Additive Manufacturing of Micromanipulator Mounted on a Glass Capillary for Biological Applications
Abstract
:1. Introduction
2. Materials and Methods
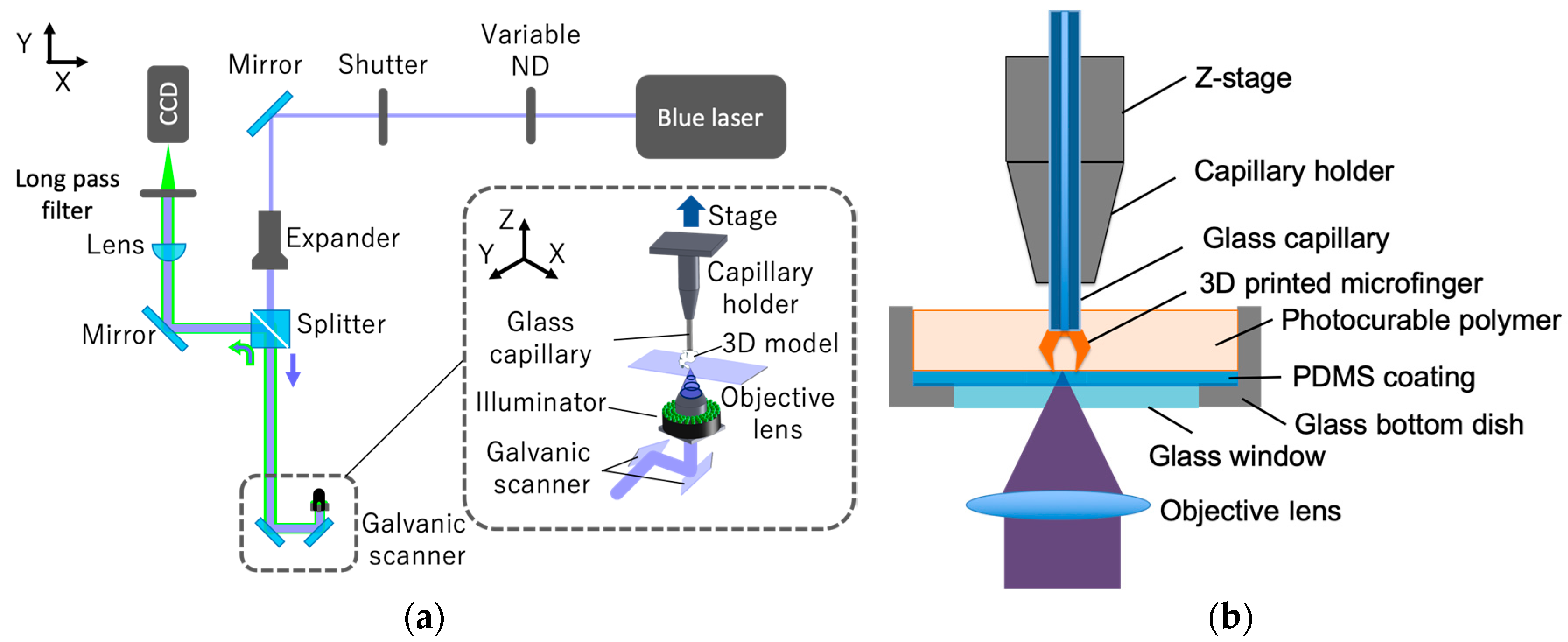
2.1. High-Resolution Microstereolithography System Using a 405-nm Blue Laser
2.2. Surface Coating of Bottom Dish and Glass Capillary
2.3. Photocurable Polymer for High-Resolution Microstereolithography Using 405-nm Blue Laser
2.4. Preparation of NIH/3T3 Spheroids
3. Results and Discussion
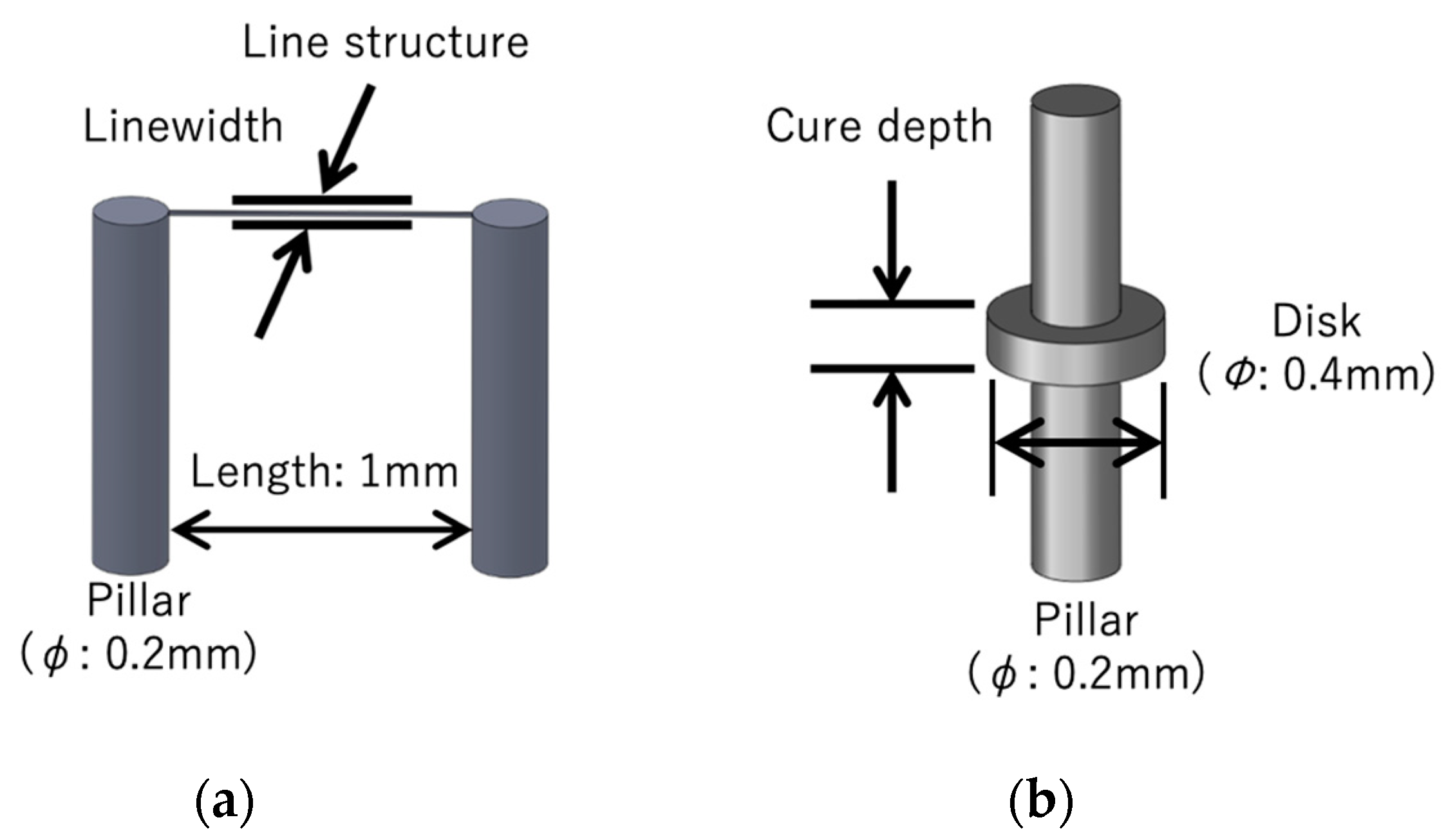
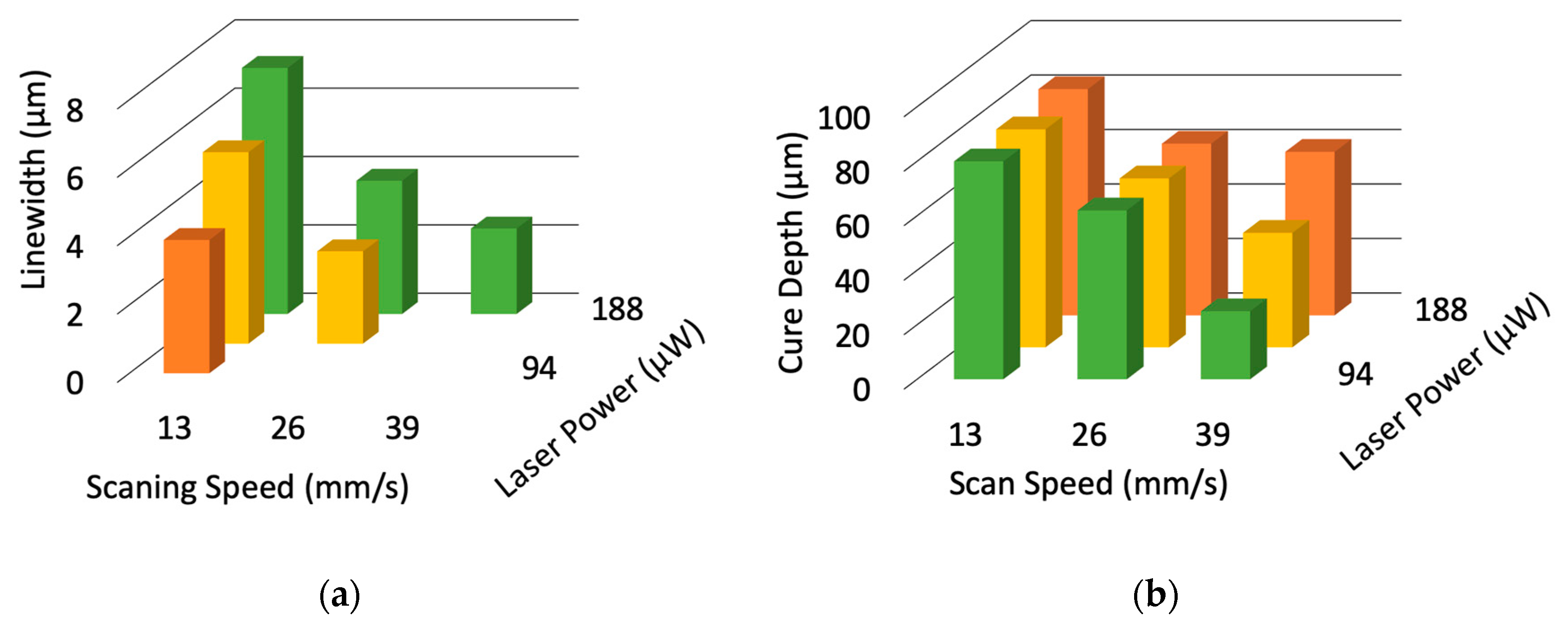
3.1. Resolution Evaluation of the Microstereolithography Using 405-nm Blue Laser
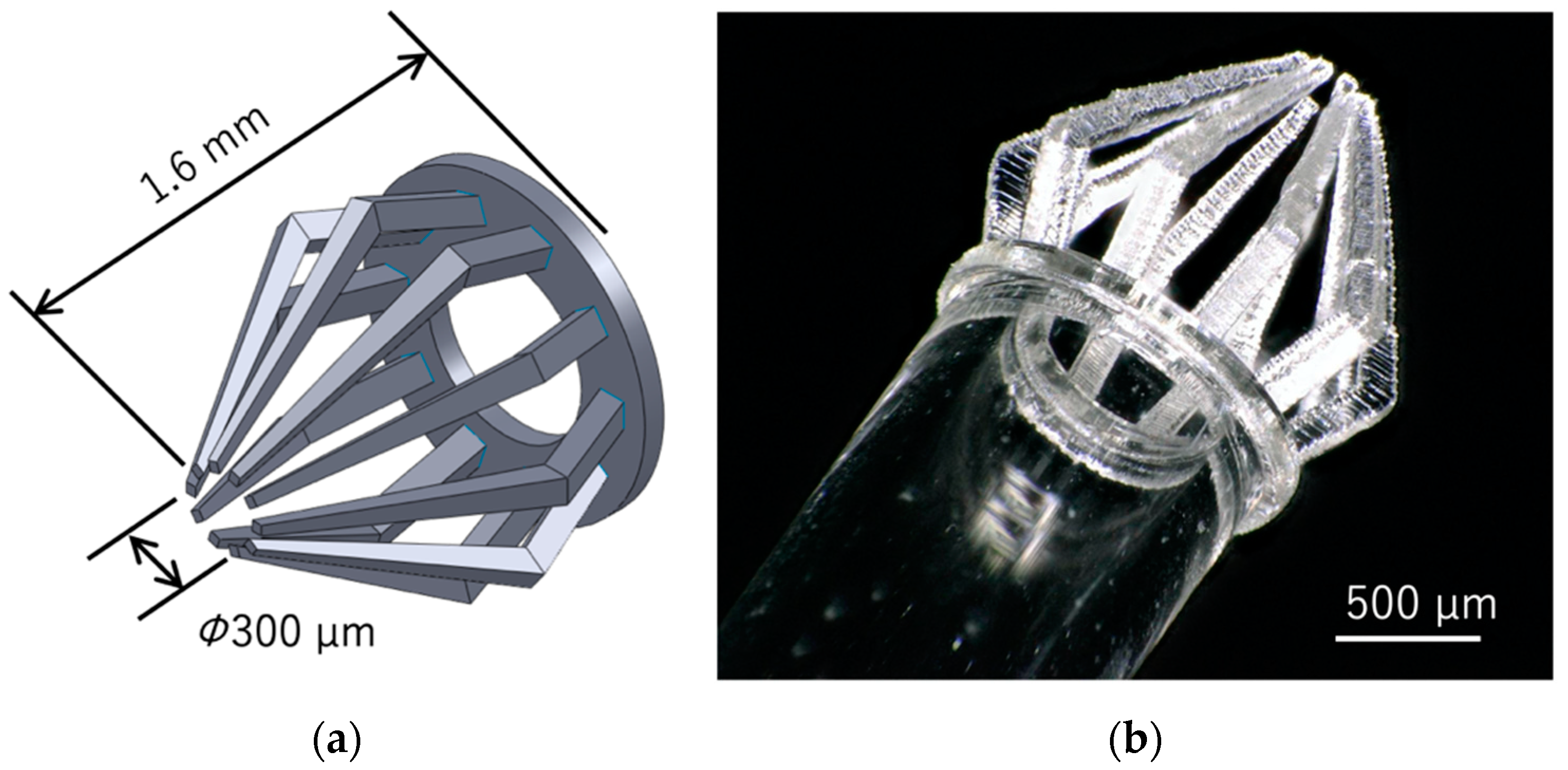
3.2. Fabrication of a Micromanipularor for Handling a Spheroid
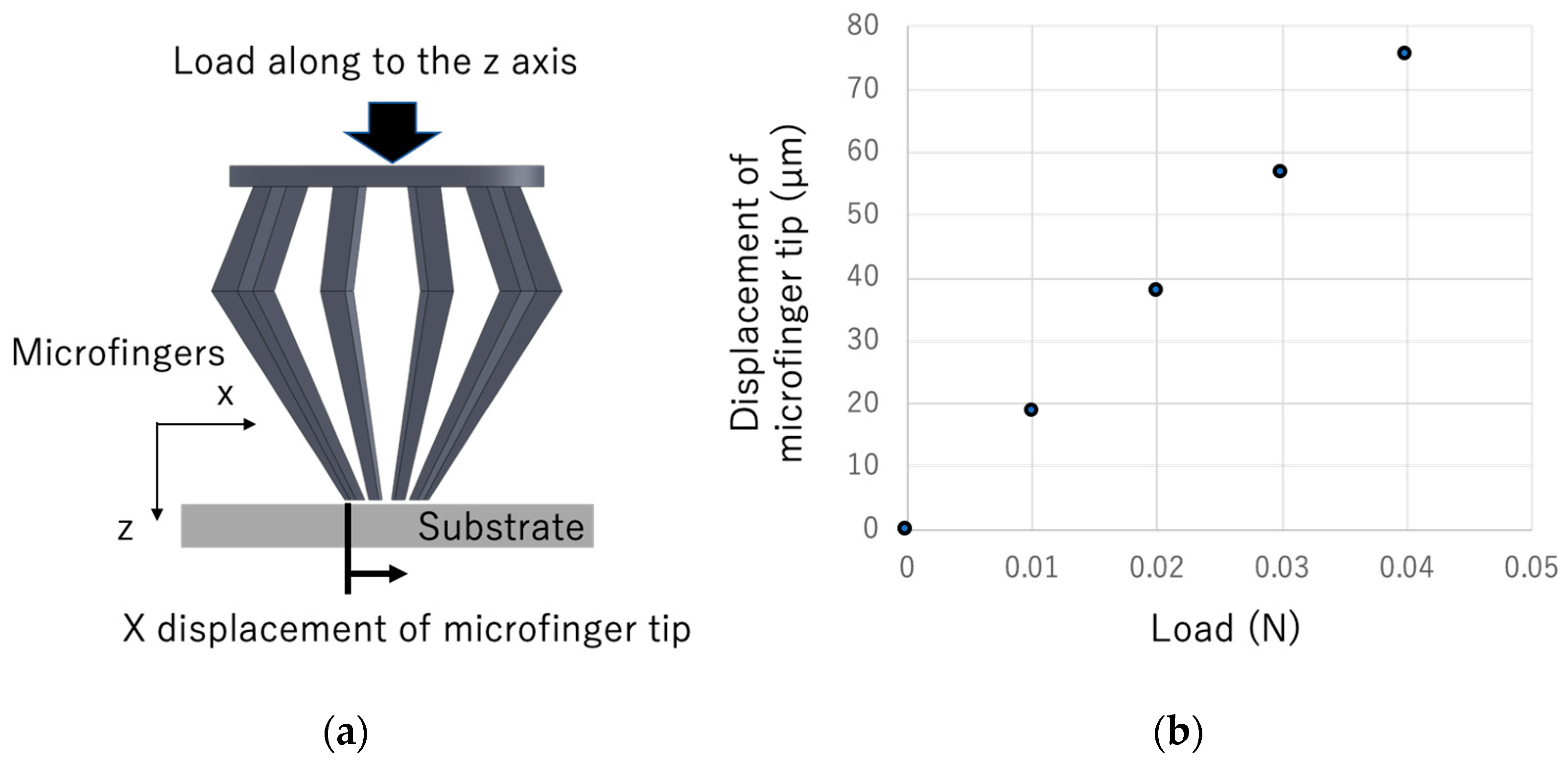
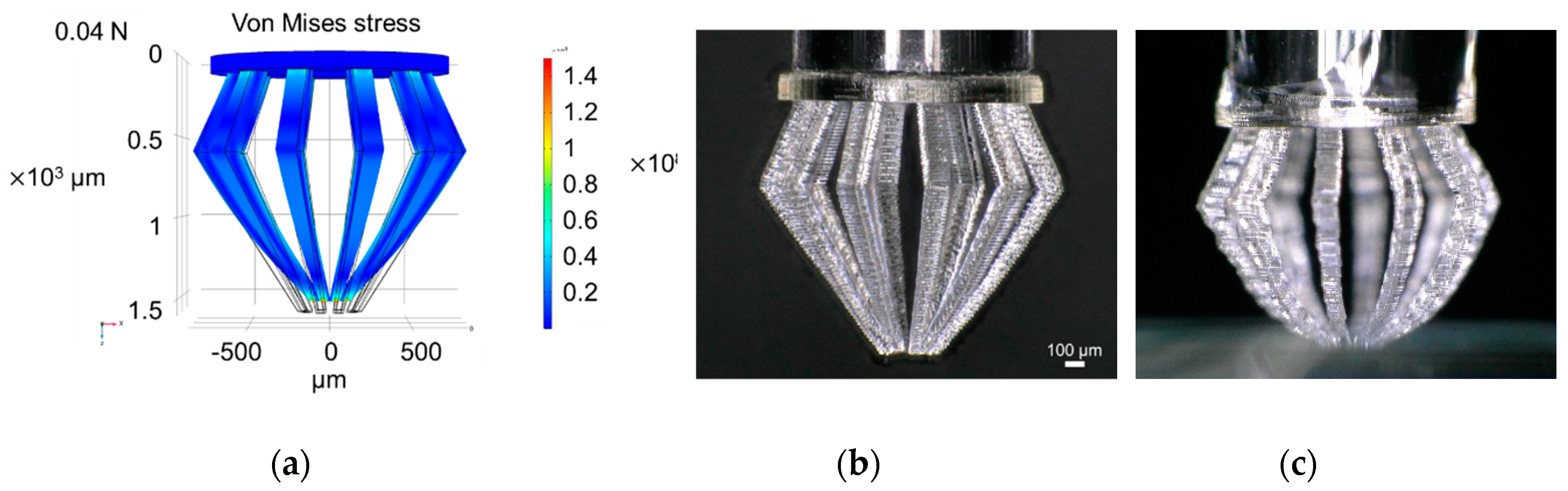
3.3. Analysis of the Mechanical Properties of Microfingers
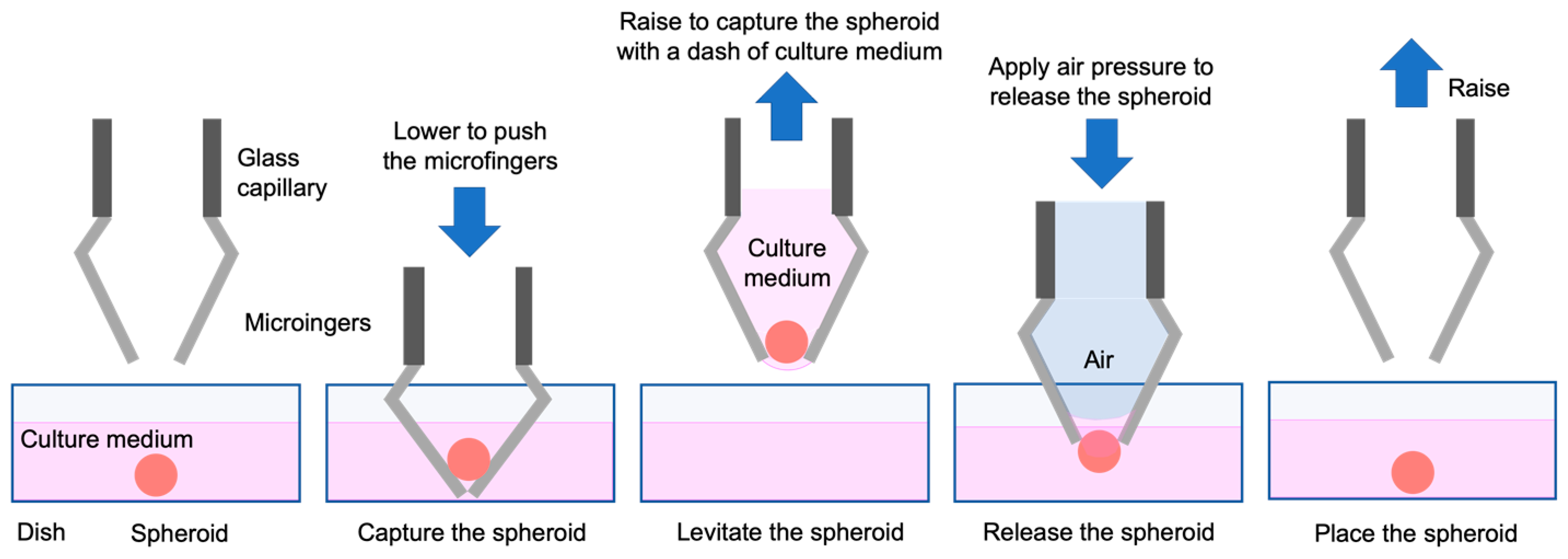
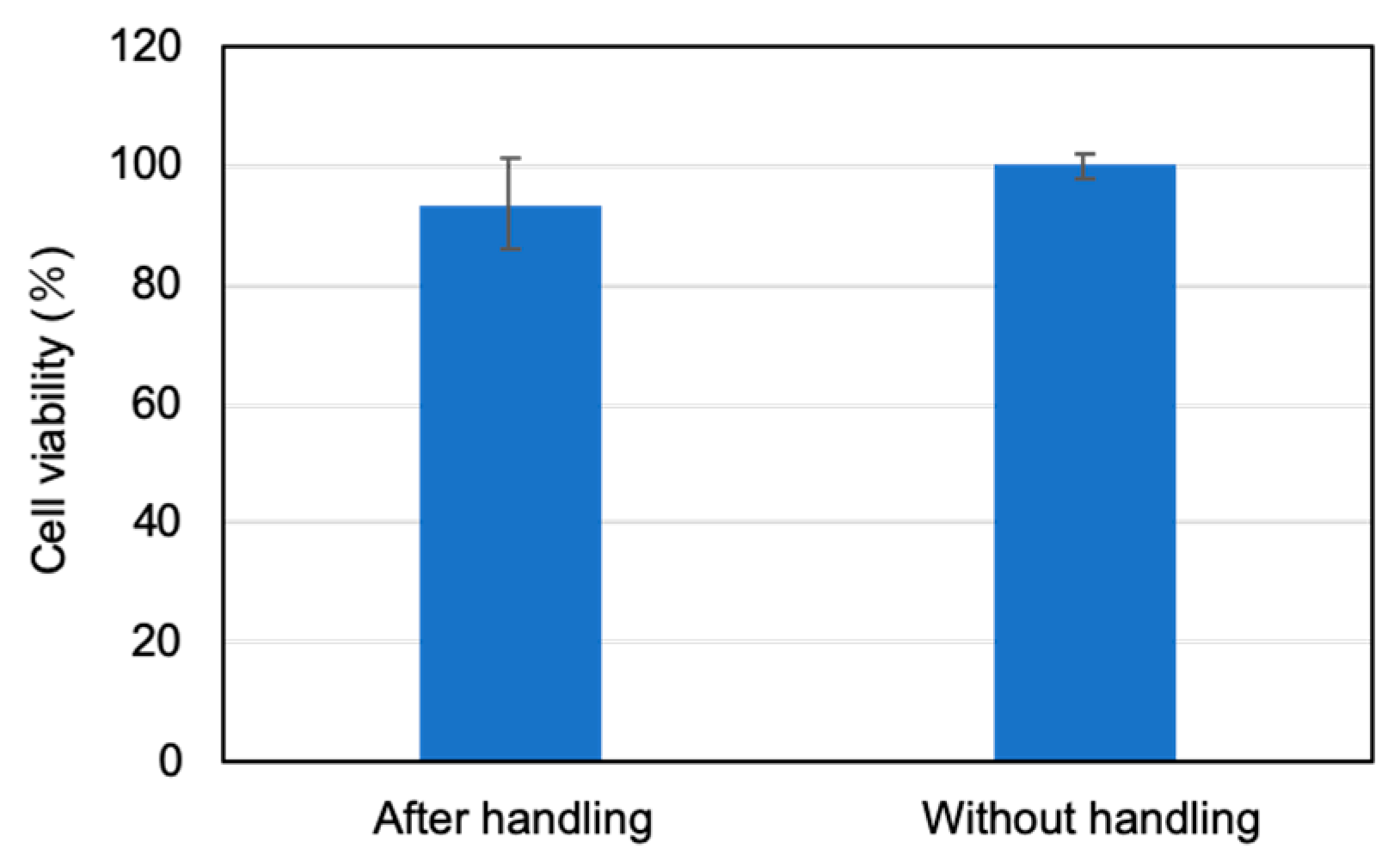
3.4. Manipulation of the Spheroid Using the Micromanipulator
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancaster, M.A.; Knoblich, M.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Kikuchi, A.; Aoyagi, T.; Okano, T. Cell sheet tissue engineering: Cell sheet preparation, harvesting/manipulation, and transplantation. J. Biomed. Mater. Res. A 2019, 107, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; Schoendube, J.; Zimmermann, S.; Steeb, M.; Zengerle, R.; Koltay, P. Technologies for single-cell isolation. Int. J. Mol. Sci. 2015, 16, 16897–16919. [Google Scholar] [CrossRef]
- Collard, D.; Takeuchi, S.; Fujita, H. MEMS technology for nanobio research. Drug Discov. Today 2008, 13, 989–996. [Google Scholar] [CrossRef]
- Hochmuth, R.M. Micropipette aspiration of living cells. J. Biomech. 2000, 33, 15–22. [Google Scholar] [CrossRef]
- Ludwig, A.; Kretzmer, G.; Schügerl, K. Determination of a “critical shear stress level” applied to adherent mammalian cells. Enzyme Microb. Technol. 1992, 14, 209–213. [Google Scholar] [CrossRef]
- Shojaei-Baghini, E.; Zheng, Y.; Sun, Y. Automated micropipette aspiration of single cells. Ann. Biomed. Eng. 2013, 41, 1208–1216. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Liu, X.; Zhang, Y.; Sun, Y. Nanonewton force-controlled manipulation of biological cells using a monolithic MEMS microgripper with two-axis force feedback. J. Micromech. Microeng. 2008, 18, 055013. [Google Scholar] [CrossRef]
- Tomizawa, Y.; Dixit, K.; Daggett, D.; Hoshino, K. Biocompatible cantilevers for mechanical characterization of zebrafish embryos using image analysis. Sensors 2019, 19, 1506. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, D.; Cowley, N.; Bian, Z.; Zheng, G.; Claffey, K.P.; Hoshino, K. Stiffness analysis of 3D spheroids using microtweezers. PLoS ONE 2017, 12, e0188346. [Google Scholar] [CrossRef]
- Svoboda, K.; Block, S.M. Biological applications of optical forces. Annu. Rev. Biophys. Biomol. Struct. 1994, 23, 247–285. [Google Scholar] [CrossRef]
- Moffitt, J.R.; Chemla, Y.R.; Smith, S.B.; Bustamante, C. Recent advances in optical tweezers. Annu. Rev. Biochem. 2008, 77, 205–228. [Google Scholar] [CrossRef] [Green Version]
- Sia, S.K.; Whitesides, G.M. Microfluidic devices fabricated in poly (dimethylsiloxane) for biological studies. Electrophoresis 2003, 24, 3563–3576. [Google Scholar] [CrossRef]
- Scheler, O.; Postek, W.; Garstecki, P. Recent developments of microfluidics as a tool for biotechnology and microbiology. Curr. Opin. Biotechnol. 2019, 55, 60–67. [Google Scholar] [CrossRef]
- Sato, M.; Levesque, M.J.; Nerem, R.M. Micropipette aspiration of cultured bovine aortic endothelial cells exposed to shear stress. Arteriosclerosis 1987, 7, 276–286. [Google Scholar] [CrossRef] [Green Version]
- Maruo, S.; Nakamura, O.; Kawata, S. Three-dimensional microfabrication with two-photon absorbed photopolymerization. Opt. Lett. 1997, 22, 132–134. [Google Scholar] [CrossRef] [Green Version]
- Maruo, S.; Ikuta, K. Submicron stereolithography for the production of freely movable mechanisms by using single-photon polymerization. Sens. Actuators A Phys. 2002, 100, 70–76. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Cordonier, C.E.J.; Noda, Y.; Nagase, F.; Enomoto, J.; Kageyama, T.; Honma, H.; Maruo, S.; Fukuda, J. Tailored cell sheet engineering using microstereolithography and electrochemical cell transfer. Sci. Rep. 2019, 9, 10415. [Google Scholar] [CrossRef] [Green Version]
- Monri, K.; Maruo, S. Three-dimensional ceramic molding based on microstereolithography for the production of piezoelectric energy harvesters. Sens. Actuators A Phys. 2013, 200, 31–36. [Google Scholar] [CrossRef]
- LaFratta, C.N.; Simoska, O.; Pelse, I.; Weng, S.; Ingram, M. A convenient direct laser writing system for the creation of microfluidic masters. Microfluid. Nanofluidics 2015, 419–426. [Google Scholar] [CrossRef]
- Christopher, B.-K.; Bastmeyer, B.; Blasco, E.; Delaittre, G.; Müller, P.; Richter, B.; Wegener, M. 3D laser micro- and nanoprinting: Challenges for chemistry. Angew. Chem. Int. Ed. 2017, 56, 15828–15845. [Google Scholar] [CrossRef]
- Legrand, C.; Bour, J.M.; Jacob, C.; Capiaumont, J.; Martial, A.; Marc, A.; Wudtke, M.; Kretzmer, G.; Demangel, C.; Duval, D. Lactate dehydrogenase (LDH) activity of the cultured eukaryotic cells as marker of the number of dead cells in the medium [corrected]. J. Biotechnol. 1992, 25, 231–243. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozaki, S.; Moritoki, Y.; Furukawa, T.; Akieda, H.; Kageyama, T.; Fukuda, J.; Maruo, S. Additive Manufacturing of Micromanipulator Mounted on a Glass Capillary for Biological Applications. Micromachines 2020, 11, 174. https://doi.org/10.3390/mi11020174
Kozaki S, Moritoki Y, Furukawa T, Akieda H, Kageyama T, Fukuda J, Maruo S. Additive Manufacturing of Micromanipulator Mounted on a Glass Capillary for Biological Applications. Micromachines. 2020; 11(2):174. https://doi.org/10.3390/mi11020174
Chicago/Turabian StyleKozaki, Shingo, Yukihito Moritoki, Taichi Furukawa, Hikaru Akieda, Tatsuto Kageyama, Junji Fukuda, and Shoji Maruo. 2020. "Additive Manufacturing of Micromanipulator Mounted on a Glass Capillary for Biological Applications" Micromachines 11, no. 2: 174. https://doi.org/10.3390/mi11020174
APA StyleKozaki, S., Moritoki, Y., Furukawa, T., Akieda, H., Kageyama, T., Fukuda, J., & Maruo, S. (2020). Additive Manufacturing of Micromanipulator Mounted on a Glass Capillary for Biological Applications. Micromachines, 11(2), 174. https://doi.org/10.3390/mi11020174





