Multi-Bit Biomemory Based on Chitosan: Graphene Oxide Nanocomposite with Wrinkled Surface
Abstract
:1. Introduction
2. Materials and Methods
3. Results
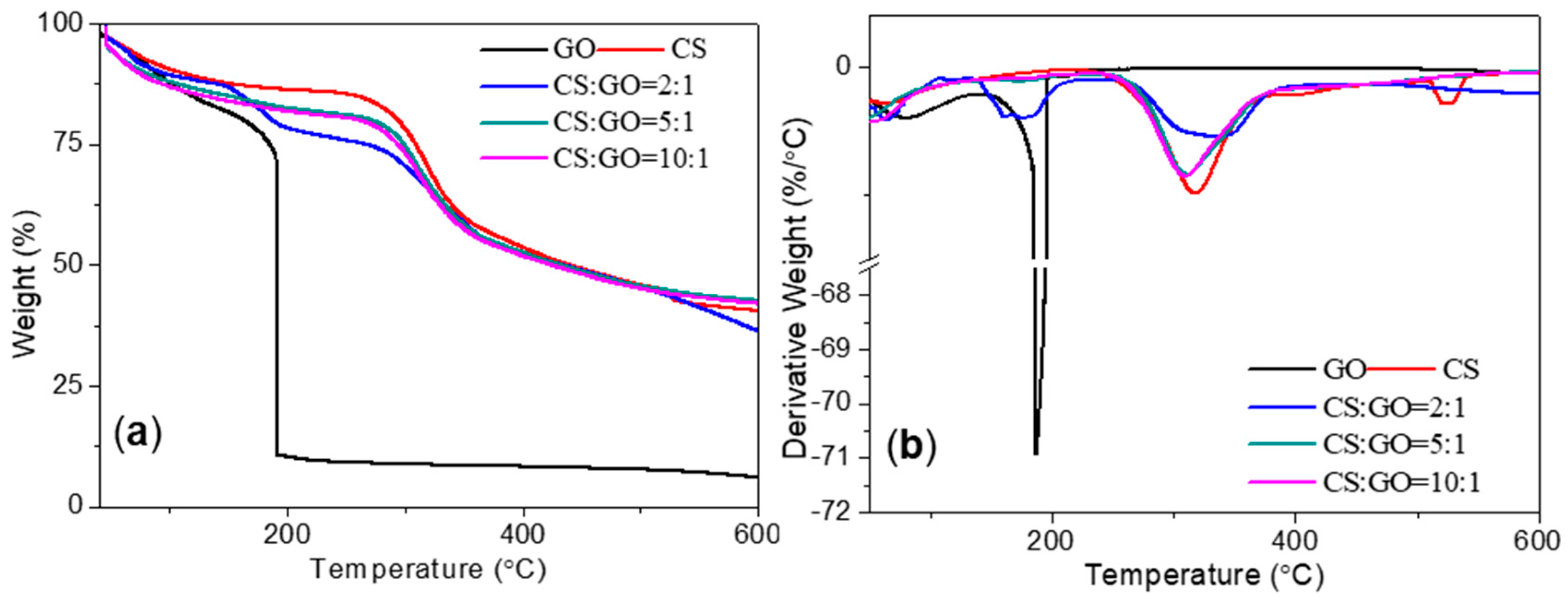
3.1. Characterization of CS:GO Nanocomposites
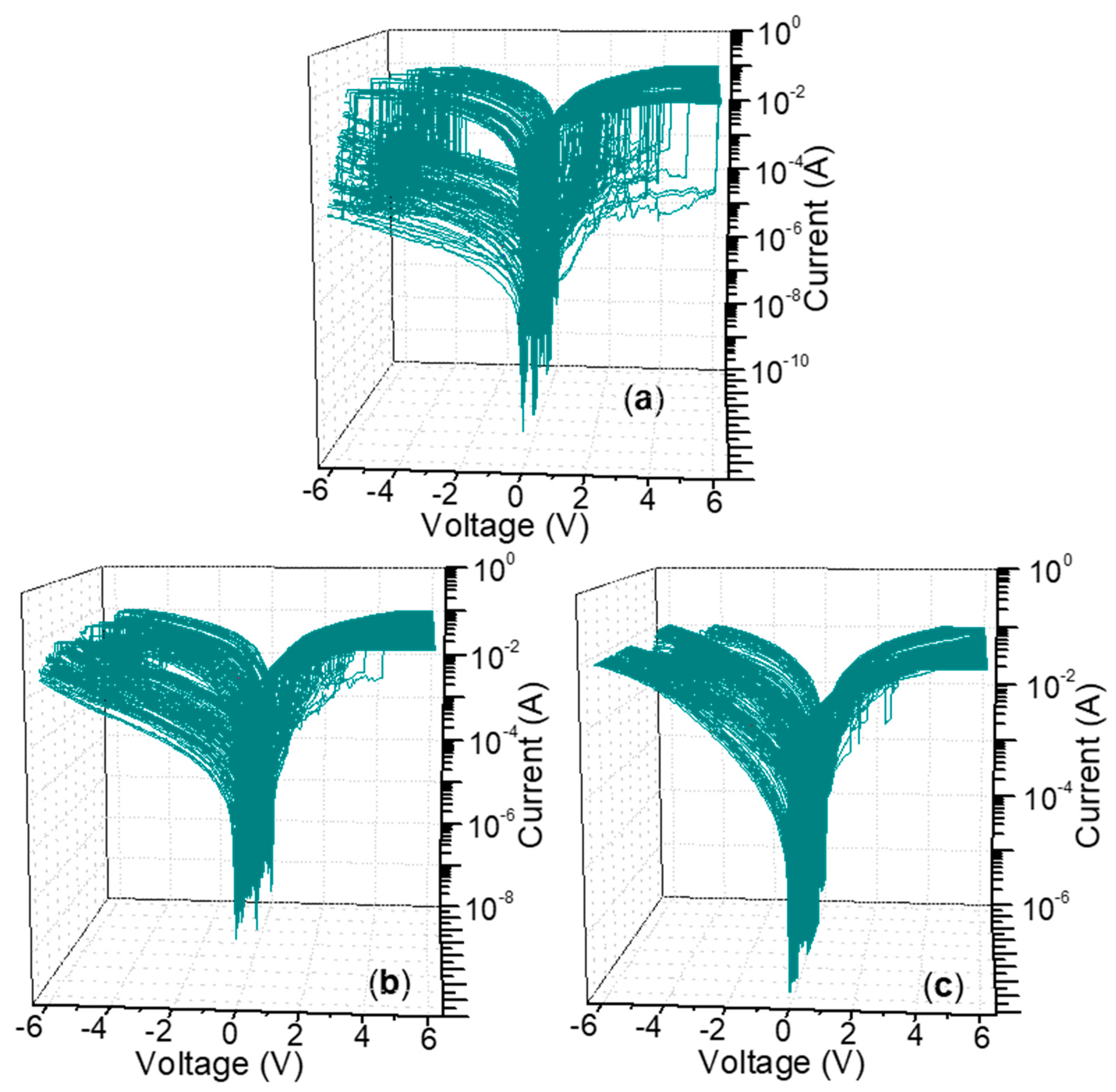
3.2. Multi-Bit Biomemory Performance of ITO/CS:GO/N
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hyun, C.J.; Woo, Y.C. Encapsulation of NEM memory switches for monolithic-three-dimensional (M3D) CMOS–NEM hybrid circuits. Micromachines 2018, 9, 317. [Google Scholar]
- Myeongsun, K.; Jongmin, H.; Ikhyeon, K.; Jae-Hee, H.; Seongjae, C.; Il, H.C. A novel one-transistor dynamic random-access memory (1T DRAM) featuring partially inserted wide-bandgap double barriers for high-temperature applications. Micromachines 2018, 9, 581. [Google Scholar]
- Li, L.; Wen, D. Memristic characteristics from bistable to tristable memory with controllable charge trap carbon nanotubes. Nanomaterials 2018, 8, 114. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wen, D. Ternary memristic effect of trilayer-structured graphene-based memory devices. Nanomaterials 2019, 9, 518. [Google Scholar] [CrossRef] [Green Version]
- Hwang, B.; Lee, J.S. Recent advances in memory devices with hybrid materials. Adv. Electron. Mater. 2019, 5, 1800519–1800540. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Mao, J.; Ren, Y.; Han, S.T.; Roy, V.A.L.; Zhou, Y. Recent advances of flexible data storage devices based on organic nanoscaled materials. Small 2018, 14, 1703126–1703152. [Google Scholar] [CrossRef]
- Jeong, D.S.; Hwang, C.S. Nonvolatile memory materials for neuromorphic intelligent machines. Adv. Mater. 2018, 30, 1704729–1704755. [Google Scholar] [CrossRef]
- Upadhyay, N.K.; Jiang, H.; Wang, Z.; Asapu, S.; Xia, Q.; Yang, J.J. Emerging memory devices for neuromorphic computing. Adv. Mater. Technol. 2019, 4, 1800589–1800601. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, S.R.; Zhou, L.; Mao, J.Y.; Han, S.T.; Ren, Y.; Yang, J.Q.; Wang, Y.; Zhai, Y.; Zhou, Y. Functional non-volatile memory devices: From fundamentals to photo-tunable properties. Phys. Status Solidi RRL 2019, 13, 1800644–1800664. [Google Scholar] [CrossRef]
- Chang, Y.C.; Lee, C.J.; Wang, L.W.; Wang, Y.H. Highly uniform resistive switching of solution-processed silver-embedded gelatin thin films. Small 2018, 14, 1703888–1703895. [Google Scholar] [CrossRef]
- Wu, W.; Han, S.T.; Venkatesh, S.; Sun, Q.; Peng, H.; Zhou, Y. Biodegradable skin-inspired nonvolatile resistive switching memory based on gold nanoparticles embedded alkali lignin. Org. Electron. 2018, 59, 382–388. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, X.; Wang, Z.; Xu, H.; Hu, J.; Ma, J.; Liu, Y. Biodegradable natural pectin-based flexible multilevel resistive switching memory for transient electronics. Small 2019, 15, 1803970–1803977. [Google Scholar] [CrossRef]
- Park, S.P.; Tak, Y.J.; Kim, H.J.; Lee, J.H.; Yoo, H.; Kim, H.J. Analysis of the bipolar resistive switching behavior of a biocompatible glucose film for resistive random access memory. Adv. Mater. 2018, 30, 1800722–1800729. [Google Scholar] [CrossRef]
- Yu, F.; Zhu, L.Q.; Gao, W.T.; Fu, Y.M.; Xiao, H.; Tao, J.; Zhou, J.M. Chitosan-based polysaccharide-gated flexible indium tin oxide synaptic transistor with learning abilities. ACS Appl. Mater. Interfaces 2018, 10, 16881–16886. [Google Scholar] [CrossRef]
- Hosseini, N.R.; Lee, J.-S. Biocompatible and flexible chitosan-based resistive switching memory with magnesium electrodes. Adv. Funct. Mater. 2015, 25, 5586–5592. [Google Scholar] [CrossRef]
- Fan, H.; Wang, L.; Zhao, K.; Li, N.; Shi, Z.; Ge, Z.; Jin, Z. Fabrication, mechanical properties, and biocompatibility of graphene-reinforced chitosan composites. Biomacromolecules 2010, 11, 2345–2351. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Koh, J. Physicochemical and optical properties of chitosan based graphane oxide bionanocomposite. Int. J. Biol. Macromol. 2014, 70, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Bikiaris, D.N. Recent modifications of chitosan for adsorption applications: A critical and systematic review. Mar. Drugs 2015, 13, 312–337. [Google Scholar] [CrossRef]
- Kima, D.S.; Choia, H.S.; Yanga, X.; Yanga, J.H.; Leeb, J.H.; Yooc, H.Y.; Leed, J.; Parke, C.; Kima, S.W. Improvement of power generation of enzyme fuel cell by novel GO/Co/chitosan electrodeposition. J. Ind. Eng. Chem. 2020, 81, 108–114. [Google Scholar] [CrossRef]
- Marroquin, J.B.; Rhee, K.; Park, S. Chitosan nanocomposite films: Enhanced electrical conductivity, thermal stability, and mechanical properties. Carbohydr. Polym. 2013, 92, 1783–1791. [Google Scholar] [CrossRef]
- Zhang, E.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Li, P. Advances in chitosan-based nanoparticles for oncotherapy. Carbohydr. Polym. 2019, 222, 115004–115015. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Deng, H.; Du, Y.; Shi, X.; Wang, Q. Emerging chitin and chitosan nanofibrous materials for biomedical applications. Nanoscale 2014, 6, 9477–9493. [Google Scholar] [CrossRef] [PubMed]
- Hanifah, M.F.R.; Jaafar, J.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Yusof, N.; Salleh, W.N.W.; Aziz, F. Facile synthesis of highly favorable graphene oxide: Effect of oxidation degree on the structural, morphological, thermal and electrochemical properties. Materialia 2019, 6, 100344–100355. [Google Scholar] [CrossRef]
- Tan, P.; Bi, Q.; Hu, Y.; Fang, Z.; Chen, Y.; Cheng, J. Effect of the degree of oxidation and defects of graphene oxide on adsorption of Cu2+ from aqueous solution. Appl. Surf. Sci. 2017, 423, 1141–1151. [Google Scholar] [CrossRef]
- Cortinez, D.; Palma, P.; Castro, R.; Palza, H. A multifunctional bi-phasic graphene oxide/chitosan paper for water treatment. Sep. Purif. Technol. 2020, 235, 116181–116188. [Google Scholar] [CrossRef]
- Li, T.T.; Yan, M.; Zhong, Y.; Ren, H.T.; Lou, C.W.; Huang, S.Y.; Lin, J.H. Processing and characterizations of rotary linear needleless electrospun polyvinyl alcohol (PVA)/chitosan(CS)/graphene(Cr). J. Mater. Res. Technol. 2019, 8, 5124–5132. [Google Scholar] [CrossRef]
- Qi, M.; Bai, L.; Xu, H.; Wang, Z.; Kang, Z.; Zhao, X.; Liu, W.; Ma, J.; Liu, Y. Oxidized carbon quantum dot-graphene oxide nanocomposites for improving data retention of resistive switching memory. J. Mater. Chem. C 2018, 6, 2026–2033. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Nayak, S.R.; Mohana, K.N.; Hegde, M.B. Anticorrosion performance of 4-fluoro phenol functionalized graphene oxide nanocompoiste coating on mild steel. J. Fluor. Chem. 2019, 228, 109392–109402. [Google Scholar] [CrossRef]
- Chen, X.; Oh, W.D.; Zhang, P.H.; Webster, R.D.; Lim, T.T. Surface construction of nitrogen-doped chitosan-derived carbon nanosheets with hierarchically porous structure for enhanced sulfacetamide degradation via peroxymonosulfate activation: Maneuverable porosity and active sites. Chem. Eng. J. 2019, 382, 122908–122920. [Google Scholar] [CrossRef]
- Alves, A.K.; Frantz, A.C.S.; Berutti, F.A. Microwave-assisted oleothermal synthesis of graphene-TiO2 quantum dots for photoelectrochemical oxygen evolution reaction. Flat. Chem. 2018, 12, 26–34. [Google Scholar] [CrossRef]
- Torres, D.; Sebastian, D.; Lazaro, M.J.; Pinilla, J.L.; Suelves, I.; Arico, A.S.; Baglio, V. Performance and stability of counter electrodes based on reduced few-layer graphene oxide sheets and reduced graphene oxide quantum dots for dye-sensitized solar cells. Electrochim. Acta 2019, 306, 396–406. [Google Scholar] [CrossRef]
- Zuo, P.P.; Feng, H.F.; Xu, Z.Z.; Zhang, L.F.; Zhang, Y.L.; Xia, W.; Zhang, W.Q. Fabrication of biocompatible and mechanically reinforced graphene oxide-chitosan nanocomposite films. Chem. Cent. J. 2013, 7, 39. [Google Scholar] [CrossRef] [Green Version]










| D Band (cm−1) | G Band (cm−1) | 2D Band (cm−1) | D + G Band (cm−1) | ID/IG | I2D/IG | |
|---|---|---|---|---|---|---|
| GO | 1347 | 1591 | 2703 | 2916 | 0.923 | 0.0567 |
| CS:GO (2:1 w/w) | 1351 | 1602 | 2697 | 2944 | 0.969 | 0.135 |
| CS:GO (5:1 w/w) | 1351 | 1604 | 2688 | 2942 | 0.954 | 0.0667 |
| CS:GO (10:1 w/w) | 1354 | 1607 | 2690 | 2943 | 0.953 | 0.0632 |
| Nanocomposites | RHRS (Ω) | RIRS (Ω) | RLRS (Ω) | |||
|---|---|---|---|---|---|---|
| mean | std | mean | std | mean | std | |
| CS:GO (2:1) | 1.151 × 105 | 1.882 × 105 | 1.170 × 104 | 1.977 × 104 | 43.492 | 7.402 |
| CS:GO (5:1) | 5.188 × 103 | 4.433 × 103 | 910.115 | 757.711 | 47.429 | 15.935 |
| CS:GO (10:1) | 1.0582 × 103 | 791.0759 | 296.611 | 219.764 | 60.310 | 22.291 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Li, G. Multi-Bit Biomemory Based on Chitosan: Graphene Oxide Nanocomposite with Wrinkled Surface. Micromachines 2020, 11, 580. https://doi.org/10.3390/mi11060580
Li L, Li G. Multi-Bit Biomemory Based on Chitosan: Graphene Oxide Nanocomposite with Wrinkled Surface. Micromachines. 2020; 11(6):580. https://doi.org/10.3390/mi11060580
Chicago/Turabian StyleLi, Lei, and Guangming Li. 2020. "Multi-Bit Biomemory Based on Chitosan: Graphene Oxide Nanocomposite with Wrinkled Surface" Micromachines 11, no. 6: 580. https://doi.org/10.3390/mi11060580
APA StyleLi, L., & Li, G. (2020). Multi-Bit Biomemory Based on Chitosan: Graphene Oxide Nanocomposite with Wrinkled Surface. Micromachines, 11(6), 580. https://doi.org/10.3390/mi11060580





