Hemispherical Microelectrode Array for Ex Vivo Retinal Neural Recording
Abstract
:1. Introduction
2. Materials and Methods
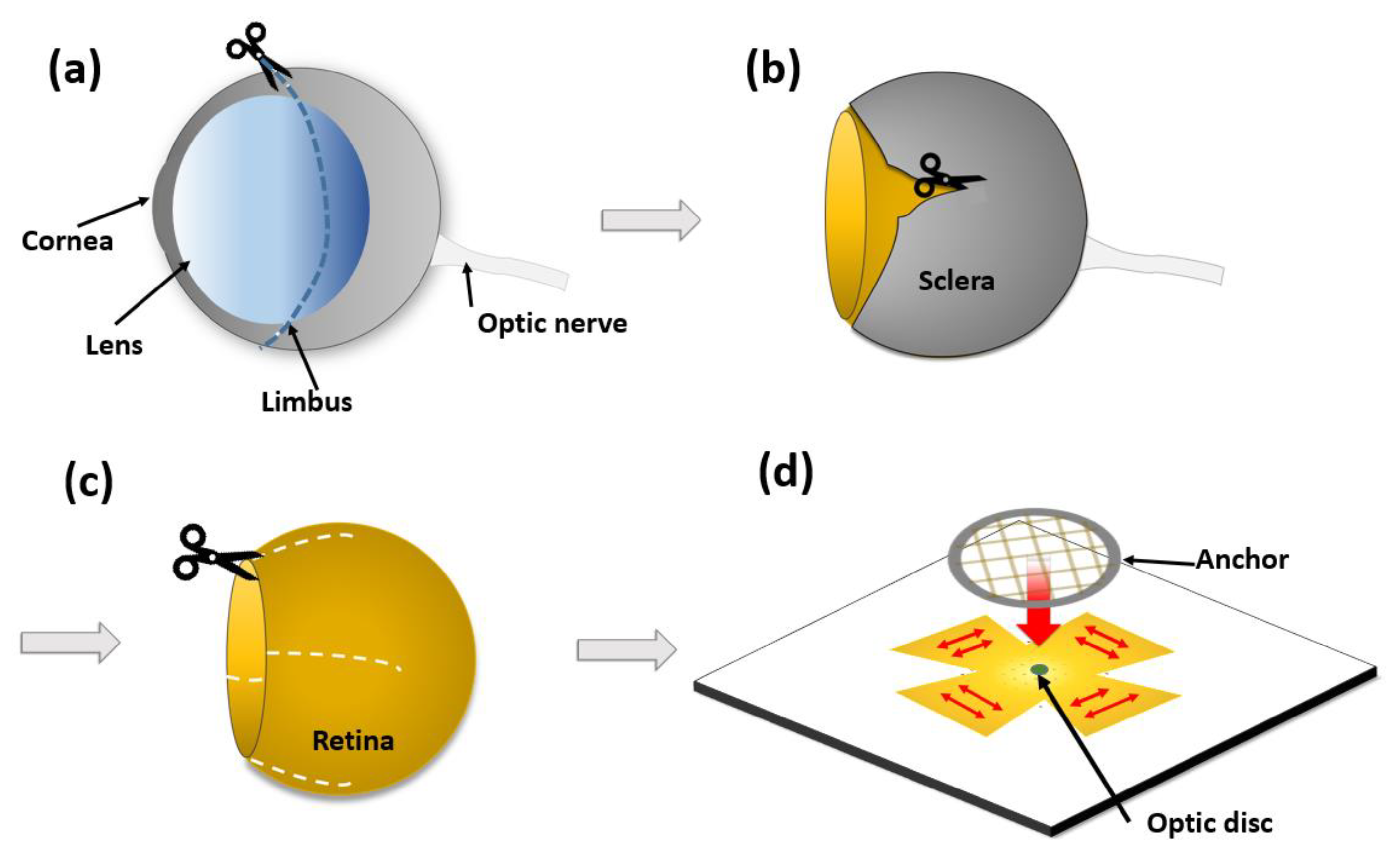
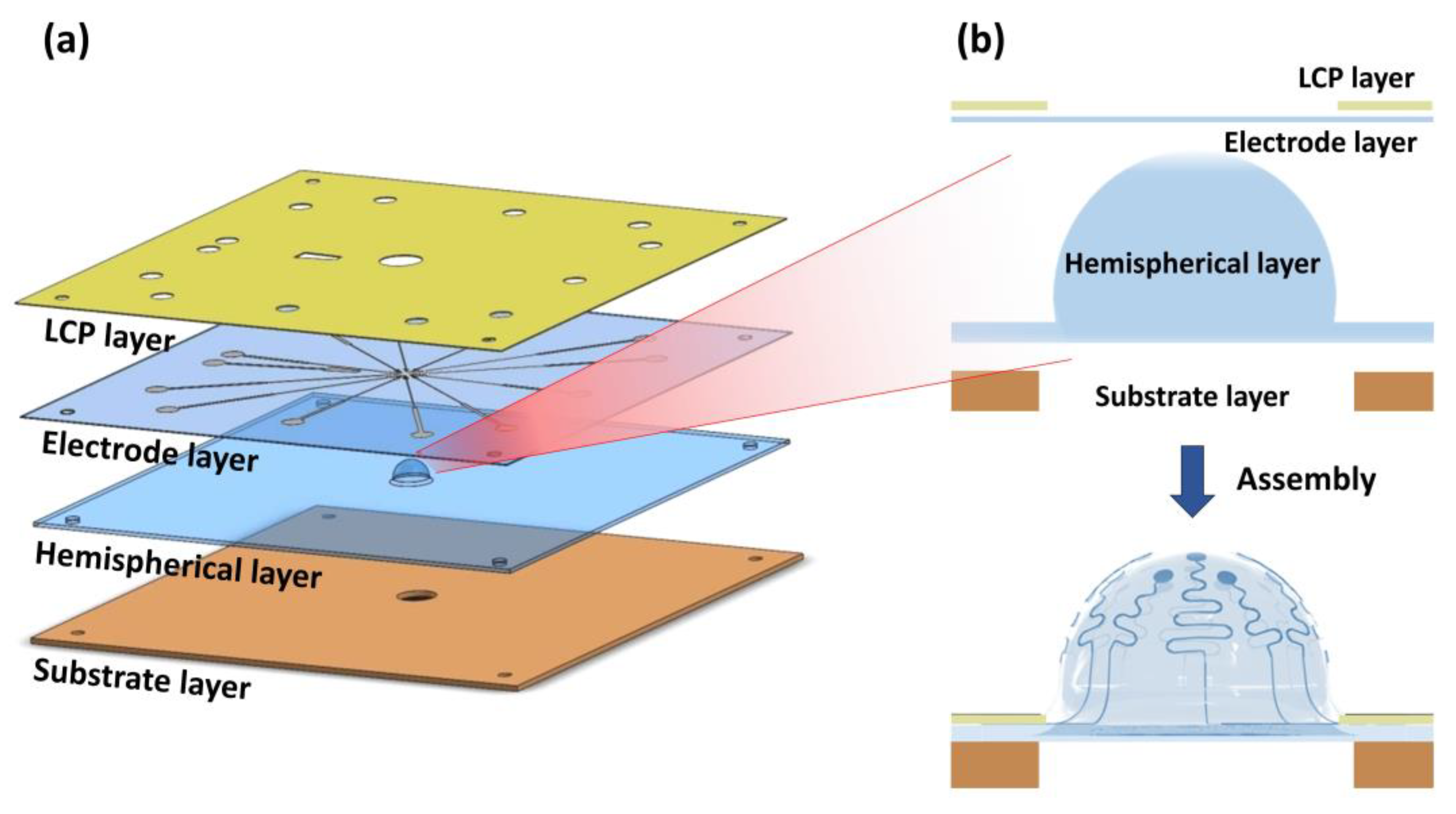
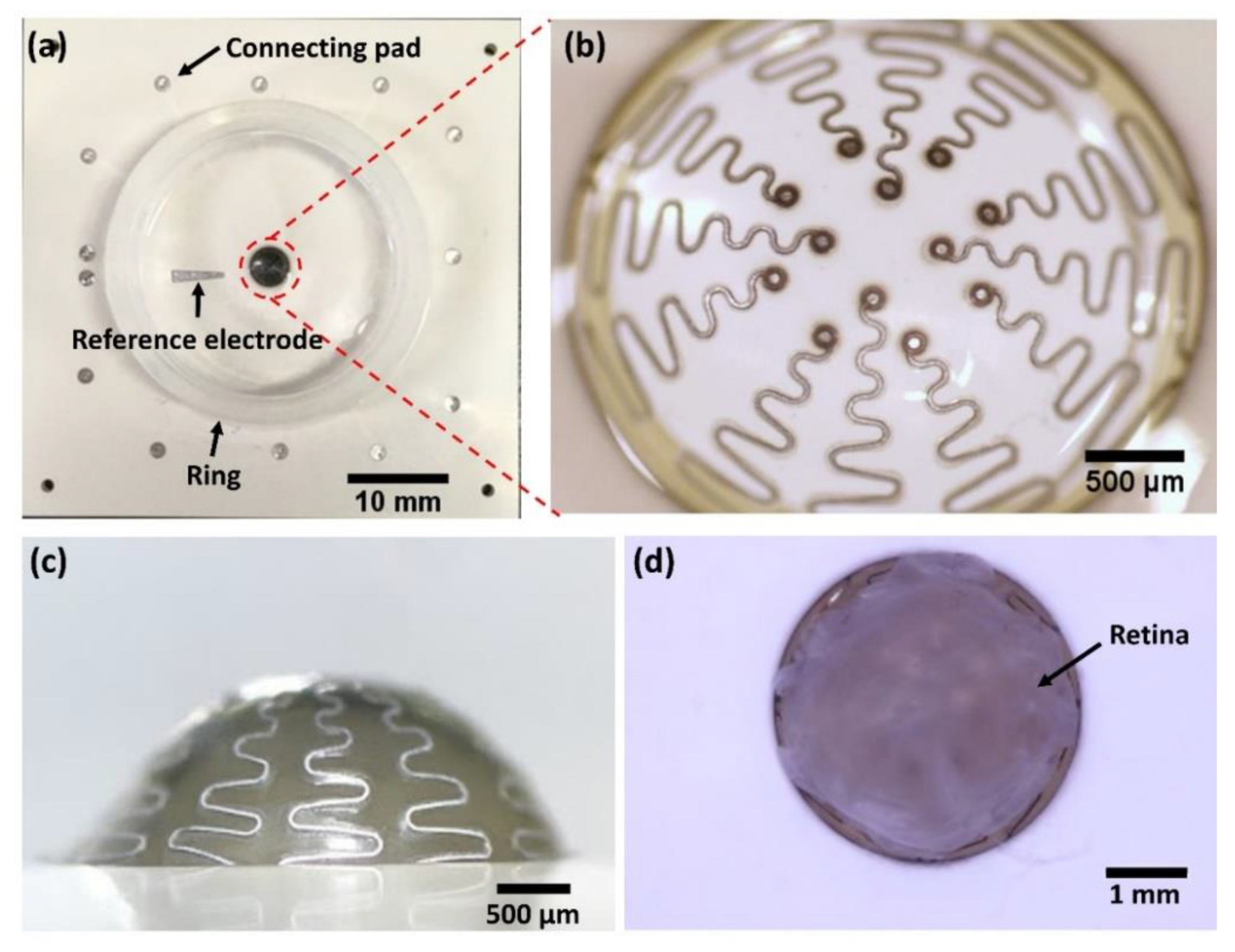
2.1. Overview of Hemispherical MEAs
2.2. Electrode Array Design
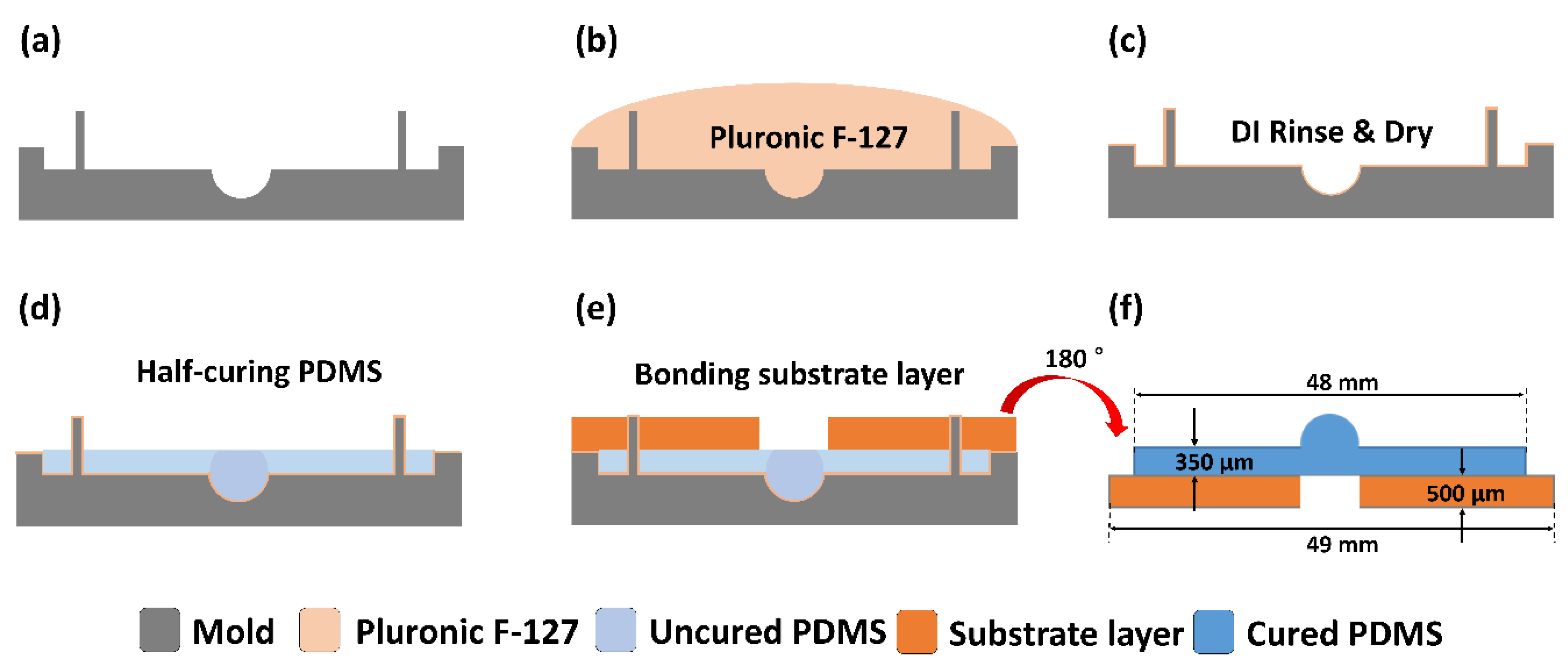
2.3. Fabrication of Hemispherical and Substrate Layers
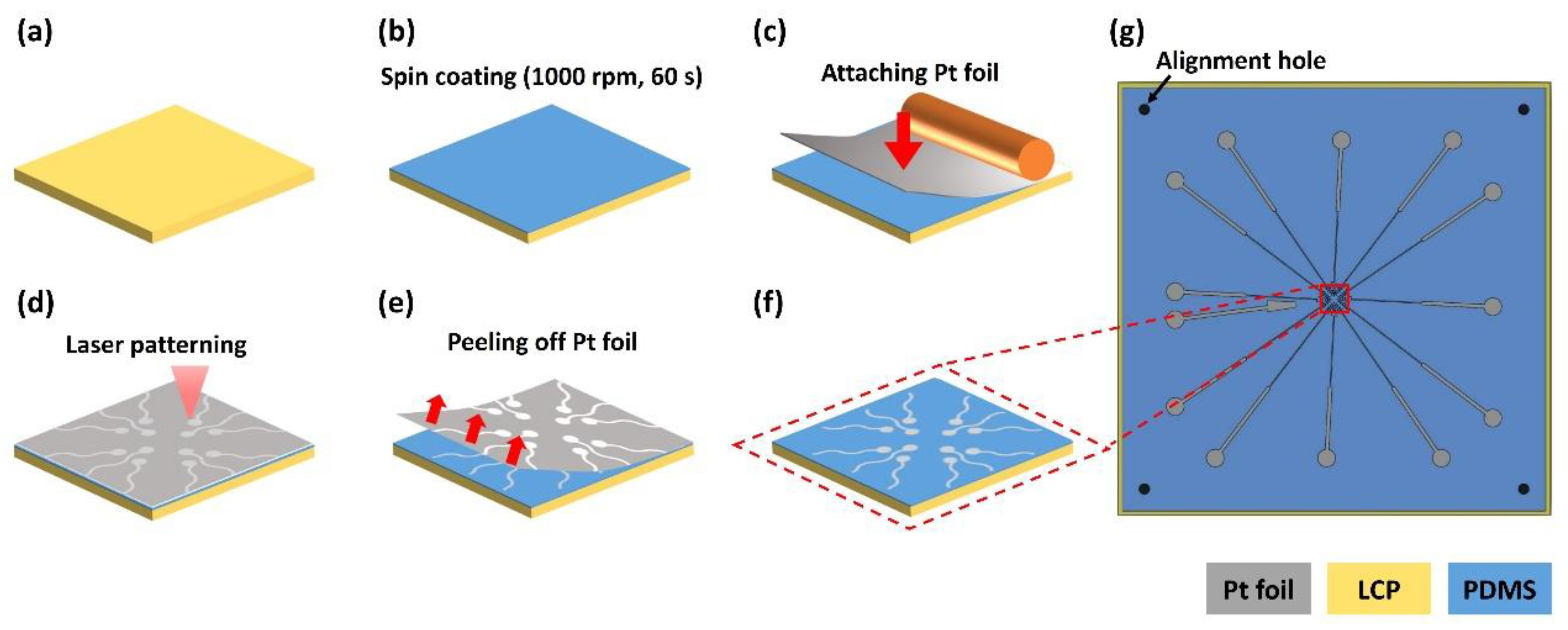
2.4. Fabrication of Electrode and Liquid Crystal Polymer (LCP) Layers
2.4.1. Bottom Layer Fabrication
2.4.2. Top Layer Fabrication
2.4.3. Bonding Between Top and Bottom Layers
2.5. Mechanical Simulation of Hemispherical Stretching
2.6. Final Assembly for Hemispherical MEA
2.7. Electrochemical Impedance Spectroscopy
2.8. Ex Vivo Retinal Neural Recording
2.8.1. Retina Preparation
2.8.2. Neural Recording
3. Results
3.1. Mechanical Simulation of Hemispherical Stretching
3.2. Electrode Fabrication
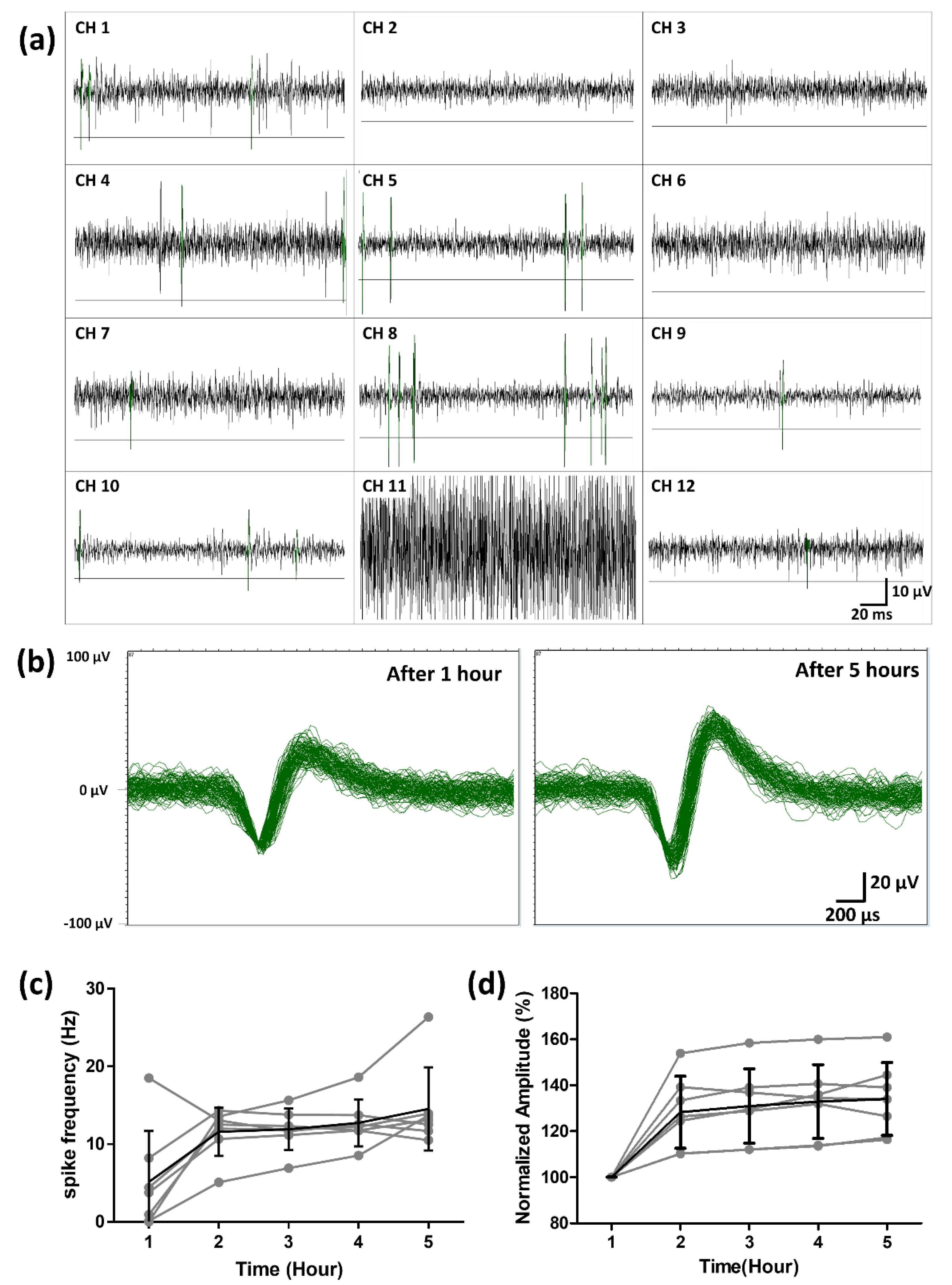
3.3. Ex Vivo Neural Recording from RGCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rizzo, S.; Belting, C.; Cinelli, L.; Allegrini, L.; Genovesi-Ebert, F.; Barca, F.; Di Bartolo, E. The Argus II Retinal Prosthesis: 12-Month Outcomes from a Single-Study Center. Am. J. Ophthalmol. 2014, 157, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Bloch, E.; Da Cruz, L. The Argus II Retinal Prosthesis System. In Prosthesis; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Ghodasra, D.H.; Chen, A.; Arevalo, J.F.; Birch, D.G.; Branham, K.; Coley, B.; Dagnelie, G.; De Juan, E.; Devenyi, R.G.; Dorn, J.D.; et al. Erratum to: Worldwide Argus II implantation: Recommendations to optimize patient outcomes. BMC Ophthalmol. 2016, 16, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zrenner, E.; Bartz-Schmidt, K.; Benav, H.; Besch, D.; Bruckmann, A.; Gabel, V.-P.; Gekeler, F.; Greppmaier, U.; Harscher, A.; Kibbel, S.; et al. Subretinal electronic chips allow blind patients to read letters and combine them to words. Proc. Biol. Sci. R. Soc. 2010, 278, 1489–1497. [Google Scholar] [CrossRef]
- Arsiero, M. Argus II Retinal Prosthesis System for peripheral retinal degeneration. Front. Neuroeng. 2014, 7. [Google Scholar] [CrossRef] [Green Version]
- Sekirnjak, C.; Hottowy, P.; Sher, A.; Dabrowski, W.; Litke, A.M.; Chichilnisky, E.J. High-Resolution Electrical Stimulation of Primate Retina for Epiretinal Implant Design. J. Neurosci. 2008, 28, 4446–4456. [Google Scholar] [CrossRef]
- Yang, F.; Yang, C.-H.; Wang, F.-M.; Cheng, Y.-T.; Teng, C.-C.; Lee, L.-J.; Yang, C.-H.; Fan, L.-S. A high-density microelectrode-tissue-microelectrode sandwich platform for application of retinal circuit study. BioMed. Eng. OnLine 2015, 14, 109. [Google Scholar] [CrossRef] [Green Version]
- Vurro, M.; Crowell, A.; Pezaris, J. Simulation of Thalamic Prosthetic Vision: Reading Accuracy, Speed and Acuity in Sighted Humans. Front. Hum. Neurosci. 2014, 8, 816. [Google Scholar] [CrossRef] [Green Version]
- Goo, Y.S.; Ye, J.H.; Lee, S.; Nam, Y.; Ryu, S.B.; Kim, K.H. Retinal ganglion cell responses to voltage and current stimulation in wild-type and rd1 mouse retinas. J. Neural Eng. 2011, 8, 035003. [Google Scholar] [CrossRef]
- Hosseinzadeh, Z.; Jalligampala, A.; Zrenner, E.; Rathbun, D.L. The Spatial Extent of Epiretinal Electrical Stimulation in the Healthy Mouse Retina. Neurosignals 2017, 25, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Adly, N.; Weidlich, S.; Seyock, S.; Brings, F.; Yakushenko, A.; Offenhäusser, A.; Wolfrum, B. Printed microelectrode arrays on soft materials: From PDMS to hydrogels. Npj Flex. Electron. 2018, 2, 15. [Google Scholar] [CrossRef]
- Agar, A.; Li, S.; Agarwal, N.; Coroneo, M.; Hill, M. Retinal ganglion cell line apoptosis induced by hydrostatic pressure. Brain Res. 2006, 1086, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Agar, A.; Yip, S.S.; Hill, M.A.; Coroneo, M.T. Pressure related apoptosis in neuronal cell lines. J. Neurosci. Res. 2000, 60, 495–503. [Google Scholar] [CrossRef]
- Armaly, M.F.; Krueger, D.E.; Maunder, L.; Becker, B.; Hetherington, J., Jr.; Kolker, A.E.; Levene, R.Z.; Maumenee, A.E.; Pollack, I.P.; Shaffer, R.N. Biostatistical analysis of the collaborative glaucoma study. I. Summary report of the risk factors for glaucomatous visual-field defects. Arch. Ophthalmol. 1980, 98, 2163–2171. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.E.; Donna, P.; Almonte, M.T.; Cordeiro, M.F. Real-Time Imaging of Retinal Ganglion Cell Apoptosis. Cells 2018, 7, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Wang, W.; Li, X.; Huang, C.; Zhang, Z.; Yuan, M.; Li, X. High hydrostatic pressure induces apoptosis of retinal ganglion cells via regulation of the NGF signalling pathway. Mol. Med. Rep. 2019, 19, 5321–5334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, M.; Sunagawa, G.A.; Kondo, M.; Takahashi, M.; Mandai, M. Evaluation of micro Electroretinograms Recorded with Multiple Electrode Array to Assess Focal Retinal Function. Sci. Rep. 2016, 6, 30719. [Google Scholar] [CrossRef]
- Nakazawa, M.; Hara, A.; Ishiguro, S.I. Optical Coherence Tomography of Animal Models of Retinitis Pigmentosa: From Animal Studies to Clinical Applications. Biomed. Res. Int. 2019, 2019, 8276140. [Google Scholar] [CrossRef] [Green Version]
- Narayan, D.S.; Ao, J.; Wood, J.P.M.; Casson, R.J.; Chidlow, G. Spatio-temporal characterization of S- and M/L-cone degeneration in the Rd1 mouse model of retinitis pigmentosa. BMC Neurosci. 2019, 20, 46. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.W.; Shin, J.Y.; Pi, K.; Goo, Y.S.; Cho, D.D. Neuron Stimulation Device Integrated with Silicon Nanowire-Based Photodetection Circuit on a Flexible Substrate. Sens. Basel 2016, 16. [Google Scholar] [CrossRef] [Green Version]
- Pennesi, M.E.; Michaels, K.V.; Magee, S.S.; Maricle, A.; Davin, S.P.; Garg, A.K.; Gale, M.J.; Tu, D.C.; Wen, Y.; Erker, L.R.; et al. Long-term characterization of retinal degeneration in rd1 and rd10 mice using spectral domain optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 2012, 53, 4644–4656. [Google Scholar] [CrossRef] [Green Version]
- Zou, T.; He, J.; Chen, X.; Sun, D.; Fan, X.; Xu, H. Rescue of Retinal Degeneration in rd1 Mice by Intravitreally Injected Metformin. Front. Mol. Neurosci. 2019, 12, 102. [Google Scholar] [CrossRef]
- Ye, J.H.; Goo, Y.S. The slow wave component of retinal activity in rd/rd mice recorded with a multi-electrode array. Physiol. Meas. 2007, 28, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.J.; Rizzo, J.F. Activation of retinal ganglion cells in wild-type and rd1 mice through electrical stimulation of the retinal neural network. Vis. Res. 2008, 48, 1562–1568. [Google Scholar] [CrossRef] [Green Version]
- Widlund, T.; Yang, S.; Hsu, Y.-Y.; Lu, N. Stretchability and compliance of freestanding serpentine-shaped ribbons. Int. J. Solids Struct. 2014, 51, 4026–4037. [Google Scholar] [CrossRef] [Green Version]
- Hocheng, H.; Chen, C.M. Design, fabrication and failure analysis of stretchable electrical routings. Sens. Basel 2014, 14, 11855–11877. [Google Scholar] [CrossRef] [PubMed]
- Schmucker, C.; Schaeffel, F. A paraxial schematic eye model for the growing C57BL/6 mouse. Vis. Res. 2004, 44, 1857–1867. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.; Min, K.S.; Kim, S.J. Microfabrication process for long-term reliable neural electrode arrays using liquid crystal polymer (LCP). Microelectron. Eng. 2019, 216, 111096. [Google Scholar] [CrossRef]
- Yang, Y.; Hwang, Y.; Cho, H.A.; Song, J.H.; Park, S.J.; Rogers, J.A.; Ko, H.C. Arrays of silicon micro/nanostructures formed in suspended configurations for deterministic assembly using flat and roller-type stamps. Small 2011, 7, 484–491. [Google Scholar] [CrossRef]
- Gwon, T.M.; Min, K.S.; Kim, J.H.; Oh, S.H.; Lee, H.S.; Park, M.H.; Kim, S.J. Fabrication and evaluation of an improved polymer-based cochlear electrode array for atraumatic insertion. Biomed. Microdevices 2015, 17, 32. [Google Scholar] [CrossRef]
- Wen, J. PDMS-PDMS Bonding Protocol—Anatech. Protoc. Rep. 2015, 8, 1–4. [Google Scholar]
- Chen, C.F.; Wharton, K. Characterization and failure mode analyses of air plasma oxidized PDMS–PDMS bonding by peel testing. RSC Adv. 2017, 7, 1286–1289. [Google Scholar] [CrossRef] [Green Version]
- Park, T.L.; Yang, M.Y.; Shin, D.G.; Kim, D.E. A study on frictional characteristics of PDMS under various conditions. J. Korean Soc. Precis. Eng. 2018, 35, 803–807. [Google Scholar] [CrossRef]
- Stasheff, S.F. Emergence of sustained spontaneous hyperactivity and temporary preservation of OFF responses in ganglion cells of the retinal degeneration (rd1) mouse. J. Neurophysiol. 2008, 99, 1408–1421. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, H.; Xiao, L.; Duan, Y.; Zhu, H.; Bian, J.; Ye, D.; Yin, Z. Assembly and applications of 3D conformal electronics on curvilinear surfaces. Mater. Horiz. 2019, 6, 642–683. [Google Scholar] [CrossRef]
- Yeo, J.C.; Lim, C.T. Emerging flexible and wearable physical sensing platforms for healthcare and biomedical applications. Microsyst. Nanoeng. 2016, 2, 16043. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, Y.; Yoo, H.-J.; Shin, S.; Jun, S.B. Hemispherical Microelectrode Array for Ex Vivo Retinal Neural Recording. Micromachines 2020, 11, 538. https://doi.org/10.3390/mi11050538
Ha Y, Yoo H-J, Shin S, Jun SB. Hemispherical Microelectrode Array for Ex Vivo Retinal Neural Recording. Micromachines. 2020; 11(5):538. https://doi.org/10.3390/mi11050538
Chicago/Turabian StyleHa, Yoonhee, Hyun-Ji Yoo, Soowon Shin, and Sang Beom Jun. 2020. "Hemispherical Microelectrode Array for Ex Vivo Retinal Neural Recording" Micromachines 11, no. 5: 538. https://doi.org/10.3390/mi11050538
APA StyleHa, Y., Yoo, H.-J., Shin, S., & Jun, S. B. (2020). Hemispherical Microelectrode Array for Ex Vivo Retinal Neural Recording. Micromachines, 11(5), 538. https://doi.org/10.3390/mi11050538




