Slicing Spheroids in Microfluidic Devices for Morphological and Immunohistochemical Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. EPOX Microfluidic Device
Design of the EPOX Microfluidic Device
2.2. Spheroids and Reagents
2.2.1. Formation of Spheroids
2.2.2. Reagents
2.3. Experimental Procedure
2.3.1. Image Acquisition and Quantification
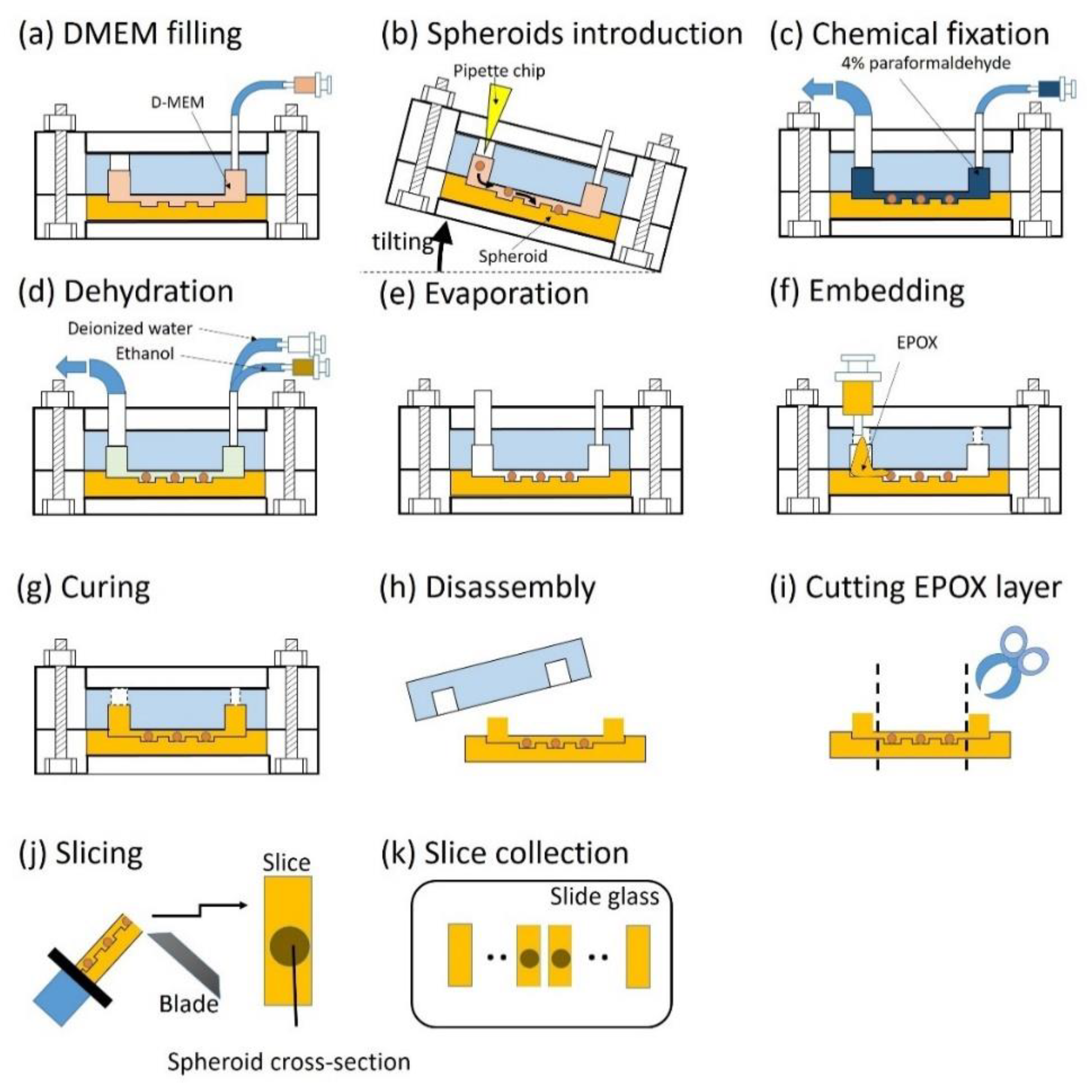
2.3.2. Operation of EPOX Device from Embedding to Sectioning of Spheroids
3. Results and Discussion
3.1. EPOX Biocompatibility
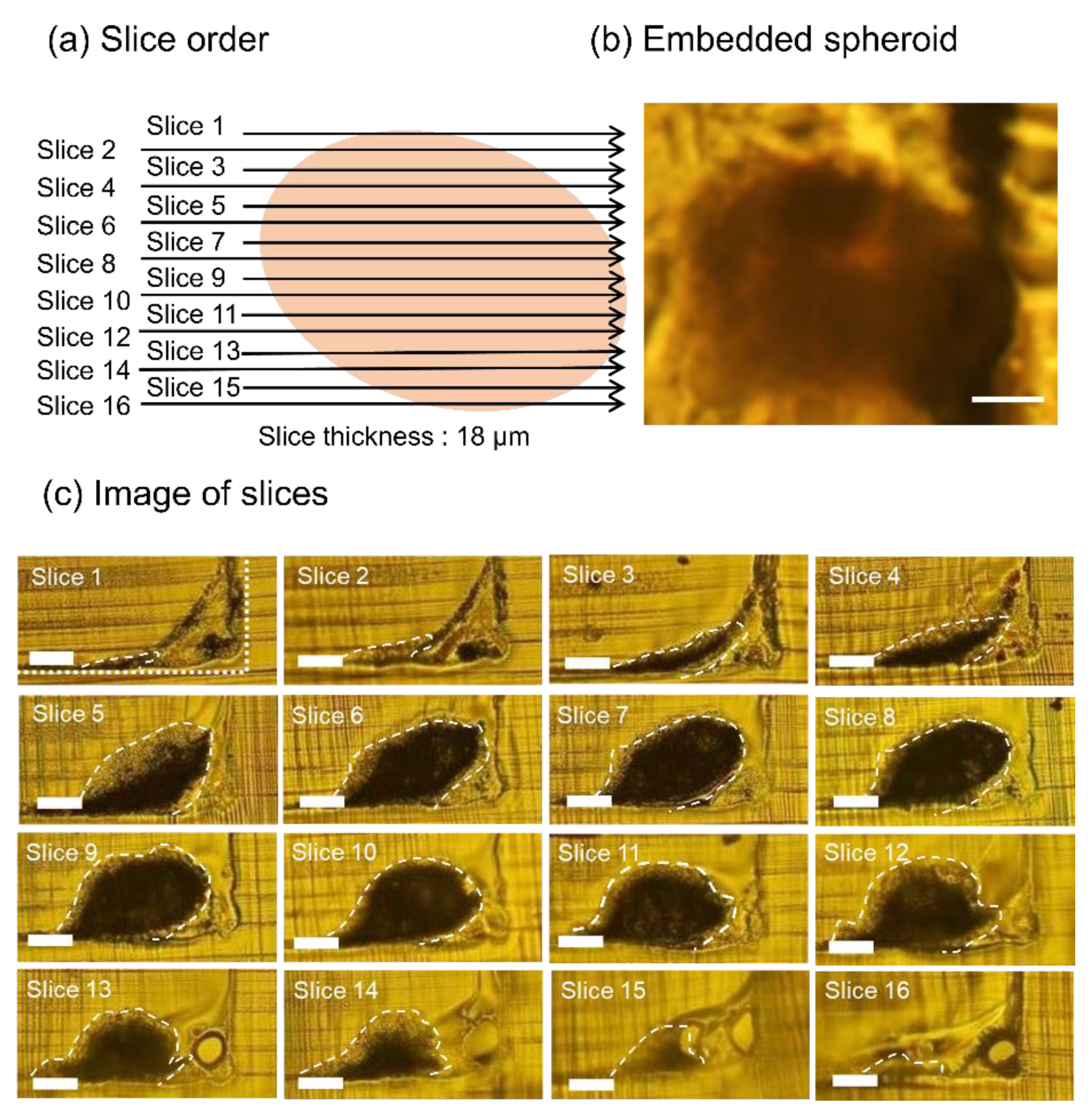
3.2. Whole Slice Imaging of Sectioned Spheroids
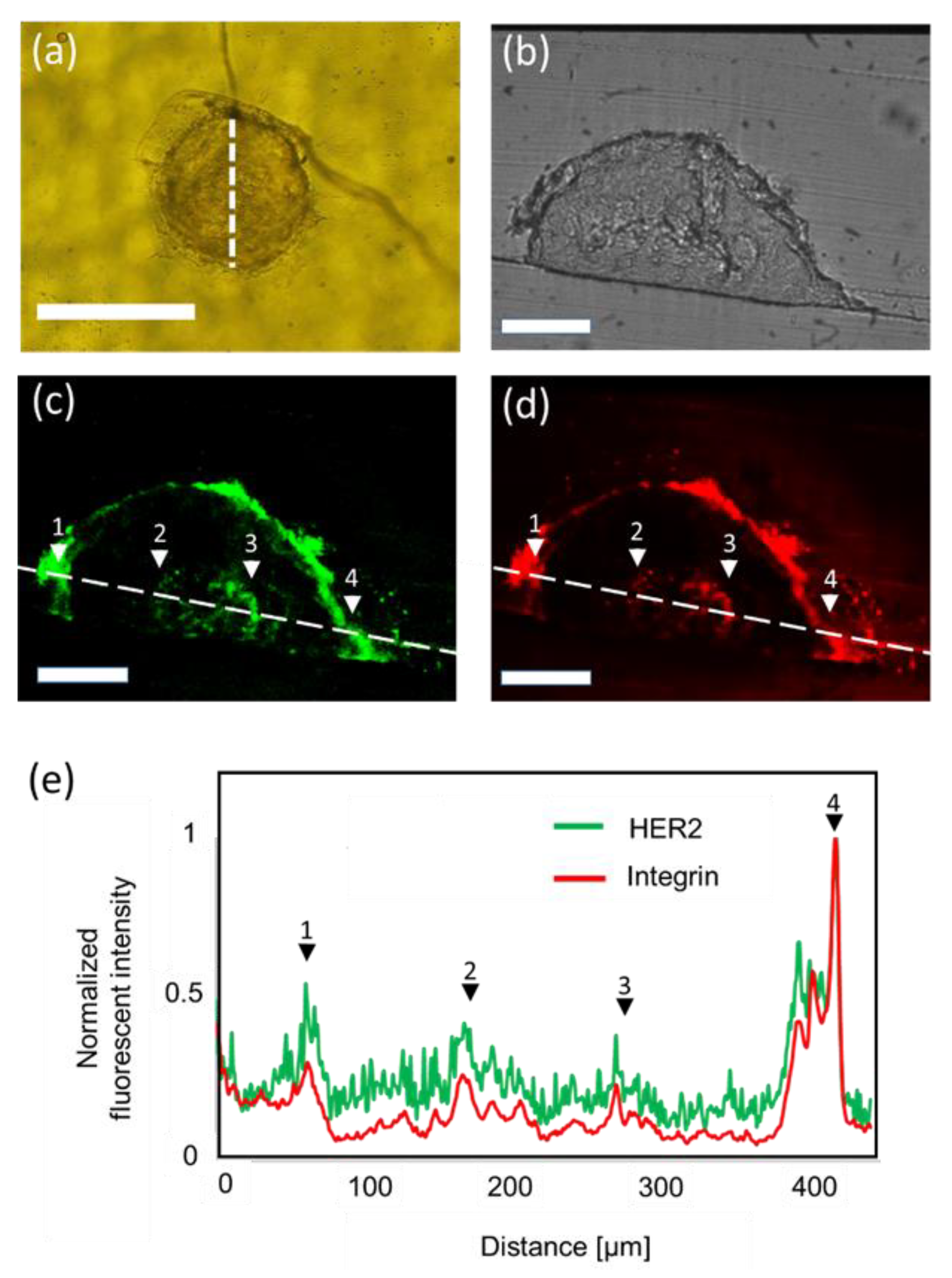
3.3. Immunohistochemistry of Spheroids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kunz-Schughart, L.A.; Freyer, J.P.; Hofsteadter, F.; Ebner, R. Use of 3-D Cultures for High-Throughput Screening: The Multicellular Spheroid Model. J. Biomol. Screen. 2004, 9, 273–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazzari, G.; Couvreur, P.; Mura, S. Multicellular tumor spheroids: A relevant 3D model for the in vitro preclinical investigation of polymer nanomedicines. Polym. Chem. 2017, 8, 4947–4969. [Google Scholar] [CrossRef] [Green Version]
- Mikhail, A.S.; Eetezadi, S.; Ekdawi, S.N.; Stewart, J.; Allen, C. Image-based analysis of the size- and time-dependent penetration of polymeric micelles in multicellular tumor spheroids and tumor xenografts. Int. J. Pharm. 2014, 464, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Tae, H.K.; Mount, C.W.; Gombotz, W.R.; Pun, S.H. The delivery of doxorbicin to 3-D multicellular spheroid and tumors in a murine xenograft model using tumor-penetrating triblock polymeric micelles. Biomaterials 2010, 31, 7386–7397. [Google Scholar]
- Wenzel, C.; Riefke, B.; Gründemann, S.; Krebs, A.; Christian, S.; Prinz, F.; Osterland, M.; Golfier, S.; Räse, S.; Ansari, N.; et al. 3D high-content screening for the identification of compounds that target cells in dormant tumor spheroid regions. Exp. Cell Res. 2014, 323, 131–143. [Google Scholar] [CrossRef] [Green Version]
- Nashimoto, Y.; Okada, R.; Hanada, S.; Arima, Y.; Nishiyama, K.; Miura, T.; Yokokawa, R. Vascularized cancer on a chip: The effect of perfusion on growth and drug delivery of tumor spheroid. Biomaterials 2020, 229, 1–13. [Google Scholar] [CrossRef]
- Albanese, A.; Lam, A.K.; Sykes, E.A.; Rocheleau, J.V.; Chan, W.C.W. Tumour-on-a-chip provides and optical window into nanoparticle tissue transport. Nat. Commun. 2013, 4, 2718–2735. [Google Scholar] [CrossRef] [Green Version]
- Richardson, D.S.; Lichtman, J.W. Clarifying Tissue Clearing. Cell 2015, 162, 246–257. [Google Scholar] [CrossRef] [Green Version]
- Keereweer, S.; Van Driel, P.B.A.A.; Snoeks, T.J.A.; Kerrebijn, J.D.F.; De Jong, R.J.B.; Vahrmeijer, A.L.; Sterenborg, H.J.C.M.; Löwik, C.W.G.M. Optical Image-Guided Cancer Surgery: Challenges and Limitations. Clin. Cancer Res. 2013, 19, 3745–3754. [Google Scholar] [CrossRef] [Green Version]
- Costa, E.C.; Silva, D.N.; Moreira, A.F.; Correia, I.J. Optical clearing methods: An overview of the techniques used for the imaging of 3D spheroids. Biotechnol. Bioeng. 2019, 116, 2742–2763. [Google Scholar] [CrossRef]
- Desmaison, A.; Guillaume, L.; Triclin, S.; Weiss, P.; Ducommun, B.; Lobjois, V. Impact of physical confinement on nuclei geometry and cell division dynamics in 3D spheroid. Sci. Rep. 2018, 8, 8785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grist, S.M.; Nasseri, S.S.; Poon, T.; Roskelley, C.; Cheung, K.C. On-chip clearing of arrays of 3-D cell cultures and micro-tissues. Biomicrofluidics 2016, 10, 044107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshioka, T.; Shien, K.; Namba, K.; Torigoe, H.; Sato, H.; Tomida, S.; Yamamoto, H.; Asano, H.; Soh, J.; Tsukuda, K.; et al. Antitumor activity of pan-HER inhibitors in HER2-positive gastric cancer. Cancer Sci. 2018, 109, 1166–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carver, K.; Ming, X.; Juliano, R.L. Multicellular Tumor Spheroids as a Model for Assessing Delivery of Oligonucleotides in Three Dimensions. Mol. Ther. Nucleic Acids 2014, 3, e153. [Google Scholar] [CrossRef]
- Parra, E.R.; Villalobos, P.; Mino, B.; Rodriguez-Canales, J. Comparison of Different Antibody Clones for Immunohistochemistry Detection of Programmed Cell Death Ligand 1 (PD-L1) on Non-Small Cell Lung Carcinoma. Appl. Immunohisto. Mol. Morphol. 2018, 26, 83–93. [Google Scholar] [CrossRef]
- Baltzarsen, P.B.; Georgsen, J.B.; Nielsen, P.S.; Steiniche, T.; Stougaard, M. Detection of mRNA of Telomerase Protein in Benign Naevi and Melanomas Using RNAscope. Appl. Immunohisito. Mol. Morphol. 2020, 28, 36–41. [Google Scholar] [CrossRef]
- Zankel, A.; Wagner, J.; Poelt, P. Serial sectioning methods for 3D investigations in materials science. Micron 2014, 62, 66–78. [Google Scholar] [CrossRef]
- Hoffpauir, B.K.; Pope, B.A.; Spirou, G.A. Serial sectioning and electron microscopy of large tissue volumes for 3D analysis and reconstruction: A case study of the calyx of Held. Nat. Protoc. 2007, 2, 9–22. [Google Scholar] [CrossRef]
- Gebhard, C.; Gabriel, C.; Walter, I. Morphological and Immunohistochemical Characterization of Canine Osteosarcoma Spheroid Cell Cultures. Anat. Histol. Embryol. 2016, 45, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Tebcherani, A.J.; De Andrade, H.F., Jr.; Sotto, M.N. Diagnostic utility of immunohistochemistry in distinguishing trichoepthelioma and basal cell carcinoma: Evaluation using tissue microarray samples. Mod. Pathol. 2012, 25, 1345–1353. [Google Scholar] [CrossRef]
- Wong, H.H.; Chu, P. Immunohistochemical features of the gastrointestinal tract tumors. J. Gastrointest. Oncol. 2012, 3, 262–284. [Google Scholar]
- Caldwell, R.L.; Caprioli, R.M. Tissue Profiling by Mass Spectrometry. Mol. Cell. Proteom. 2013, 4, 394–401. [Google Scholar] [CrossRef] [Green Version]
- Shirure, V.S.; Bi, Y.; Curtis, M.B.; Lezia, A.; Goedegebuure, M.M.; Goedegebuure, S.P.; Aft, R.; Fields, R.C.; George, S.C. Tumor-on-a-chip platform to investigate progression and drug sensitivity in cell lines and patient-derived organoids. Lab Chip 2018, 18, 3687–3702. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, D.P.; Grabowska, A.M. In Vitro Tissue Microarrays for Quick and Efficient Spheroid Characterization. SLAS Discov. 2018, 23, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balmana, M.; Mereiter, S.; Diniz, F.; Feijao, T.; Barrias, C.C.; Reis, C.A. Multicellular Human Gastrix Cancer Spheroids Mimic the Glycosylation Phenotype of Gastric Carcinomas. Molecules 2018, 23, 2815. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.Z.; Chang, H.Y. Recent advances in there-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008, 3, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.Y.; Hou, C.W.; Li, X.J.; Jin, Z.J. Actuation Mechanism of Microvalves: A Review. Micromachines 2020, 11, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, W.; Park, S. A Microfluidic Spheroid Culture Device with a Concentration Gradient Generator for High-Throughput Screening of Drug Efficacy. Molecules 2018, 23, 3355. [Google Scholar] [CrossRef] [Green Version]
- Junttila, M.R.; De Sauvae, F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013, 501, 346–354. [Google Scholar] [CrossRef]
- Riffle, S.; Hedge, R.S. Modeling tumor cell adaptations to hypoxia in multicellular tumor spheroids. Clin. Cancer Res. 2017, 36, 102. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuriu, S.; Kadonosono, T.; Kizaka-Kondoh, S.; Ishida, T. Slicing Spheroids in Microfluidic Devices for Morphological and Immunohistochemical Analysis. Micromachines 2020, 11, 480. https://doi.org/10.3390/mi11050480
Kuriu S, Kadonosono T, Kizaka-Kondoh S, Ishida T. Slicing Spheroids in Microfluidic Devices for Morphological and Immunohistochemical Analysis. Micromachines. 2020; 11(5):480. https://doi.org/10.3390/mi11050480
Chicago/Turabian StyleKuriu, Satoru, Tetsuya Kadonosono, Shinae Kizaka-Kondoh, and Tadashi Ishida. 2020. "Slicing Spheroids in Microfluidic Devices for Morphological and Immunohistochemical Analysis" Micromachines 11, no. 5: 480. https://doi.org/10.3390/mi11050480






