lactic-co-glycolic acid) for use in bone regeneration. Tissue Eng. Part B Rev. 2013, 19, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, R.N.; Aldabbagh, F.; Ronan, W.; Erxleben, A.; Rochev, Y.; McHugh, P. Effects of material thickness and processing method on poly(lactic-co-glycolic acid) degradation and mechanical performance. J. Mater. Sci. Mater. Med. 2016, 27, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [Green Version]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Böstman, O.; Päivärinta, U.; Partio, E.; Manninen, M.; Majola, A.; Vasenius, J.; Rokkanen, P. Absorbable polyglycolide screws in internal fixation of femoral osteotomies in rabbits. Acta Orthop. Scand. 1991, 62, 587–591. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Slamovich, E.B.; Webster, T.J. Less harmful acidic degradation of poly(lacticco-glycolic acid) bone tissue engineering scaffolds through titania nanoparticle addition. Int. J. Nanomed. 2006, 1, 541–545. [Google Scholar] [CrossRef] [Green Version]
- Böstman, O.; Hirvensalo, E.; Mäkinen, J.; Rokkanen, P. Foreign-body reactions to fracture fixation implants of biodegradable synthetic polymers. J. Bone Jt. Surg. Br. 1990, 72, 592–596. [Google Scholar] [CrossRef]
- Böstman, O.M.; Pihlajamäki, H.K. Adverse tissue reactions to bioabsorbable fixation devices. Clin. Orthop. Relat. Res. 2000, 317, 216–227. [Google Scholar] [CrossRef] [Green Version]
- Böstman, O.M. Osteolytic changes accompanying degradation of absorbable fracture fixation implants. J. Bone Jt. Surg. Br. 1991, 73, 679–682. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. FOREIGN BODY REACTION TO BIOMATERIALS. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.M. Chapter 4 Mechanisms of inflammation and infection with implanted devices. Cardiovasc. Pathol. 1993, 2, 33–41. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Rokkanen, P. Absorbable polyglycolide devices in trauma and bone surgery. Biomaterials 1997, 18, 3–9. [Google Scholar] [CrossRef]
- Böstman, O.; Pihlajamäki, H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: A review. Biomaterials 2000, 21, 2615–2621. [Google Scholar] [CrossRef]
- Rokkanen, P.U.; Böstman, O.; Hirvensalo, E.; Mäkelä, E.A.; Partio, E.K.; Pätiälä, H.; Vainionpää, S.; Vihtonen, K.; Törmälä, P. Bioabsorbable fixation in orthopaedic surgery and traumatology. Biomaterials 2000, 21, 2607–2613. [Google Scholar] [CrossRef]
- Anderson, J.M.; Shive, M.S. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 1997, 28, 5–24. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chang, C.-H.; Lu, Y.-C.; Chang, C.-H.; Tsai, C.-C.; Huang, C.-H. Late foreign-body reaction after treatment of distal radial fractures with poly-L-lactic acid bioabsorbable implants: A report of three cases. J. Bone Jt. Surg. Am. 2010, 92, 2719–2724. [Google Scholar] [CrossRef]
- Landes, C.A.; Ballon, A.; Roth, C. Maxillary and mandibular osteosyntheses with PLGA and P(L/DL)LA implants: A 5-year inpatient biocompatibility and degradation experience. Plast. Reconstr. Surg. 2006, 117, 2347–2360. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Törmälä, P. From Past to Present and Future Is Today: From Inert to Multifunctional Biomaterials. J. Craniofacial Surg. 2004, 15, 897. [Google Scholar] [CrossRef]
- Hickey, T.; Kreutzer, D.; Burgess, D.J.; Moussy, F. In vivo evaluation of a dexamethasone/PLGA microsphere system designed to suppress the inflammatory tissue response to implantable medical devices. J. Biomed. Mater. Res. 2002, 61, 180–187. [Google Scholar] [CrossRef]
- Yoon, J.J.; Kim, J.H.; Park, T.G. Dexamethasone-releasing biodegradable polymer scaffolds fabricated by a gas-foaming/salt-leaching method. Biomaterials 2003, 24, 2323–2329. [Google Scholar] [CrossRef]
- Khaled, K.A.; Sarhan, H.A.; Ibrahim, M.A.; Ali, A.H.; Naguib, Y.W. Prednisolone-loaded PLGA microspheres. in vitro characterization and in vivo application in adjuvant-induced arthritis in mice. Aaps Pharmscitech 2010, 11, 859–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, C.V.; Ben-David, D.; Dhillon, A.; Kuhn, G.; Gould, T.W.A.; Müller, R.; Rose, F.R.A.J.; Shakesheff, K.M.; Livne, E. Controlled release of BMP-2 from a sintered polymer scaffold enhances bone repair in a mouse calvarial defect model. J. Tissue Eng. Regen. Med. 2014, 8, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Riggin, C.N.; Qu, F.; Kim, D.H.; Huegel, J.; Steinberg, D.R.; Kuntz, A.F.; Soslowsky, L.J.; Mauck, R.L.; Bernstein, J. Electrospun PLGA Nanofiber Scaffolds Release Ibuprofen Faster and Degrade Slower After In Vivo Implantation. Ann. Biomed. Eng. 2017, 45, 2348–2359. [Google Scholar] [CrossRef] [Green Version]
- Chvatal, S.A.; Kim, Y.-T.; Bratt-Leal, A.M.; Lee, H.; Bellamkonda, R.V. Spatial distribution and acute anti-inflammatory effects of Methylprednisolone after sustained local delivery to the contused spinal cord. Biomaterials 2008, 29, 1967–1975. [Google Scholar] [CrossRef] [Green Version]
- Huolman, R.; Ashammakhi, N. New Multifunctional Anti-Osteolytic Releasing Bioabsorbable Implant. J. Craniofacial Surg. 2007, 18, 295–301. [Google Scholar] [CrossRef]
- Viitanen, P.; Suokas, E.; Törmälä, P.; Ashammakhi, N. Release of diclofenac sodium from polylactide-co-glycolide 80/20 rods. J. Mater. Sci. Mater. Med. 2006, 17, 1267–1274. [Google Scholar] [CrossRef]
- Nikkola, L.; Viitanen, P.; Ashammakhi, N. Temporal control of drug release from biodegradable polymer: Multicomponent diclofenac sodium releasing PLGA 80/20 rod. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 89, 518–526. [Google Scholar] [CrossRef]
- Nikkola, L.; Morton, T.; Balmayor, E.R.; Jukola, H.; Harlin, A.; Redl, H.; van Griensven, M.; Ashammakhi, N. Fabrication of electrospun poly(D,L lactide-co-glycolide)80/20 scaffolds loaded with diclofenac sodium for tissue engineering. Eur. J. Med. Res. 2015, 20, 54. [Google Scholar] [CrossRef] [Green Version]
- Donath, K. Die Trenn-Dünnschliff-Technik zur Herstellung histologischer Präparate von nicht schneidbaren Geweben und Materialien. Der Präparator 1988, 34, 10. [Google Scholar]
- Laczko, J.; Levai, G. A simple differential staining method for semi-thin sections of ossifying cartilage and bone tissues embedded in epoxy resin. Mikroskopie 1975, 31, 1–4. [Google Scholar]
- Al-Maawi, S.; Orlowska, A.; Sader, R.; James Kirkpatrick, C.; Ghanaati, S. In vivo cellular reactions to different biomaterials—Physiological and pathological aspects and their consequences. Semin. Immunol. 2017, 29, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Aherne, W.; Dunnill, M. Point counting and the estimation of volume fraction. Morphometry 1982, 33, 45. [Google Scholar]
- Merz, W.; Schenk, R. A quantitative histological study on bone formation in human cancellous bone. Cells Tissues Organs 1970, 76, 1–15. [Google Scholar] [CrossRef]
- Kimmel, D.B.; Jee, W.S.S. A quantitative histologic analysis of the growing long bone metaphysis. Calcif. Tissue Int. 1980, 32, 113–122. [Google Scholar] [CrossRef]
- Ranjan, V.; Chakrabarty, S.; Arora, P.; Rastogi, T. Classifying giant cell lesions: A review. J. Indian Acad. Oral Med. Radiol. 2018, 30, 297. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. J. Math. Methods Biosci. 2008, 50, 346–363. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing (Version 3.6.1.); R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Trindade, R.; Albrektsson, T.; Tengvall, P.; Wennerberg, A. Foreign Body Reaction to Biomaterials: On Mechanisms for Buildup and Breakdown of Osseointegration. Clin. Implant Dent. Relat. Res. 2016, 18, 192–203. [Google Scholar] [CrossRef]
- Kolar, P.; Schmidt-Bleek, K.; Schell, H.; Gaber, T.; Toben, D.; Schmidmaier, G.; Perka, C.; Buttgereit, F.; Duda, G.N. The Early Fracture Hematoma and Its Potential Role in Fracture Healing. Tissue Eng. Part B Rev. 2010, 16, 427–434. [Google Scholar] [CrossRef]
- Pape, H.-C.; Marcucio, R.; Humphrey, C.; Colnot, C.; Knobe, M.; Harvey, E.J. Trauma-Induced Inflammation and Fracture Healing. J. Orthop. Trauma 2010, 24, 522–525. [Google Scholar] [CrossRef]
- Mountziaris, P.M.; Spicer, P.P.; Kasper, F.K.; Mikos, A.G. Harnessing and Modulating Inflammation in Strategies for Bone Regeneration. Tissue Eng. Part B Rev. 2011, 17, 393–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mountziaris, P.M.; Mikos, A.G. Modulation of the Inflammatory Response for Enhanced Bone Tissue Regeneration. Tissue Eng. Part B Rev. 2008, 14, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Boehler, R.M.; Graham, J.G.; Shea, L.D. Tissue engineering tools for modulation of the immune response. BioTechniques 2011, 51, 239–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shields, C.W.; Wang, L.L.-W.; Evans, M.A.; Mitragotri, S. Materials for Immunotherapy. Adv. Mater. 2020, 32, 1901633. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Triffitt, J.T. A review on macrophage responses to biomaterials. Biomed. Mater. 2006, 1, R1–R9. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Booms, P.; Unger, R.; Hoffmann, V.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Multinucleated giant cells in the implant bed of bone substitutes are foreign body giant cells—New insights into the material-mediated healing process. J. Biomed. Mater. Res. Part A 2017, 105, 1105–1111. [Google Scholar] [CrossRef]
- Piattelli, A.; Franco, M.; Ferronato, G.; Santello, M.T.; Martinetti, R.; Scarano, A. Resorption of composite polymer—hydroxyapatite membranes: A time-course study in rabbit. Biomaterials 1997, 18, 629–633. [Google Scholar] [CrossRef]
- Solheim, E.; Sudmann, B.; Bang, G.; Sudmann, E. Biocompatibility and effect on osteogenesis of poly(ortho ester) compared to poly(DL-lactic acid). J. Biomed. Mater. Res. 2000, 49, 257–263. [Google Scholar] [CrossRef]
- Miron, R.J.; Bosshardt, D.D. Multinucleated Giant Cells: Good Guys or Bad Guys? Tissue Eng. Part B Rev. 2017, 24, 53–65. [Google Scholar] [CrossRef]
- Lorenz, J.; Barbeck, M.; Sader, R.A.; Kirkpatrick, C.J.; Russe, P.; Choukroun, J.; Ghanaati, S. Foreign Body Giant Cell–Related Encapsulation of a Synthetic Material Three Years After Augmentation. J. Oral Implant. 2016, 42, 273–277. [Google Scholar] [CrossRef]
- Sidney, L.E.; Heathman, T.R.J.; Britchford, E.R.; Abed, A.; Rahman, C.V.; Buttery, L.D.K. Investigation of Localized Delivery of Diclofenac Sodium from Poly(D,L-Lactic Acid-co-Glycolic Acid)/Poly(Ethylene Glycol) Scaffolds Using an In Vitro Osteoblast Inflammation Model. Tissue Eng. Part A 2014, 21, 362–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuolteenaho, K.; Moilanen, T.; Moilanen, E. Non-Steroidal Anti-Inflammatory Drugs, Cyclooxygenase-2 and the Bone Healing Process. Basic Clin. Pharmacol. Toxicol. 2008, 102, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Aspenberg, P. Drugs and fracture repair. Acta Orthop. 2005, 76, 741–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisowska, B.; Kosson, D.; Domaracka, K. Lights and shadows of NSAIDs in bone healing: The role of prostaglandins in bone metabolism. Drug Des. Dev. Ther. 2018, 12, 1753–1758. [Google Scholar] [CrossRef] [Green Version]
- Harder, A.T.; An, Y.H. The Mechanisms of the Inhibitory Effects of Nonsteroidal Anti-Inflammatory Drugs on Bone Healing: A Concise Review. J. Clin. Pharmacol. 2003, 43, 807–815. [Google Scholar] [CrossRef]
- Ho, M.-L.; Chang, J.-K.; Chuang, L.-Y.; Hsu, H.-K.; Wang, G.-J. Effects of nonsteroidal anti-inflammatory drugs and prostaglandins on osteoblastic functions. Biochem. Pharmacol. 1999, 58, 983–990. [Google Scholar] [CrossRef]
- Krischak, G.D.; Augat, P.; Blakytny, R.; Claes, L.; Kinzl, L.; Beck, A. The non-steroidal anti-inflammatory drug diclofenac reduces appearance of osteoblasts in bone defect healing in rats. Arch Orthop. Trauma Surg. 2007, 127, 453–458. [Google Scholar] [CrossRef]
- García-Martínez, O.; De Luna-Bertos, E.; Ramos-Torrecillas, J.; Manzano-Moreno, F.J.; Ruiz, C. Repercussions of NSAIDS drugs on bone tissue: The osteoblast. Life Sci. 2015, 123, 72–77. [Google Scholar] [CrossRef]
- Karakawa, A.; Sano, T.; Amano, H.; Yamada, S. Inhibitory Mechanism of Non-steroidal Anti-inflammatory Drugs on Osteoclast Differentiation and Activation. J. Oral Biosci. 2010, 52, 119–124. [Google Scholar] [CrossRef]
- Lumawig, J.M.T.; Yamazaki, A.; Watanabe, K. Dose-dependent inhibition of diclofenac sodium on posterior lumbar interbody fusion rates. Spine J. 2009, 9, 343–349. [Google Scholar] [CrossRef]
- Gerstenfeld, L.C.; Einhorn, T.A. COX inhibitors and their effects on bone healing. Expert Opin. Drug Saf. 2004, 3, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.; MacDonald, D.A.; Matthews, S.J.; Smith, R.M.; Furlong, A.J.; De Boer, P. Nonunion of the femoral diaphysis|The Bone & Joint Journal. J. Bone Jt. Surg. Br. 2000, 82, 655–658. [Google Scholar]
- Bhattacharyya, T.; Levin, R.; Vrahas, M.S.; Solomon, D.H. Nonsteroidal antiinflammatory drugs and nonunion of humeral shaft fractures. Arthritis Care Res. Off. J. Am. Coll. Rheumatol. 2005, 53, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Krischak, G.; Sorg, T.; Augat, P.; Farker, K.; Merkel, U.; Kinzl, L.; Claes, L. Influence of diclofenac (group of nonsteroidal anti-inflammatory drugs) on fracture healing. Arch Orthop. Trauma Surg. 2003, 123, 327–332. [Google Scholar] [CrossRef]
- Mouriño, V.; Boccaccini, A.R. Bone tissue engineering therapeutics: Controlled drug delivery in three-dimensional scaffolds. J. R. Soc. Interface 2010, 7, 209–227. [Google Scholar] [CrossRef] [Green Version]
- Simon, A.M.; O’Connor, J.P. Dose and Time-Dependent Effects of Cyclooxygenase-2 Inhibition on Fracture-Healing. JBJS 2007, 89, 500–511. [Google Scholar] [CrossRef]
- Gerstenfeld, L.C.; Al-Ghawas, M.; Alkhiary, Y.M.; Cullinane, D.M.; Krall, E.A.; Fitch, J.L.; Webb, E.G.; Thiede, M.A.; Einhorn, T.A. Selective and Nonselective Cyclooxygenase-2 Inhibitors and Experimental Fracture-Healing: Reversibility of Effects After Short-Term Treatment. JBJS 2007, 89, 114–125. [Google Scholar] [CrossRef]
- Miller, R.A.; Brady, J.M.; Cutright, D.E. Degradation rates of oral resorbable implants (polylactates and polyglycolates): Rate modification with changes in PLA/PGA copolymer ratios. J. Biomed. Mater. Res. 1977, 11, 711–719. [Google Scholar] [CrossRef]
- Tiainen, J.; Soini, Y.; Törmälä, P.; Waris, T.; Ashammakhi, N. Self-reinforced polylactide/polyglycolide 80/20 screws take more than 1½ years to resorb in rabbit cranial bone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 70B, 49–55. [Google Scholar] [CrossRef]
- Landes, C.A.; Ballon, A.; Roth, C. In-patient versus in vitro degradation of P(L/DL)LA and PLGA. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 76B, 403–411. [Google Scholar] [CrossRef]
- Weiser, J.R.; Saltzman, W.M. Controlled release for local delivery of drugs: Barriers and models. J. Control. Release 2014, 190, 664–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltzman, W.M.; Radomsky, M.L. Drugs released from polymers: Diffusion and elimination in brain tissue. Chem. Eng. Sci. 1991, 46, 2429–2444. [Google Scholar] [CrossRef]
- Carnicer-Lombarte, A.; Barone, D.G.; Dimov, I.B.; Hamilton, R.S.; Prater, M.; Zhao, X.; Rutz, A.L.; Malliaras, G.G.; Lacour, S.P.; Bryant, C.E.; et al. Mechanical matching of implant to host minimises foreign body reaction. bioRxiv 2019. [Google Scholar] [CrossRef] [Green Version]
- Bosshardt, D.D.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Lang, N.P. The role of bone debris in early healing adjacent to hydrophilic and hydrophobic implant surfaces in man. Clin. Oral Implant. Res. 2011, 22, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Donath, K.; Laaß, M.; Günzl, H.-J. The histopathology of different foreign-body reactions in oral soft tissue and bone tissue. Vichows Arch. A Pathol. Anat. 1992, 420, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Kastellorizios, M.; Papadimitrakopoulos, F.; Burgess, D.J. Prevention of foreign body reaction in a pre-clinical large animal model. J. Control. Release 2015, 202, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Khouw, I.M.S.L.; van Wachem, P.B.; Molema, G.; Plantinga, J.A.; de Leij, L.F.; van Luyn, M.J. The foreign body reaction to a biodegradable biomaterial differs between rats and mice. J. Biomed. Mater. Res. 2000, 52, 439–446. [Google Scholar] [CrossRef]
- Kidd, K.R.; Ponte, D.B.D.; Kellar, R.S.; Williams, S.K. A comparative evaluation of the tissue responses associated with polymeric implants in the rat and mouse. J. Biomed. Mater. Res. 2002, 59, 682–689. [Google Scholar] [CrossRef]




| Treatment Group | Follow-up Period | Difference | Std. Error | p-Value | Statistical Significance |
|---|---|---|---|---|---|
| PLGA | 3 weeks vs. 6 weeks | −0.19 | 0.06 | 0.0110 | * |
| PLGA | 3 weeks vs. 12 weeks | −0.20 | 0.06 | 0.0068 | * |
| PLGA | 6 weeks vs. 12 weeks | −0.01 | 0.06 | 0.9999 | |
| PLGA+DS | 3 weeks vs. 6 weeks | −0.11 | 0.07 | 0.3902 | |
| PLGA+DS | 3 weeks vs. 12 weeks | −0.24 | 0.06 | <0.001 | * |
| PLGA+DS | 6 weeks vs. 12 weeks | −0.13 | 0.06 | 0.1502 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reich, K.M.; Viitanen, P.; Apu, E.H.; Tangl, S.; Ashammakhi, N. The Effect of Diclofenac Sodium-Loaded Poly(Lactide-co-Glycolide) Rods on Bone Formation and Inflammation: A Histological and Histomorphometric Study in the Femora of Rats. Micromachines 2020, 11, 1098. https://doi.org/10.3390/mi11121098
Reich KM, Viitanen P, Apu EH, Tangl S, Ashammakhi N. The Effect of Diclofenac Sodium-Loaded Poly(Lactide-co-Glycolide) Rods on Bone Formation and Inflammation: A Histological and Histomorphometric Study in the Femora of Rats. Micromachines. 2020; 11(12):1098. https://doi.org/10.3390/mi11121098
Chicago/Turabian StyleReich, Karoline M., Petrus Viitanen, Ehsanul Hoque Apu, Stefan Tangl, and Nureddin Ashammakhi. 2020. "The Effect of Diclofenac Sodium-Loaded Poly(Lactide-co-Glycolide) Rods on Bone Formation and Inflammation: A Histological and Histomorphometric Study in the Femora of Rats" Micromachines 11, no. 12: 1098. https://doi.org/10.3390/mi11121098







