Label-Free, High-Throughput Assay of Human Dendritic Cells from Whole-Blood Samples with Microfluidic Inertial Separation Suitable for Resource-Limited Manufacturing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Device Design and Simulation Studies
2.2. Device Fabrication in Resource-Limited Settings
2.3. Device Validation with Assay of Polystyrene Microparticles
2.4. Blood Cells Sample Preparation
2.5. Blood Cell Selection Assay
2.6. Statistical Analysis
3. Results
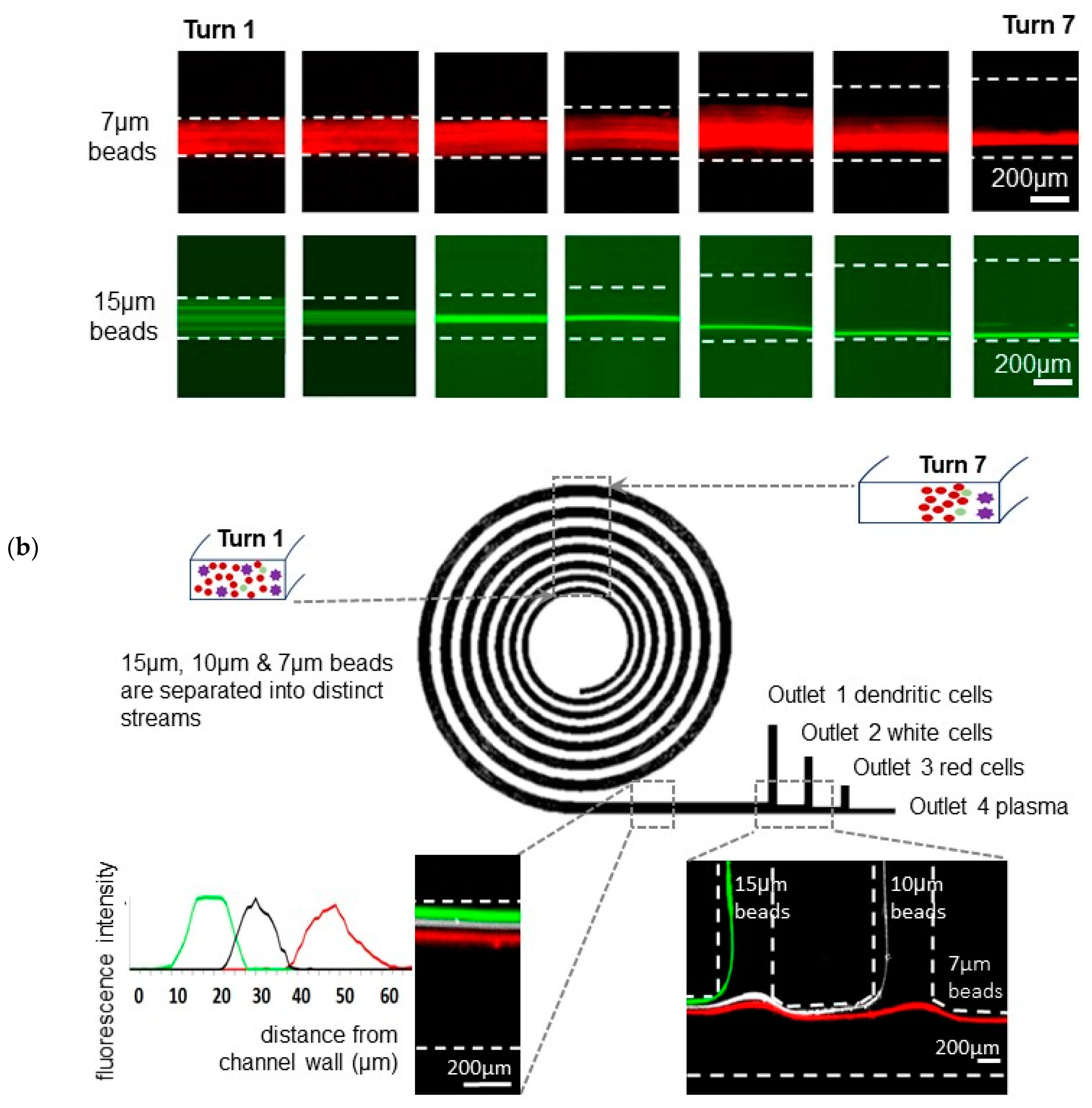
3.1. A Spiral Microfluidic Device with Channels Widths Exceeding 200 μm, for Focused Fractionation of Multiple Particle Sizes
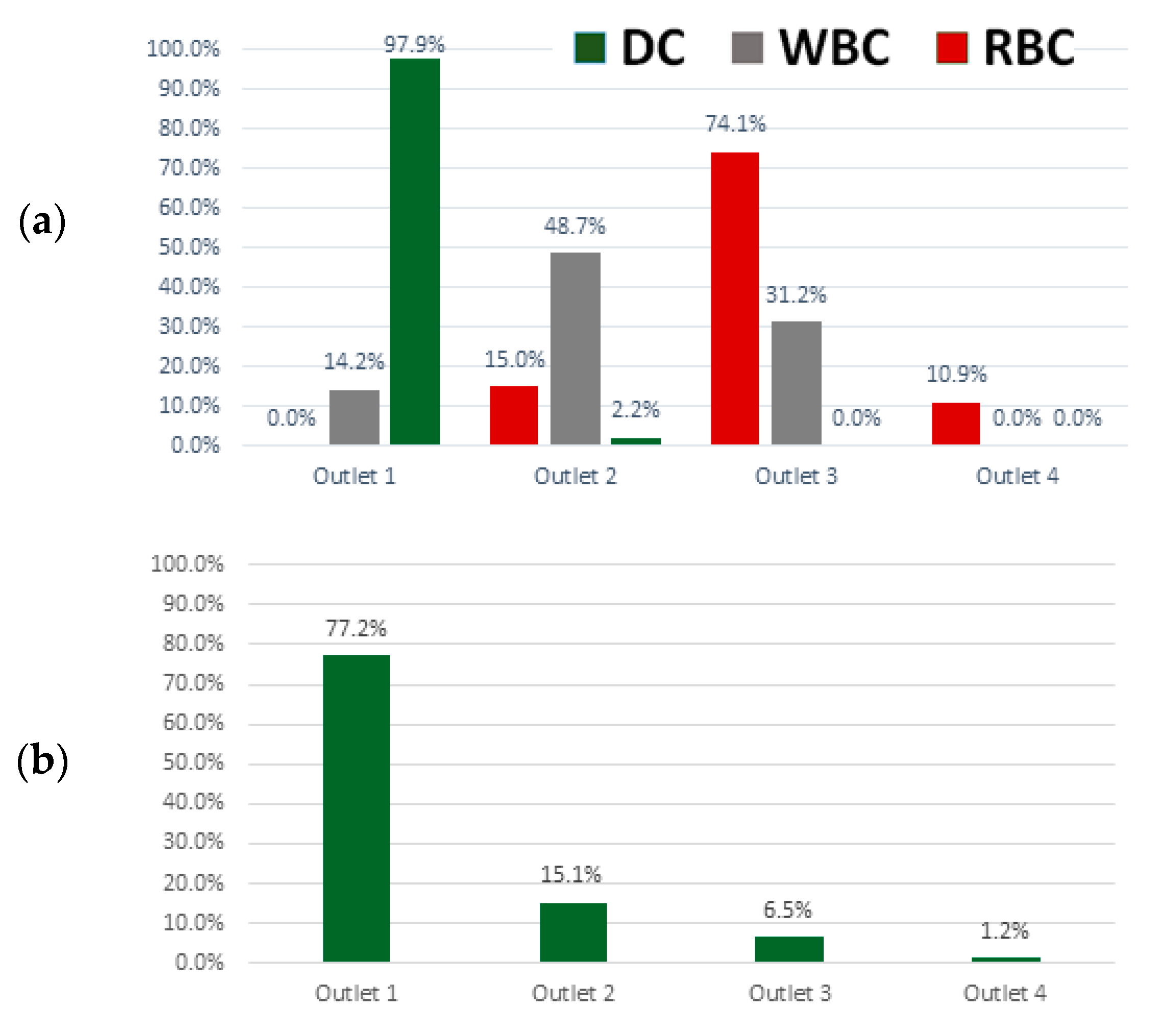
3.2. Red, White Blood and Dendritic Cell Separation Assay
3.3. Device Performance under Variations in Flow Rate and Cell Concentration
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Convery, N.; Gadegaard, N. 30 years of microfluidics. Micro Nano Eng. 2019, 2, 76–91. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Littman, D.R. Releasing the Brakes on Cancer Immunotherapy. Cell 2015, 162, 1186–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drain, P.K.; Hyle, E.P.; Noubary, F.; Freedberg, K.A.; Wilson, D.; Bishai, W.R.; Rodriguez, W.; Bassett, I. V Diagnostic point-of-care tests in resource-limited settings. Lancet Infect. Dis. 2014, 14, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Chin, C.D.; Linder, V.; Sia, S.K. Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip 2012, 12, 2118–2134. [Google Scholar] [CrossRef] [PubMed]
- Yousuff, C.; Ho, E.; Hussain, K.I.; Hamid, N. Microfluidic Platform for Cell Isolation and Manipulation Based on Cell Properties. Micromachines 2017, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Yan, S.; Miao, C.; Li, L.; Shi, W.; Liu, X.; Luo, Y.; Liu, T.; Lin, B.; Wu, W.; et al. Paper Microfluidics for Cell Analysis. Adv. Healthc. Mater. 2019, 8, 1801084. [Google Scholar] [CrossRef]
- Carrell, C.; Kava, A.; Nguyen, M.; Menger, R.; Munshi, Z.; Call, Z.; Nussbaum, M.; Henry, C. Beyond the lateral flow assay: A review of paper-based microfluidics. Microelectron. Eng. 2019, 206, 45–54. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the Developing World: Microfluidic Paper-Based Analytical Devices. Anal. Chem. 2010, 82, 3–10. [Google Scholar] [CrossRef]
- Palucka, K.; Banchereau, J. Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer 2012, 12. [Google Scholar] [CrossRef]
- Gelao, L.; Locatelli, M.A.; Minchella, I.; Placido, S. De Dendritic cell-based vaccines: Clinical applications in breast cancer. Immunotherapy 2014, 6, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Bol, K.F.; Schreibelt, G.; Gerritsen, W.R.; de Vries, I.J.M.; Figdor, C.G. Dendritic Cell-Based Immunotherapy: State of the Art and Beyond. Clin. Cancer Res. 2016, 22, 1897–1906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strasser, E.F.; Eckstein, R. Optimization of Leukocyte Collection and Monocyte Isolation for Dendritic Cell Culture. Transfus. Med. Rev. 2010, 24, 130–139. [Google Scholar] [CrossRef]
- Thurner, B.; Röder, C.; Dieckmann, D.; Heuer, M.; Kruse, M.; Glaser, A.; Keikavoussi, P.; Kämpgen, E.; Bender, A.; Schuler, G. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J. Immunol. Methods 1999, 223, 1–15. [Google Scholar] [CrossRef]
- Schreibelt, G.; Bol, K.F.; Westdorp, H.; Wimmers, F.; Aarntzen, E.H.J.G.; Boer, T.D.; Van De Rakt, M.W.M.M.; Scharenborg, N.M.; De Boer, A.J.; Pots, J.M.; et al. Effective Clinical Responses in Metastatic Melanoma Patients after Vaccination with Primary Myeloid Dendritic Cells. Clin. Cancer Res. 2016, 22, 2155–2167. [Google Scholar] [CrossRef] [Green Version]
- Prue, R.L.; Vari, F.; Radford, K.J.; Tong, H.; Hardy, M.Y.; Rozario, R.D.; Waterhouse, N.J.; Rossetti, T.; Coleman, R.; Tracey, C.; et al. A Phase I Clinical Trial of CD1c ( BDCA-1 ) + Dendritic Cells Pulsed With HLA-A * 0201 Peptides for Immunotherapy of Metastatic Hormone Refractory Prostate Cancer. J. Immunother. 2015, 38, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Archer, G.E.; Tedder, T.F. Isolation and generation of human dendritic cells. Curr Protoc Immunol. 2012, Chapter 7, 1–31. [Google Scholar] [CrossRef]
- Nathamgari, S.S.P.; Dong, B.; Zhou, F.; Kang, W.; Giraldo-vela, J.P. Isolating single cells in a neurosphere assay using inertial microfluidics. Lab Chip 2015, 15, 4591–4597. [Google Scholar] [CrossRef]
- Son, J.; Murphy, K.; Samuel, R.; Gale, B.K.; Carrell, D.T.; Hotaling, J.M. Non-motile sperm cell separation using a spiral channel. Anal. Methods 2015, 7, 8041–8047. [Google Scholar] [CrossRef]
- Lee, W.C.; Bhagat, A.A.S.; Huang, S.; Van Vliet, K.J.; Han, J.; Lim, C.T. High-throughput cell cycle synchronization using inertial forces in spiral microchannels. Lab Chip 2011, 11, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Warkiani, M.E.; Wu, L.; Kah, A.; Tay, P.; Han, J. Large-Volume Microfluidic Cell Sorting For Biomedical Applications. Annu. Rev. Biomed. Eng. 2015, 17, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, M.; Liu, C.; Zhang, Y.; Liu, D.; Liu, W.; Hu, G.; Jiang, X. Double spiral microchannel for label-free tumor cell separation and enrichment. Lab Chip 2012, 12, 3952–3960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuntaegowdanahalli, S.S.; Bhagat, A.A.S.; Kumar, G.; Papautsky, I. Inertial microfluidics for continuous particle separation in spiral microchannels. Lab Chip 2009, 9, 2973–2980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoo, B.L.; Warkiani, M.E.; Tan, D.S.W.; Bhagat, A.A.S.; Irwin, D.; Lau, D.P.; Lim, A.S.T.; Lim, K.H.; Krisna, S.S.; Lim, W.T.; et al. Clinical validation of an ultra high-throughput spiral microfluidics for the detection and enrichment of viable circulating tumor cells. PLoS ONE 2014, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Guan, G.; Wu, L.; Bhagat, A.A.; Li, Z.; Chen, P.C.Y.; Chao, S.; Ong, C.J.; Han, J. Spiral microchannel with rectangular and trapezoidal cross-sections for size based particle separation. Sci. Rep. 2013, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Nivedita, N.; Papautsky, I. Continuous separation of blood cells in spiral microfluidic devices. Biomicrofluidics 2013, 7, 054101. [Google Scholar] [CrossRef] [Green Version]
- Pinto, E.; Faustino, V.; Rodrigues, R.O.; Pinho, D.; Garcia, V.; Miranda, J.M.; Lima, R. A Rapid and Low-Cost Nonlithographic Method to Fabricate Biomedical Microdevices for Blood. Micromachinesi 2015, 6, 121–135. [Google Scholar] [CrossRef] [Green Version]
- Bartholomeusz, D.A.; Boutte, R.W.; Andrade, J.D. Xurography: Rapid prototyping of microstructures using a cutting plotter. J. Microelectromechanical Syst. 2005, 14, 1364–1374. [Google Scholar] [CrossRef]
- Speller, N.C.; Morbioli, G.G.; Cato, M.E.; Cantrell, T.P.; Leydon, E.M.; Schmidt, B.E.; Stockton, A.M. Cutting edge microfluidics: Xurography and a microwave. Sensors Actuators B Chem. 2019, 291, 250–256. [Google Scholar] [CrossRef]
- Mohamed Yousuff, C.; B Hamid, N.H.; Kamal Basha, I.H.; Wei Ho, E.T. Output channel design for collecting closely-spaced particle streams from spiral inertial separation devices. AIP Adv. 2017, 7, 085004. [Google Scholar] [CrossRef] [Green Version]
- Bhagat, A.A.S.; Kuntaegowdanahalli, S.S.; Papautsky, I. Continuous particle separation in spiral microchannels using dean flows and differential migration. Lab Chip 2008, 8, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, D.; Irimia, D.; Tompkins, R.G.; Toner, M. Continuous inertial focusing, ordering, and separation of particles in microchannels. Proc. Natl. Acad. Sci. USA 2007, 104, 18892–18897. [Google Scholar] [CrossRef] [Green Version]
- Di Carlo, D.; Edd, J.F.; Humphry, K.J.; Stone, H.A.; Toner, M. Particle Segregation and Dynamics in Confined Flows. Phys. Rev. Lett. 2009, 102, 094503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahkeshani, S.; Haddadi, H.; Carlo, D. Di Preferred interparticle spacings in trains of particles in inertial microchannel flows. Cambridge Univ. Press 2016, 786, R3. [Google Scholar]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid Prototyping of Microfluidic Systems in Poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Li, M.; Alici, G.; Nguyen, N.T. Particle inertial focusing and its mechanism in a serpentine microchannel. Microfluid. Nanofluidics 2014, 17, 305–316. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Yuan, D.; Alici, G.; Nguyen, N.-T.; Majid Ebrahimi Warkianic, W.L. Fundamentals and applications of inertial microfluidics: A review. Lab Chip 2016, 16, 10–34. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.T.; Xu, Y.Q.; Tang, X.Y. A Numerical Simulation of Cell Separation by Simplified Asymmetric Pinched Flow Fractionation. Comput. Math. Methods Med. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Rafeie, M.; Zhang, J.; Asadnia, M.; Li, W.; Warkiani, M.E. Multiplexing slanted spiral microchannels for ultra-fast blood plasma separation. Lab Chip 2016, 16, 2791–2802. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, D.; Sluyter, R.; Yan, S.; Zhao, Q.; Xia, H.; Tan, S.H.; Nguyen, N.T.; Li, W. High-Throughput Separation of White Blood Cells from Whole Blood Using Inertial Microfluidics. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 1422–1430. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Amini, H.; Stone, H.A.; Di Carlo, D. Dynamic self-assembly and control of microfluidic particle crystals. Proc. Natl. Acad. Sci. USA 2010, 107, 22413–22418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martel, J.M.; Toner, M. Inertial focusing dynamics in spiral microchannels. Phys. Fluids 2012, 24, 032001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reece, A.E.; Oakey, J.; Reece, A.E.; Oakey, J. Long-range forces affecting equilibrium inertial focusing behavior in straight high aspect ratio microfluidic channels Long-range forces affecting equilibrium inertial focusing behavior in straight high aspect ratio microfluidic channels. Phys. Fluids 2016, 28, 043303. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Position | Channel Depth (µm) | Desired Channel Width (µm) | Fabricated Channel Width (µm) | Error (%) |
|---|---|---|---|---|
| Turn 2 | 100 | 200 | 208 | 4 |
| Turn 2 | 100 | 200 | 193 | 3.5 |
| Turn 4 | 100 | 400 | 390 | 2.5 |
| Turn 4 | 100 | 400 | 412 | 3 |
| Turn 6 | 100 | 600 | 589 | 1.8 |
| Turn 6 | 100 | 600 | 608 | 1.3 |
| Spiral Position | Fluid Velocity, Uf (m/s) | Reynolds Number, Re | Dean Number, De | Particle Reynolds Number, Rp | ||
|---|---|---|---|---|---|---|
| 7 μm | 10 μm | 15 μm | ||||
| Inlet | 1.50 | 200 | 36.51 | 0.60 | 1.13 | 2.53 |
| Turn 1 | 1.0 | 150 | 27.09 | 0.36 | 0.67 | 1.5 |
| Turn 2 | 0.86 | 133 | 22.84 | 0.30 | 0.55 | 1.24 |
| Turn 3 | 0.75 | 120 | 19.75 | 0.25 | 0.47 | 1.05 |
| Turn 4 | 0.67 | 109 | 16.91 | 0.22 | 0.41 | 0.92 |
| Turn 5 | 0.60 | 100 | 14.61 | 0.19 | 0.36 | 0.81 |
| Turn 6 | 0.55 | 92 | 12.72 | 0.17 | 0.32 | 0.73 |
| Turn 7 | 0.50 | 86 | 11.16 | 0.16 | 0.29 | 0.66 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caffiyar, M.Y.; Lim, K.P.; Basha, I.H.K.; Hamid, N.H.; Cheong, S.C.; Ho, E.T.W. Label-Free, High-Throughput Assay of Human Dendritic Cells from Whole-Blood Samples with Microfluidic Inertial Separation Suitable for Resource-Limited Manufacturing. Micromachines 2020, 11, 514. https://doi.org/10.3390/mi11050514
Caffiyar MY, Lim KP, Basha IHK, Hamid NH, Cheong SC, Ho ETW. Label-Free, High-Throughput Assay of Human Dendritic Cells from Whole-Blood Samples with Microfluidic Inertial Separation Suitable for Resource-Limited Manufacturing. Micromachines. 2020; 11(5):514. https://doi.org/10.3390/mi11050514
Chicago/Turabian StyleCaffiyar, Mohamed Yousuff, Kue Peng Lim, Ismail Hussain Kamal Basha, Nor Hisham Hamid, Sok Ching Cheong, and Eric Tatt Wei Ho. 2020. "Label-Free, High-Throughput Assay of Human Dendritic Cells from Whole-Blood Samples with Microfluidic Inertial Separation Suitable for Resource-Limited Manufacturing" Micromachines 11, no. 5: 514. https://doi.org/10.3390/mi11050514
APA StyleCaffiyar, M. Y., Lim, K. P., Basha, I. H. K., Hamid, N. H., Cheong, S. C., & Ho, E. T. W. (2020). Label-Free, High-Throughput Assay of Human Dendritic Cells from Whole-Blood Samples with Microfluidic Inertial Separation Suitable for Resource-Limited Manufacturing. Micromachines, 11(5), 514. https://doi.org/10.3390/mi11050514





