Design and Implementation of a Multifunction Wearable Device to Monitor Sleep Physiological Signals
Abstract
:1. Introduction
2. Materials and Methods
2.1. System Architecture
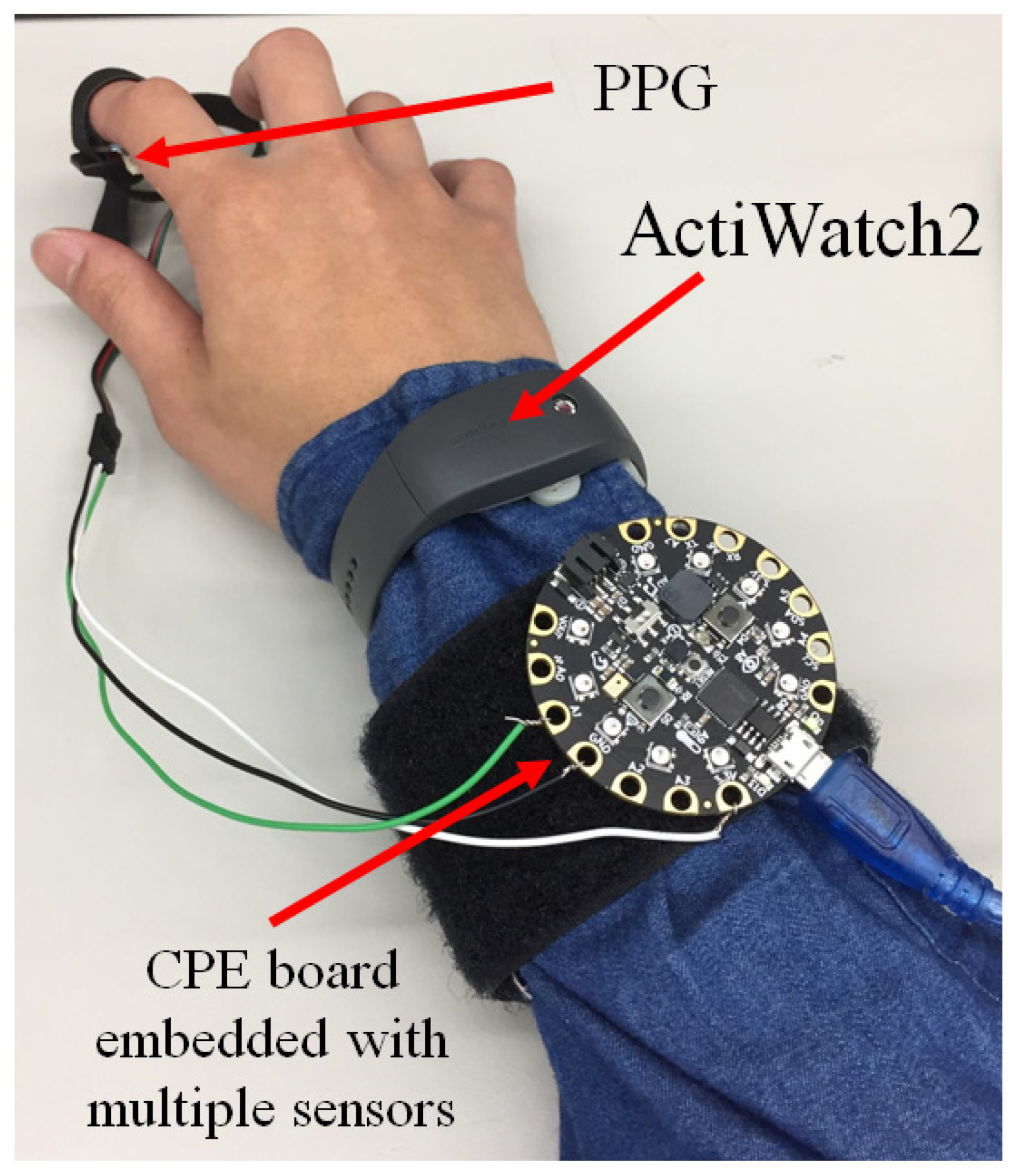
2.2. A Tiny Microcontroller Integrated with Multiple Sensors
2.3. Motion Detection
2.4. Heartbeat Monitor
2.5. Environment Detection
3. Results and Discussion
3.1. Core Function
3.2. Calibration
3.3. Sleep Analysis Using MATLAB
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liao, L.D.; Wang, Y.; Tsao, Y.C.; Wang, I.J.; Jhang, D.F.; Chu, T.S.; Tsao, C.H.; Tsai, C.N.; Chen, S.F.; Chuang, C.C.; et al. Design and Validation of a Multifunctional Android-Based Smart Home Control and Monitoring System. IEEE Access 2019, 7, 163313–163322. [Google Scholar] [CrossRef]
- Banerjee, S.; Hemphill, T.; Longstreet, P. Wearable devices and healthcare: Data sharing and privacy. Inf. Soc. 2018, 34, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Razjouyan, J.; Najafi, B.; Horstman, M.; Sharafkhaneh, A.; Amirmazaheri, M.; Zhou, H.; Kunik, M.E.; Naik, A. Toward Using Wearables to Remotely Monitor Cognitive Frailty in Community-Living Older Adults: An Observational Study. Sensors 2020, 20, 2218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Campbell, A.S.; de Avila, B.E.F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Lee, K.; Yeom, J.; Lee, T.H.; Kim, D.H.; Kim, J.J. Wearable Multi-Biosignal Analysis Integrated Interface With Direct Sleep-Stage Classification. IEEE Access 2020, 8, 46131–46140. [Google Scholar] [CrossRef]
- Kumar, D.; Adarsh, A.; Chandrika, S.; Kishor, N.; Mala, R. Analysis of Vital Signals Acquired from Wearable Device. Emerg. Res. Electron. Comput. Sci. Technol. Icerect 2018 2019, 545, 423–436. [Google Scholar]
- Guillodo, E.; Lemey, C.; Simonnet, M.; Walter, M.; Baca-Garcia, E.; Masetti, V.; Moga, S.; Larsen, M.; Network, H.U.; Ropars, J.; et al. Clinical Applications of Mobile Health Wearable-Based Sleep Monitoring: Systematic Review. JMIR mHealth uHealth 2020, 8, e10733. [Google Scholar] [CrossRef] [PubMed]
- Chemparathy, A.; Kassiri, H.; Salam, M.T.; Boyce, R.; Bekmambetova, F.; Adamantidis, A.; Genov, R. Wearable Low-Latency Sleep Stage Classifier. In Proceedings of the 2014 IEEE Biomedical Circuits and Systems Conference (Biocas), Lausanne, Switzerland, 22–24 October 2014; pp. 592–595. [Google Scholar]
- Yang, C.C.; Hsu, Y.L. A Review of Accelerometry-Based Wearable Motion Detectors for Physical Activity Monitoring. Sensors 2010, 10, 7772–7788. [Google Scholar] [CrossRef]
- Halson, S.L. Sleep Monitoring in Athletes: Motivation, Methods, Miscalculations and Why it Matters. Sports Med. 2019, 49, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Ferber, R.; Millman, R.; Coppola, M.; Fleetham, J.; Murray, C.F.; Iber, C.; McCall, W.V.; Nino-Murcia, G.; Pressman, M.; Sanders, M.; et al. Portable Recording in the Assessment of Obstructive Sleep-Apnea. Sleep 1994, 17, 378–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collop, N.A.; Anderson, W.M.; Boehlecke, B.; Claman, D.; Goldberg, R.; Gottlieb, D.J.; Hudgel, D.; Sateia, M.; Schwab, R. Clinical Guidelines for the Use of Unattended Portable Monitors in the Diagnosis of Obstructive Sleep Apnea in Adult Patients. J. Clin. Sleep Med. 2007, 3, 737–747. [Google Scholar] [PubMed]
- Yildirim, O.; Baloglu, U.B.; Acharya, U.R. A Deep Learning Model for Automated Sleep Stages Classification Using PSG Signals. Int. J. Environ. Res. Public Health 2019, 16, 599. [Google Scholar] [CrossRef] [Green Version]
- Norman, M.B.; Pithers, S.M.; Teng, A.Y.; Waters, K.A.; Sullivan, C.E. Validation of the Sonomat Against PSG and Quantitative Measurement of Partial Upper Airway Obstruction in Children With Sleep-Disordered Breathing. Sleep 2017, 40, zsx017. [Google Scholar] [CrossRef] [PubMed]
- Fallmann, S.; Chen, L.M. Computational Sleep Behavior Analysis: A Survey. IEEE Access 2019, 7, 142421–142440. [Google Scholar] [CrossRef]
- Crivello, A.; Barsocchi, P.; Girolami, M.; Palumbo, F. The Meaning of Sleep Quality: A Survey of Available Technologies. IEEE Access 2019, 7, 167374–167390. [Google Scholar] [CrossRef]
- Cosoli, G.; Spinsante, S.; Scalise, L. Wrist-worn and chest-strap wearable devices: Systematic review on accuracy and metrological characteristics. Measurement 2020, 159, 107789. [Google Scholar] [CrossRef]
- Jin, K.H.; Eglowstein, H.; Sabin, M. Using Physical Computing Projects in Teaching Introductory Programming. In Proceedings of the 19th Annual Sig Conference on Information Technology Education, Fort Lauderdale, FL, USA, 3–6 October 2018; p. 155. [Google Scholar]
- Puranik, S.; Morales, A.W. Heart Rate Estimation of PPG Signals With Simultaneous Accelerometry Using Adaptive Neural Network Filtering. IEEE Trans. Consum. Electron. 2020, 66, 69–76. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, E.; Kim, J.; Park, P.; Cho, S. A Sleep Apnea Monitoring IC for Respiration, Heart-Rate, SpO(2) and Pulse-Transit Time Measurement Using Thermistor, PPG and Body-Channel Communication. IEEE Sens. J. 2020, 20, 1997–2007. [Google Scholar] [CrossRef]
- Schnelle, J.F.; Alessi, C.A.; Al-Samarrai, N.R.; Fricker, R.D.; Ouslander, J.G. The nursing home at night: Effects of an intervention on noise, light, and sleep. J. Am. Geriatr. Soc. 1999, 47, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Kinnunen, H.; Rantanen, A.; Kentta, T.; Koskimaki, H. Feasible assessment of recovery and cardiovascular health: Accuracy of nocturnal HR and HRV assessed via ring PPG in comparison to medical grade ECG. Physiol. Meas. 2020, 41, 04NT01. [Google Scholar] [CrossRef]
- Lee, S.; Kim, D. Effect of Color Light Stimulation Using LED on Sleep Induction Time. J. Healthc. Eng. 2017, 2017, 6030268. [Google Scholar] [CrossRef]
- Al-Naggar, N.Q.; Al-Hammadi, H.M.; Al-Fusail, A.M.; Al-Shaebi, Z.A. Design of a Remote Real-Time Monitoring System for Multiple Physiological Parameters Based on Smartphone. J. Healthc. Eng. 2019, 2019, 5674673. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.T.; Prasad, M.; Chung, C.H.; Puthal, D.; El-Sayed, H.; Sankar, S.; Wang, Y.K.; Singh, J.; Sangaiah, A.K. IoT-Based Wireless Polysomnography Intelligent System for Sleep Monitoring. IEEE Access 2018, 6, 405–414. [Google Scholar] [CrossRef]
- Jeon, S.; Park, T.; Paul, A.; Lee, Y.S.; Son, S.H. A Wearable Sleep Position Tracking System Based on Dynamic State Transition Framework. IEEE Access 2019, 7, 135742–135756. [Google Scholar] [CrossRef]
- Lin, W.Y.; Verma, V.K.; Lee, M.Y.; Lai, C.S. Activity Monitoring with a Wrist-Worn, Accelerometer-Based Device. Micromachines 2018, 9, 450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tollervey, N.H. Programming with MicroPython: Embedded Programming with Microcontrollers and Python, 1st ed.; O’Reilly: Sebastopol, CA, USA, 2018. [Google Scholar]
- Mack, D.C.; Patrie, J.T.; Suratt, P.M.; Felder, R.A.; Alwan, M. Development and Preliminary Validation of Heart Rate and Breathing Rate Detection Using a Passive, Ballistocardiography-Based Sleep Monitoring System. IEEE Trans. Inf. Technol. Biomed. 2009, 13, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.T.; Chuang, C.H.; Cao, Z.H.; Singh, A.K.; Hung, C.S.; Yu, Y.H.; Nascimben, M.; Liu, Y.T.; King, J.T.; Su, T.P.; et al. Forehead EEG in support of Future Feasible Personal Healthcare Solutions: Sleep Management, Headache Prevention, and Depression Treatment. IEEE Access 2017, 5, 10612–10621. [Google Scholar] [CrossRef]
- Ma, Q.D.Y.; Ji, C.; Xie, H.; Yin, K.; Ma, Z.; Bian, C. Transitions in physiological coupling between heartbeat and pulse across sleep stages. Physiol. Meas. 2018, 39, 3. [Google Scholar] [CrossRef]
- Li, S.Y.; Wang, T.J.; Wu, S.F.V.; Liang, S.Y.; Tung, H.H. Efficacy of controlling night-time noise and activities to improve patients’ sleep quality in a surgical intensive care unit. J. Clin. Nurs. 2011, 20, 396–407. [Google Scholar] [CrossRef]
- Shin, M.; Swan, P.; Chow, C.M. The validity of Actiwatch2 and SenseWear armband compared against polysomnography at different ambient temperature conditions. Sleep Sci. 2015, 8, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Guo, B.; Shen, Y.; Tang, X.D.; Zhang, T.; Li, D.; Jiang, Z. Sleep staging algorithm based on multichannel data adding and multifeature screening. Comput. Methods Programs Biomed. 2020, 187, 105253. [Google Scholar] [CrossRef]
- Neil-Sztramko, S.E.; Rafn, B.S.; Gotay, C.C.; Campbell, K.L. Determining activity count cut-points for measurement of physical activity using the Actiwatch2 accelerometer. Physiol. Behav. 2017, 173, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Feder, K.; Keith, S.E.; Voicescu, S.A.; Marro, L.; Than, J.; Guay, M.; Denning, A.; Murray, B.J.; Weiss, S.K.; et al. Effects of Wind Turbine Noise on Self-Reported and Objective Measures of Sleep. Sleep 2016, 39, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.C.; Su, C.L.; Ong, J.H.; Tsai, K.L.; Chen, Y.W.; Lin, C.Y.; Hung, C.H. Pulse Oximetry Monitor Feasible for Early Screening of Obstructive Sleep Apnea (OSA). J. Med Biol. Eng. 2020, 40, 62–70. [Google Scholar] [CrossRef]
- Zhou, G.X.; Li, R.Z.; Zhang, S.; Wang, J.; Ma, J.Z. Multimodal Sleep Signals-Based Automated Sleep Arousal Detection. IEEE Access 2020, 8, 106157–106164. [Google Scholar] [CrossRef]
- Lewicke, A.; Sazonov, E.; Corwin, M.J.; Neuman, M.; Schuckers, S.; Grp, C.S. Sleep versus wake classification from heart rate variability using computational intelligence: Consideration of rejection in classification models. IEEE Trans. Biomed. Eng. 2008, 55, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Metcalf, D.; Milliard, S.T.J.; Gomez, M.; Schwartz, M. Wearables and the Internet of Things for Health Wearable, interconnected devices promise more efficient and comprehensive health care. IEEE Pulse 2016, 7, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Zhang, X.M.; Liu, W.H.; Luo, Y.X.; Yu, E.J.; Zou, K.J.; Liu, X. Automatic Sleep Staging using Multi-dimensional Feature Extraction and Multi-kernel Fuzzy Support Vector Machine. J. Healthc. Eng. 2014, 5, 505–520. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, L.-D.; Wang, Y.; Tsao, Y.-C.; Wang, I.-J.; Jhang, D.-F.; Chuang, C.-C.; Chen, S.-F. Design and Implementation of a Multifunction Wearable Device to Monitor Sleep Physiological Signals. Micromachines 2020, 11, 672. https://doi.org/10.3390/mi11070672
Liao L-D, Wang Y, Tsao Y-C, Wang I-J, Jhang D-F, Chuang C-C, Chen S-F. Design and Implementation of a Multifunction Wearable Device to Monitor Sleep Physiological Signals. Micromachines. 2020; 11(7):672. https://doi.org/10.3390/mi11070672
Chicago/Turabian StyleLiao, Lun-De, Yuhling Wang, Yung-Chung Tsao, I-Jan Wang, De-Fu Jhang, Chiung-Cheng Chuang, and Sheng-Fu Chen. 2020. "Design and Implementation of a Multifunction Wearable Device to Monitor Sleep Physiological Signals" Micromachines 11, no. 7: 672. https://doi.org/10.3390/mi11070672




