Fabrication of Subretinal 3D Microelectrodes with Hexagonal Arrangement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of Subretinal Electrode Array
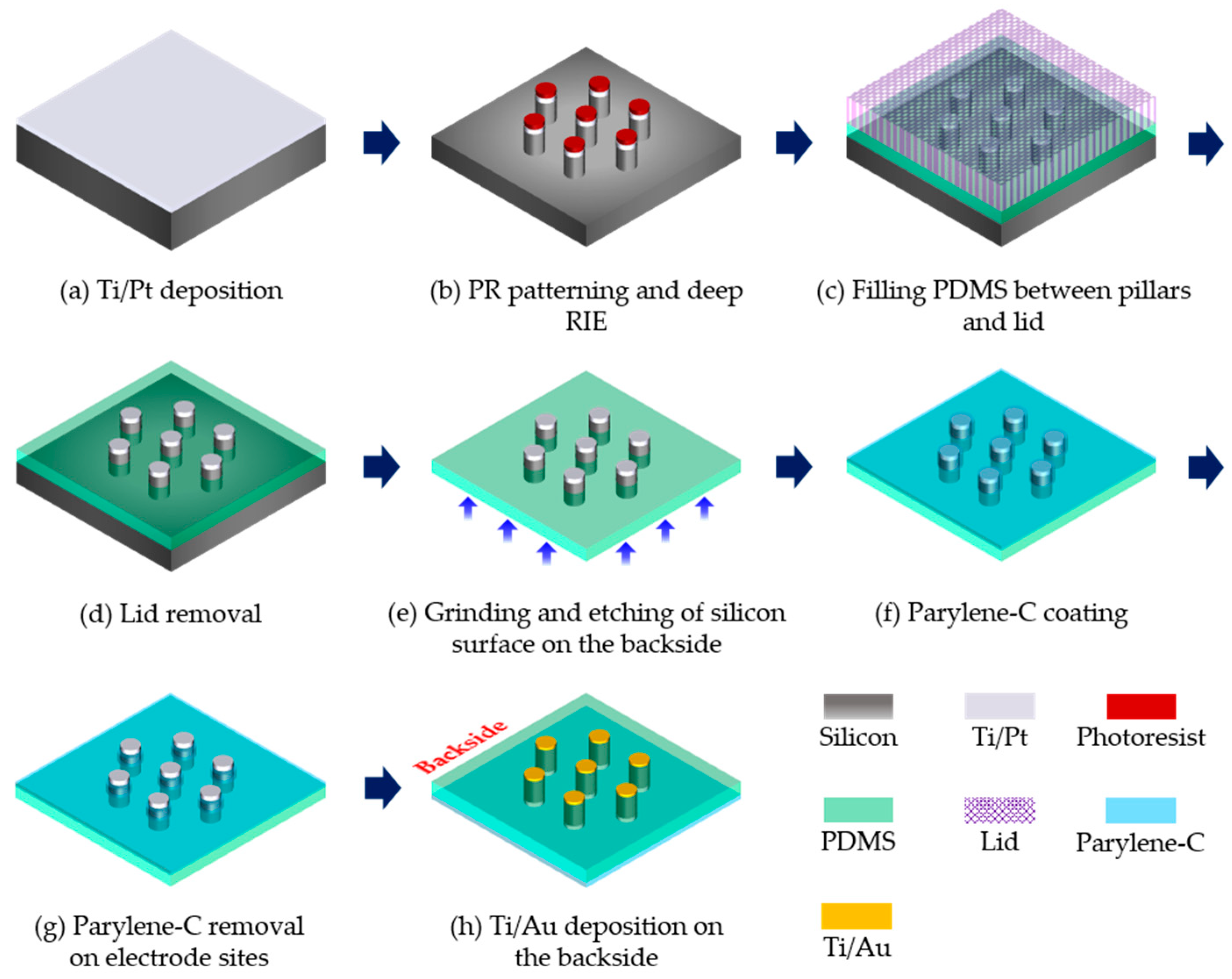
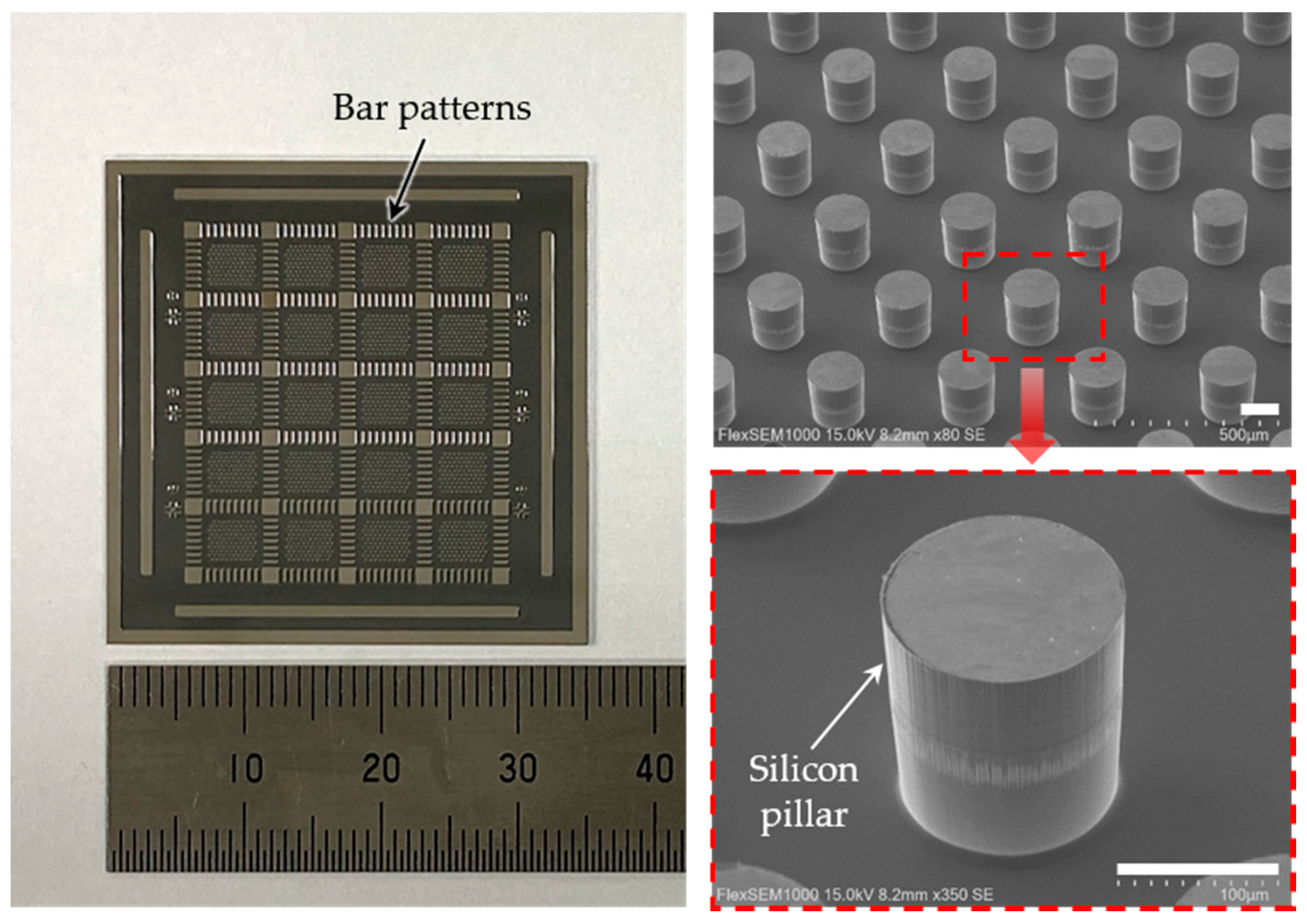
2.2. Generation of Silicon Pillars and Transparent Base
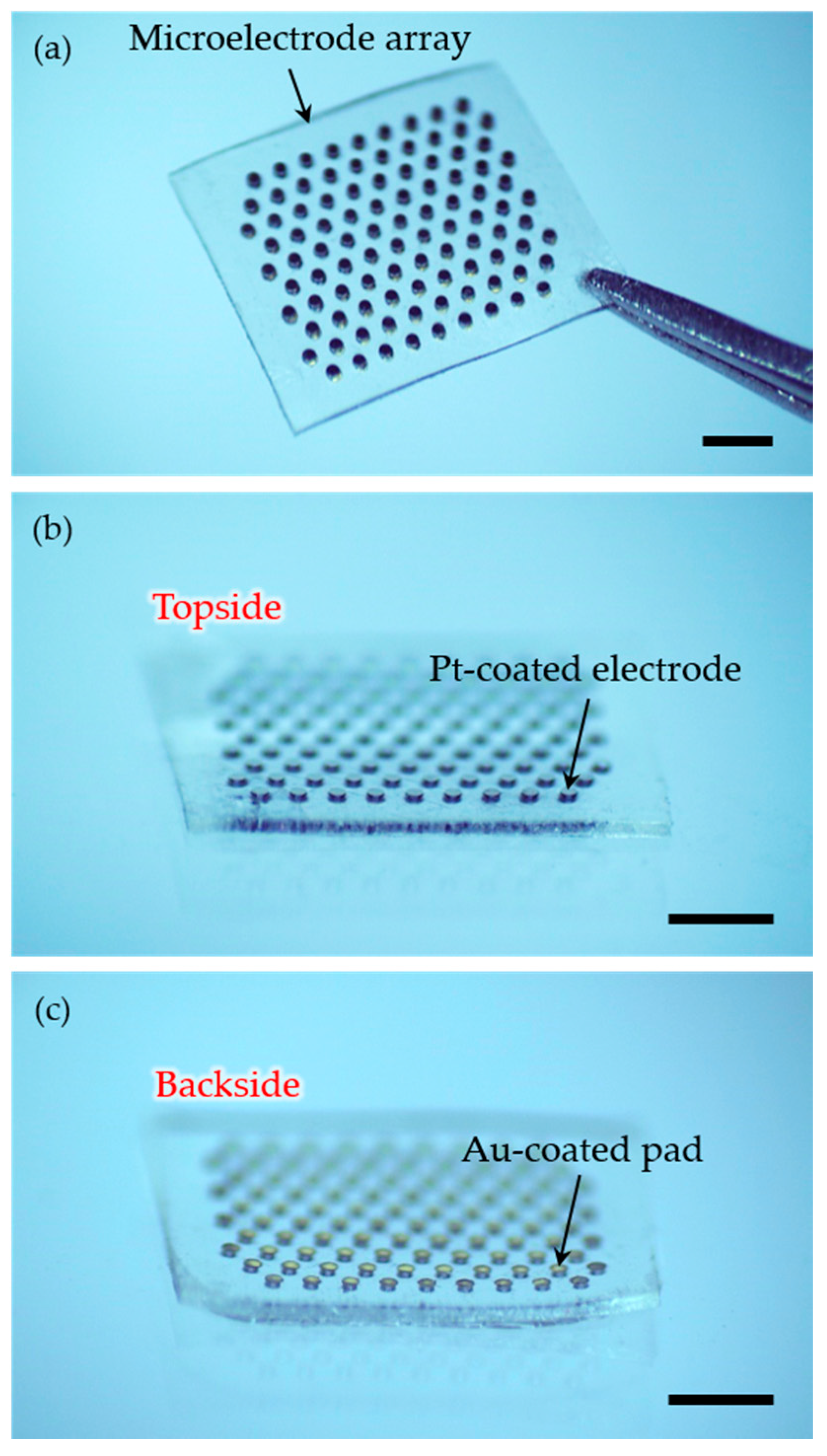
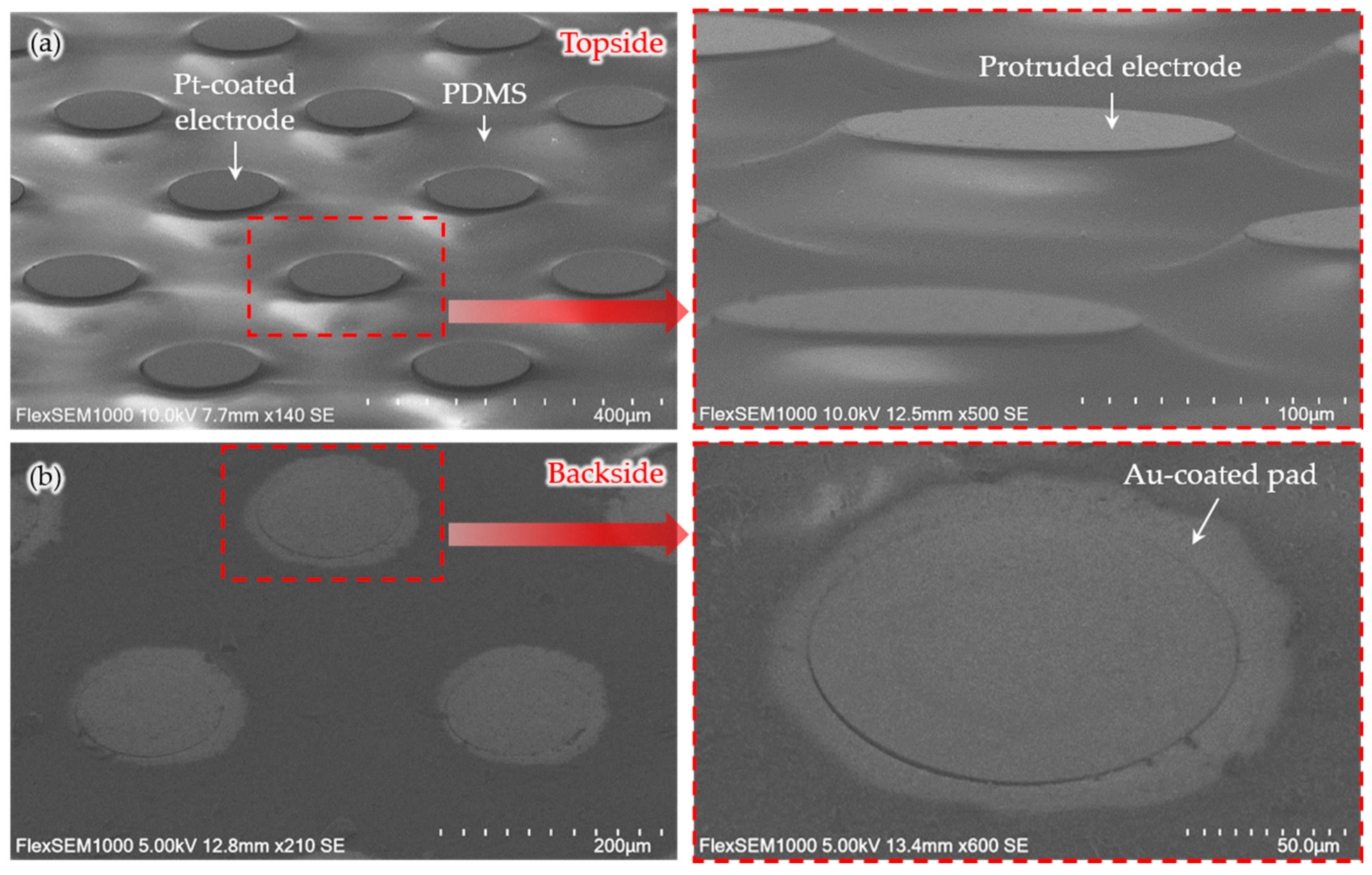
2.3. Generation of Connection Pads and Parylene Insulation
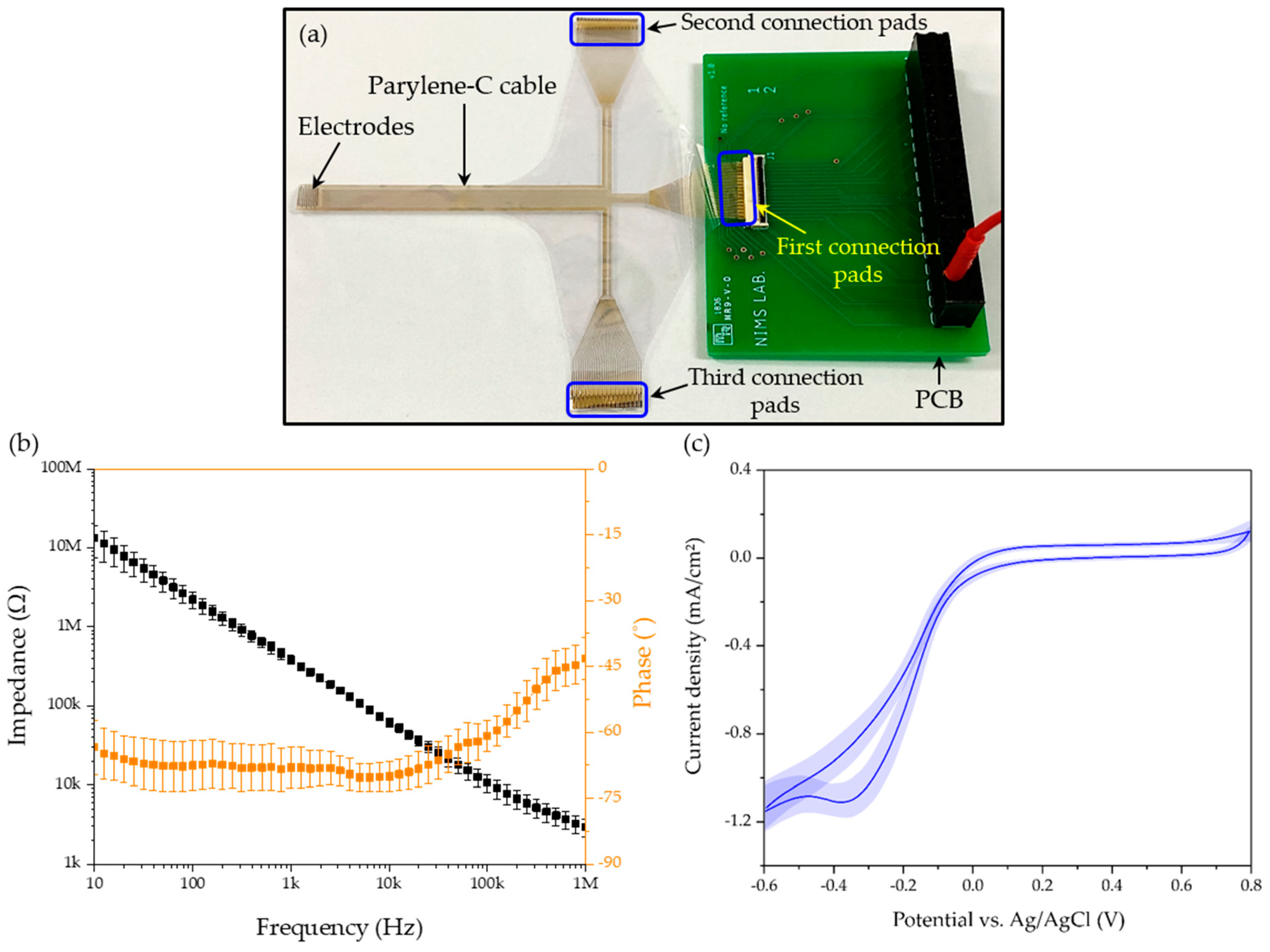
2.4. Electrochemical Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Kim, S.Y.; Sadda, S.; Pearlman, J.; Humayun, M.S.; de Juan, E.; Melia, B.M.; Green, W.R. Morphometric analysis of the macula in eyes with disciform age-related macular degeneration. Retina 2002, 22, 471–477. [Google Scholar] [CrossRef]
- Bressler, N.M. Age-related macular degeneration is the leading cause of blindness. JAMA 2004, 291, 1900–1901. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.S.; O’Colmain, B.J.; Muñoz, B.; Tomany, S.C.; McCarty, C.; de Jong, P.T.; Nemesure, B.; Mitchell, P.; Kempen, J. Prevalence of age-related macular degeneration in the united states. Arch. Ophthalmol. 2004, 122, 564–572. [Google Scholar]
- Goetz, G.A.; Palanker, D.V. Electronic approaches to restoration of sight. Reports Prog. Phys. 2016, 79, 96701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlauto, L.; Airaghi Leccardi, M.J.I.; Chenais, N.A.L.; Gilliéron, S.C.A.; Vagni, P.; Bevilacqua, M.; Wolfensberger, T.J.; Sivula, K.; Ghezzi, D. Design and validation of a foldable and photovoltaic wide-field epiretinal prosthesis. Nat. Commun. 2018, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Ho, E.; Lei, X.; Flores, T.; Lorach, H.; Huang, T.; Galambos, L.; Kamins, T.; Harris, J.; Mathieson, K.; Palanker, D. Characteristics of prosthetic vision in rats with subretinal flat and pillar electrode arrays. J. Neural Eng. 2019, 16, 066027. [Google Scholar] [CrossRef]
- Tong, W.; Stamp, M.; Apollo, N.V.; Ganesan, K.; Meffin, H.; Prawer, S.; Garrett, D.J.; Ibbotson, M.R. Improved visual acuity using a retinal implant and an optimized stimulation strategy. J. Neural Eng. 2019, 17, 016018. [Google Scholar] [CrossRef]
- Davidsen, R.S.; Hemanth, S.; Keller, S.S.; Bek, T.; Hansen, O. Evaluation of the capacitive behavior of 3D carbon electrodes for sub-retinal photovoltaic prosthesis. Micro Nano Eng. 2019, 2, 110–116. [Google Scholar] [CrossRef]
- Losada, P.G.; Rousseau, L.; Grzeskowiak, M.; Valet, M.; Nguyen, D.; Dégardin, J.; Dubus, E.; Picaud, S.; Lissorgues, G. Protuberant electrode structures for subretinal electrical stimulation: Modeling, fabrication and in vivo evaluation. Front. Neurosci. 2019, 13, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Lohmann, T.K.; Haiss, F.; Schaffrath, K.; Schnitzler, A.C.; Waschkowski, F.; Barz, C.; Van Der Meer, A.M.; Werner, C.; Johnen, S.; Laube, T.; et al. The very large electrode array for retinal stimulation (VLARS)-A concept study. J. Neural Eng. 2019, 16, 066031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bareket, L.; Barriga-Rivera, A.; Zapf, M.P.; Lovell, N.H.; Suaning, G.J. Progress in artificial vision through suprachoroidal retinal implants. J. Neural Eng. 2017, 14, 045002. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Zhao, S.; Yang, H.; Zhang, Y.; Wu, T. Micro/nano technologies for high-density retinal implant. Micromachines 2019, 10, 419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabel, V.P. Artificial Vision; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 9783319418742. [Google Scholar]
- Luo, Y.H.L.; da Cruz, L. The Argus® II retinal prosthesis system. Prog. Retin. Eye Res. 2016, 50, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, A.K.; Dorn, J.D.; Caspi, A.; McMahon, M.J.; Dagnelie, G.; DaCruz, L.; Stanga, P.; Humayun, M.S.; Greenberg, R.J. Blind subjects implanted with the Argus II retinal prosthesis are able to improve performance in a spatial-motor task. Br. J. Ophthalmol. 2011, 95, 539–543. [Google Scholar] [CrossRef]
- Dorn, J.D.; Ahuja, A.K.; Caspi, A.; Da Cruz, L.; Dagnelie, G.; Sahel, J.A.; Greenberg, R.J.; McMahon, M.J. The detection of motion by blind subjects with the epiretinal 60-electrode (Argus II) retinal prosthesis. JAMA Ophthalmol. 2013, 131, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Humayun, M.S.; Dorn, J.D.; Da Cruz, L.; Dagnelie, G.; Sahel, J.A.; Stanga, P.E.; Cideciyan, A.V.; Duncan, J.L.; Eliott, D.; Filley, E.; et al. Interim results from the international trial of second sight’s visual prosthesis. Ophthalmology 2012, 119, 779–788. [Google Scholar] [CrossRef] [Green Version]
- Zrenner, E.; Bartz-Schmidt, K.U.; Benav, H.; Besch, D.; Bruckmann, A.; Gabel, V.P.; Gekeler, F.; Greppmaier, U.; Harscher, A.; Kibbel, S.; et al. Subretinal electronic chips allow blind patients to read letters and combine them to words. Proc. R. Soc. B Biol. Sci. 2011, 278, 1489–1497. [Google Scholar] [CrossRef]
- Kelly, S.K.; Shire, D.B.; Chen, J.; Doyle, P.; Cogan, S.F.; Gingerich, M.D.; Drohan, W.A.; Behan, S.; Theogarajan, L.; Wyatt, J.L.; et al. A hermetic wireless subretinal neurostimulator for vision prostheses. IEEE Trans. Biomed. Eng. 2011, 58, 3197–3205. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Mathieson, K.; Kamins, T.I.; Loudin, J.D.; Galambos, L.; Goetz, G.; Sher, A.; Mandel, Y.; Huie, P.; Lavinsky, D.; et al. Photovoltaic retinal prosthesis: Implant fabrication and performance. J. Neural Eng. 2012, 9, 046014. [Google Scholar] [CrossRef] [Green Version]
- Mathieson, K.; Loudin, J.; Goetz, G.; Huie, P.; Wang, L.; Kamins, T.I.; Galambos, L.; Smith, R.; Harris, J.S.; Sher, A.; et al. Photovoltaic retinal prosthesis with high pixel density. Nat. Photonics 2012, 6, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Flores, T.; Lei, X.; Huang, T.; Lorach, H.; Dalal, R.; Galambos, L.; Kamins, T.; Mathieson, K.; Palanker, D. Optimization of pillar electrodes in subretinal prosthesis for enhanced proximity to target neurons. J. Neural Eng. 2018, 15, 036011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stingl, K.; Schippert, R.; Bartz-Schmidt, K.U.; Besch, D.; Cottriall, C.L.; Edwards, T.L.; Gekeler, F.; Greppmaier, U.; Kiel, K.; Koitschev, A.; et al. Interim results of a multicenter trial with the new electronic subretinal implant alpha AMS in 15 patients blind from inherited retinal degenerations. Front. Neurosci. 2017, 11, 445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boinagrov, D.; Pangratz-Fuehrer, S.; Goetz, G.; Palanker, D. Selectivity of direct and network-mediated stimulation of the retinal ganglion cells with epi-, sub- and intraretinal electrodes. J. Neural Eng. 2014, 11, 026008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, R.J.; Ziv, O.R.; Rizzo, J.F. Thresholds for activation of rabbit retinal ganglion cells with relatively large, extracellular microelectrodes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.J.; Rizzo, J.F. Thresholds for activation of rabbit retinal ganglion cells with a subretinal electrode. Exp. Eye Res. 2006, 83, 367–373. [Google Scholar] [CrossRef]
- Jensen, R.J.; Rizzo, J.F. Activation of ganglion cells in wild-type and rd1 mouse retinas with monophasic and biphasic current pulses. J. Neural Eng. 2009, 6, 035004. [Google Scholar] [CrossRef]
- Tsai, D.; Morley, J.W.; Suaning, G.J.; Lovell, N.H. Direct activation and temporal response properties of rabbit retinal ganglion cells following subretinal stimulation. J. Neurophysiol. 2009, 102, 2982–2993. [Google Scholar] [CrossRef]
- Seo, H.W.; Kim, N.; Ahn, J.; Cha, S.; Goo, Y.S.; Kim, S. A 3D flexible microelectrode array for subretinal stimulation. J. Neural Eng. 2019, 16, 056016. [Google Scholar] [CrossRef]
- Wilke, R.G.H.; Moghadam, G.K.; Lovell, N.H.; Suaning, G.J.; Dokos, S. Electric crosstalk impairs spatial resolution of multi-electrode arrays in retinal implants. J. Neural Eng. 2011, 8, 046016. [Google Scholar] [CrossRef]
- Abramian, M.; Lovell, N.H.; Habib, A.; Morley, J.W.; Suaning, G.J.; Dokos, S. Quasi-monopolar electrical stimulation of the retina: A computational modelling study. J. Neural Eng. 2014, 11, 025002. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Hallum, L.E.; Lovell, N.H.; Suaning, G.J. Visual acuity measurement of prosthetic vision: A virtual-reality simulation study. J. Neural Eng. 2005, 2, S135–S145. [Google Scholar] [CrossRef] [PubMed]
- Vurro, M.; Baselli, G.; Orabona, F.; Sandini, G. Simulation and assessment of bioinspired visual processing system for epi-retinal prostheses. Annu. Int. Conf. IEEE Eng. Med. Biol. - Proc. 2006, 3278–3281. [Google Scholar]
- Hamzah, A.A.; Abd Aziz, N.; Yeop Majlis, B.; Yunas, J.; Dee, C.F.; Bais, B. Optimization of HNA etching parameters to produce high aspect ratio solid silicon microneedles. J. Micromechan. Microeng. 2012, 22, 095017. [Google Scholar] [CrossRef]
- Kang, Y.N.; Chou, N.; Jang, J.; Byun, D.; Kang, H.; Moon, D.; Kim, J.; Kim, S. An intrafascicular neural interface with enhanced interconnection for recording of peripheral nerve signals. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1312–1319. [Google Scholar] [CrossRef]
- Negi, S.; Bhandari, R.; Rieth, L.; Solzbacher, F. In vitro comparison of sputtered iridium oxide and platinum-coated neural implantable microelectrode arrays. Biomed. Mater. 2010, 5, 015007. [Google Scholar] [CrossRef]
- Hocheng, H.; Chen, C.M.; Chou, Y.C.; Lin, C.H. Study of novel electrical routing and integrated packaging on bio-compatible flexible substrates. Microsyst. Technol. 2010, 16, 423–430. [Google Scholar] [CrossRef]
- Wang, Z.; Volinsky, A.A.; Gallant, N.D. Crosslinking effect on polydimethylsiloxane elastic modulus measured by custom-built compression instrument. J. Appl. Polym. Sci. 2014, 131, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Lee, D.S.; Kim, I.G.; Sohn, D.W.; Park, J.Y.; Choi, B.K.; Kim, S.W. Evaluation of the biocompatibility of a coating material for an implantable bladder volume sensor. Kaohsiung J. Med. Sci. 2012, 28, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.Z.; Rieth, L.; Tathireddy, P.; Solzbacher, F. Long-term in-vivo investigation of parylene-C as encapsulation material for neural interfaces. Procedia Eng. 2011, 25, 483–486. [Google Scholar] [CrossRef] [Green Version]
- Montezuma, S.R.; Loewenstein, J.; Scholz, C.; Rizzo, J.F. Biocompatibility of materials implanted into the subretinal space of Yucatan pigs. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3514–3522. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Wang, X.; Zhao, C.; Yang, Z.; Dai, R.; Dong, F. Biocompatibility of subretinal parylene-based Ti/Pt microelectrode array in rabbit for further artificial vision studies. J. Ocul. Biol. Dis. Infor. 2009, 2, 33–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorach, H.; Goetz, G.; Smith, R.; Lei, X.; Mandel, Y.; Kamins, T.; Mathieson, K.; Huie, P.; Harris, J.; Sher, A.; et al. Photovoltaic restoration of sight with high visual acuity. Nat. Med. 2015, 21, 476–482. [Google Scholar] [CrossRef] [Green Version]
- Cogan, S.F.; Troyk, P.R.; Ehrlich, J.; Gasbarro, C.M.; Plante, T.D. The influence of electrolyte composition on the in vitro charge-injection limits of activated iridium oxide (AIROF) stimulation electrodes. J. Neural Eng. 2007, 4, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Cogan, S.F.; Ehrlich, J.; Plante, T.D.; Smirnov, A.; Shire, D.B.; Gingerich, M.; Rizzo, J.F. Sputtered iridium oxide films for neural stimulation electrodes. J. Biomed. Mater. Res. - Part B Appl. Biomater. 2009, 89, 353–361. [Google Scholar] [CrossRef]
- Loudin, J.D.; Simanovskii, D.M.; Vijayraghavan, K.; Sramek, C.K.; Butterwick, A.F.; Huie, P.; McLean, G.Y.; Palanker, D.V. Optoelectronic retinal prosthesis: System design and performance. J. Neural Eng. 2007, 4, S72–S84. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Chang, M.-Y.; Yang, C.-H.; Teng, C.-C.; Fan, L.-S. Flexible, high-density microphotodiode array with integrated sputtered iridium oxide electrodes for retinal stimulation. J. Micro/Nanolithography, MEMS, MOEMS 2016, 15, 015002. [Google Scholar] [CrossRef]
- Corna, A.; Herrmann, T.; Zeck, G. Electrode-size dependent thresholds in subretinal neuroprosthetic stimulation. J. Neural Eng. 2018, 15, 045003. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, H.W.; Kim, N.; Kim, S. Fabrication of Subretinal 3D Microelectrodes with Hexagonal Arrangement. Micromachines 2020, 11, 467. https://doi.org/10.3390/mi11050467
Seo HW, Kim N, Kim S. Fabrication of Subretinal 3D Microelectrodes with Hexagonal Arrangement. Micromachines. 2020; 11(5):467. https://doi.org/10.3390/mi11050467
Chicago/Turabian StyleSeo, Hee Won, Namju Kim, and Sohee Kim. 2020. "Fabrication of Subretinal 3D Microelectrodes with Hexagonal Arrangement" Micromachines 11, no. 5: 467. https://doi.org/10.3390/mi11050467
APA StyleSeo, H. W., Kim, N., & Kim, S. (2020). Fabrication of Subretinal 3D Microelectrodes with Hexagonal Arrangement. Micromachines, 11(5), 467. https://doi.org/10.3390/mi11050467




