Continuous ES/Feeder Cell-Sorting Device Using Dielectrophoresis and Controlled Fluid Flow
Abstract
:1. Introduction
2. Materials and Methods
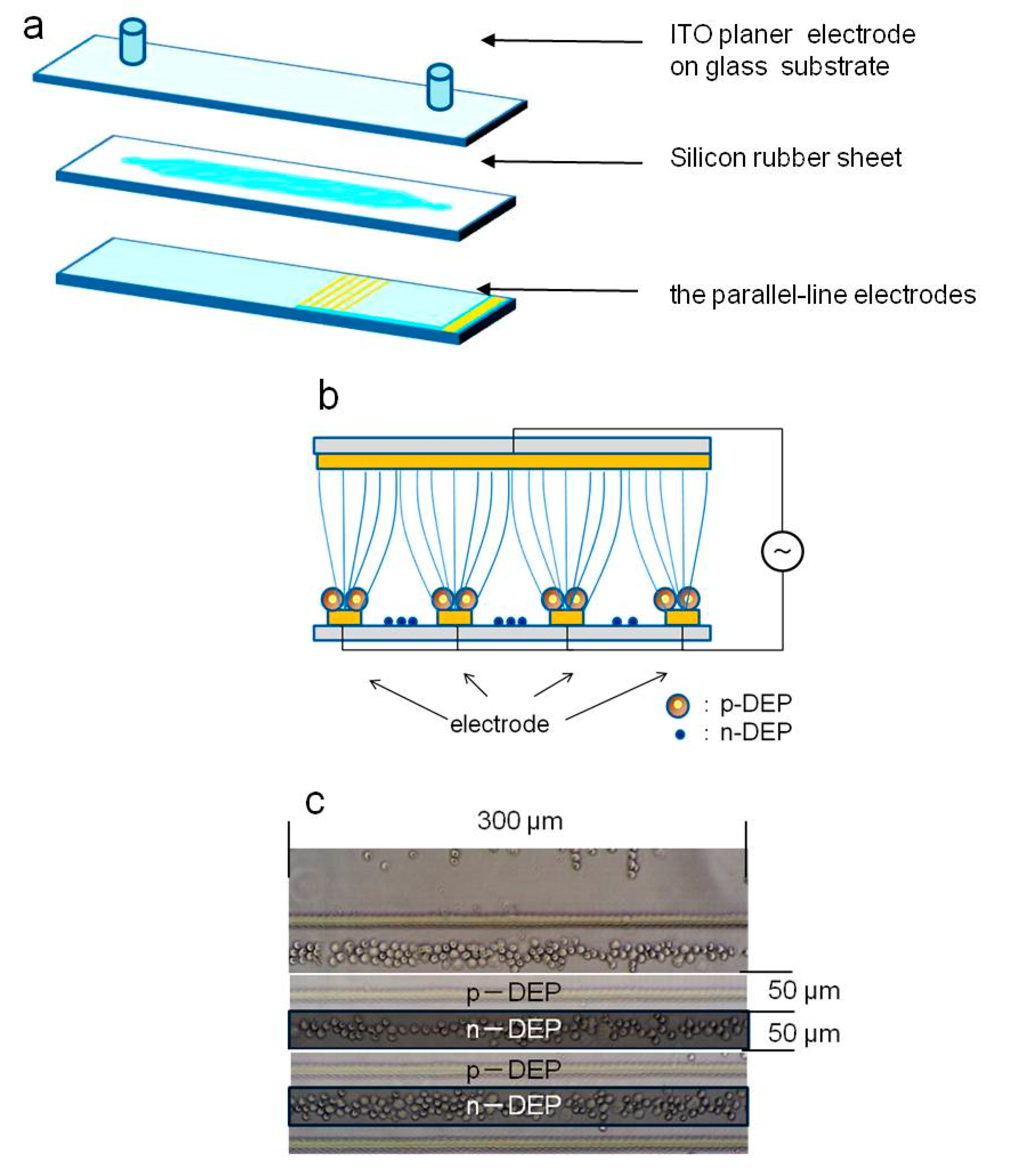
2.1. Dielectrophoresis
2.2. Cell Culture
2.3. DEP Characterization of ES-B3 and MEF Cells
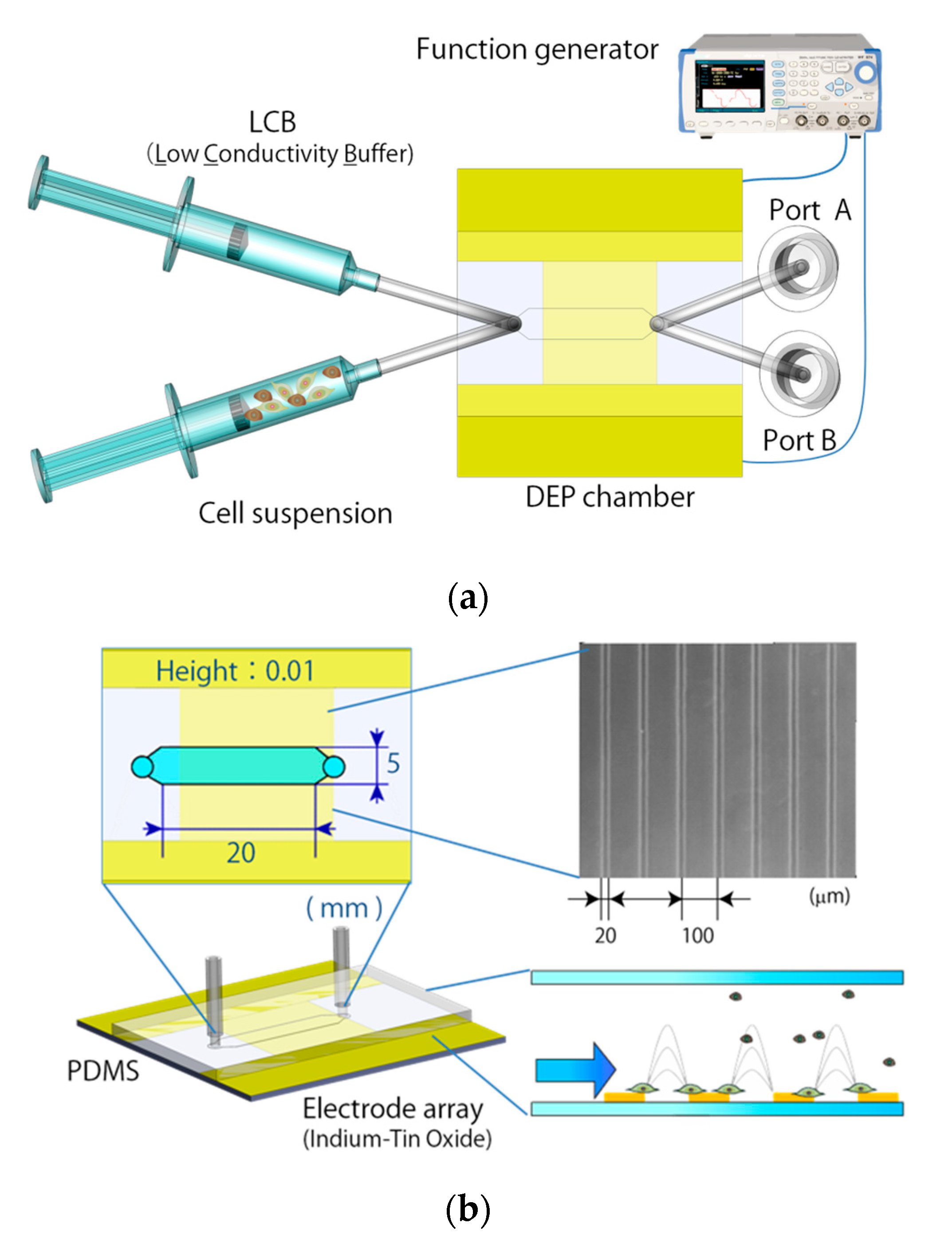
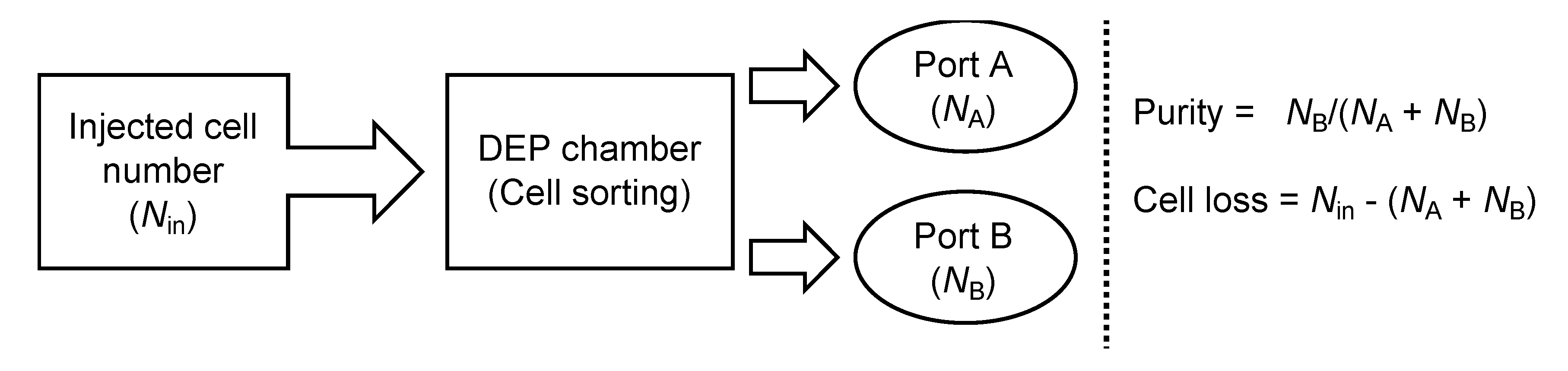
2.4. Continuous Cell Sorting Using DEP and Fluid Shear Forces
3. Results and Discussion
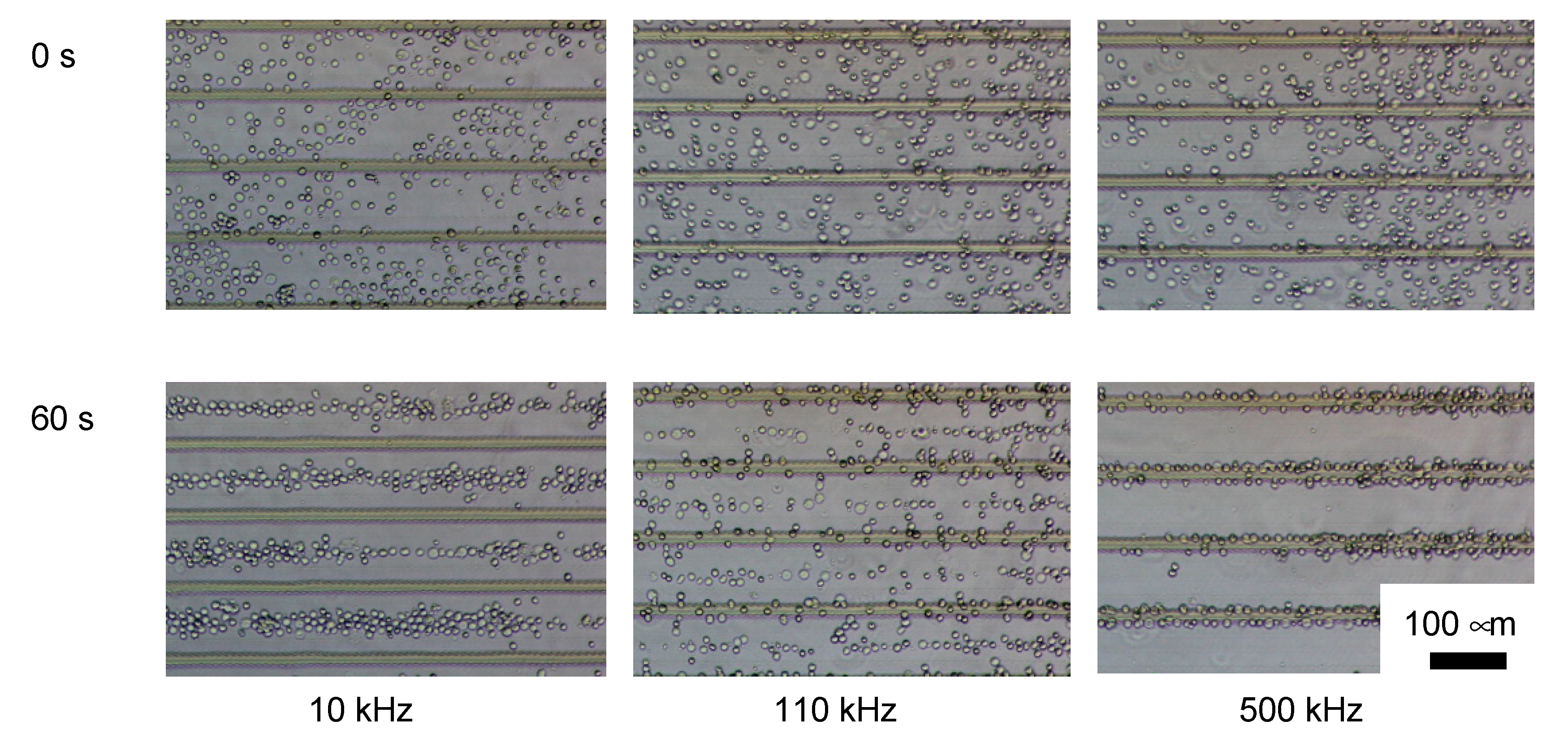
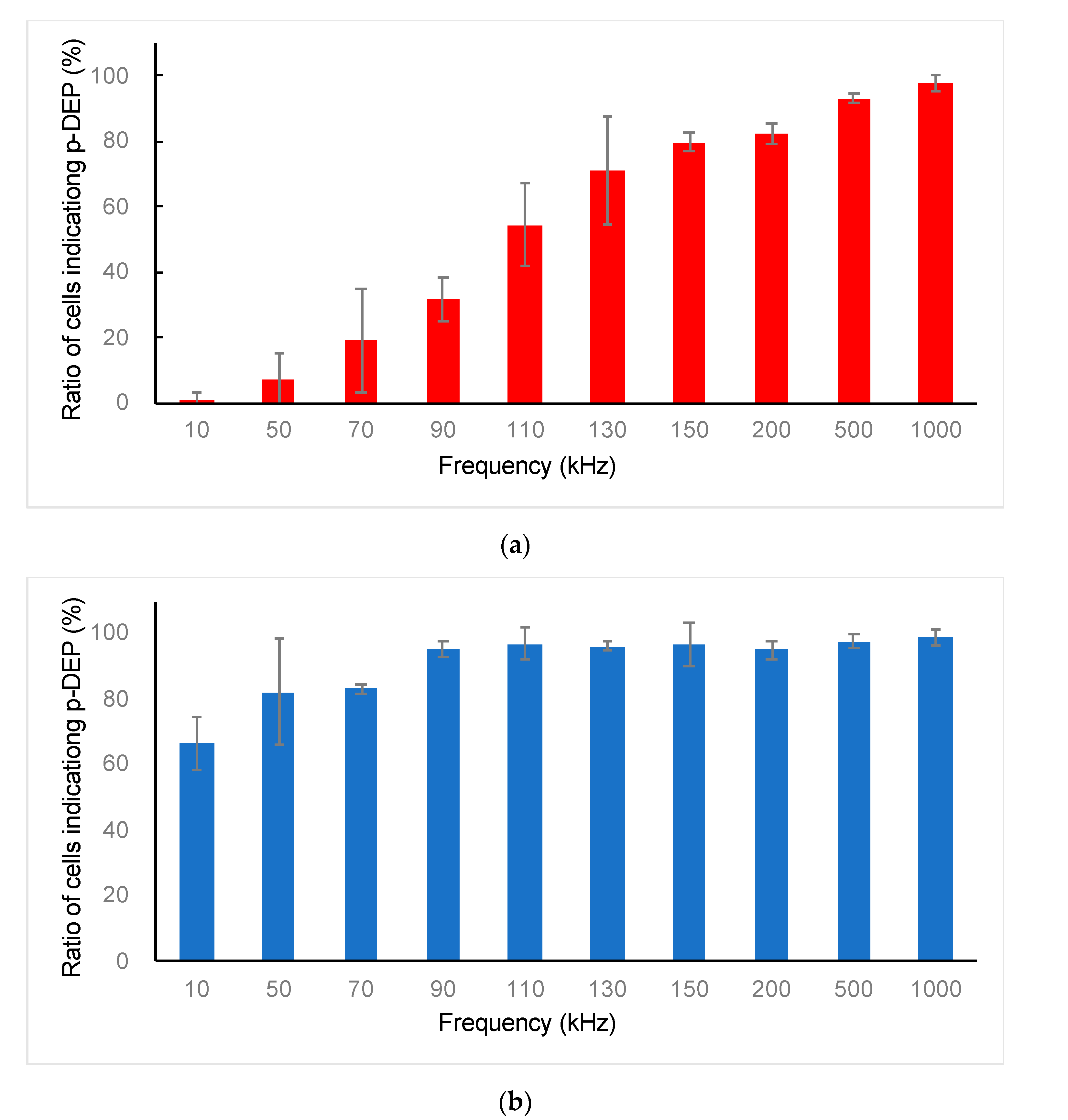
3.1. DEP Characterization of ES-B3 and MEF
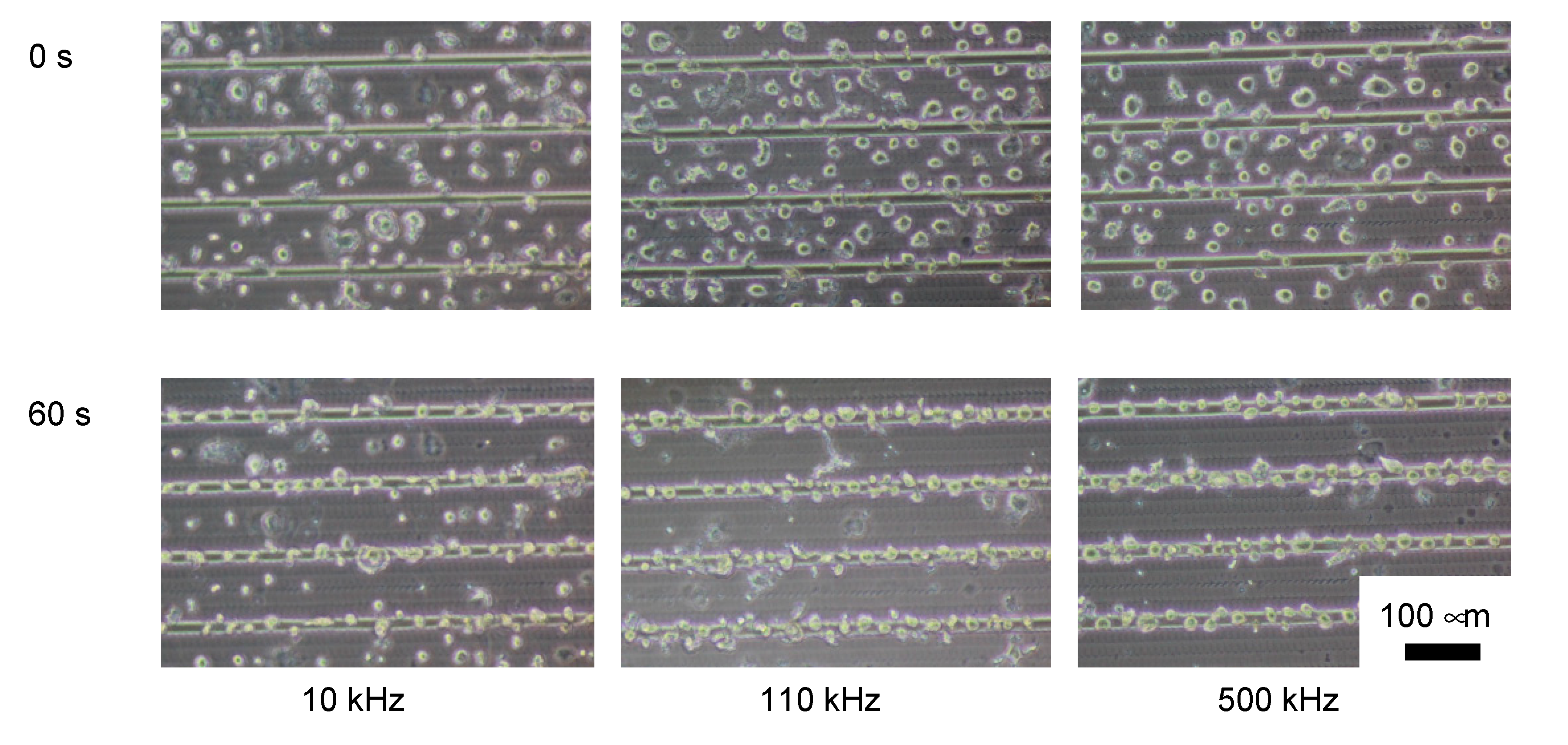
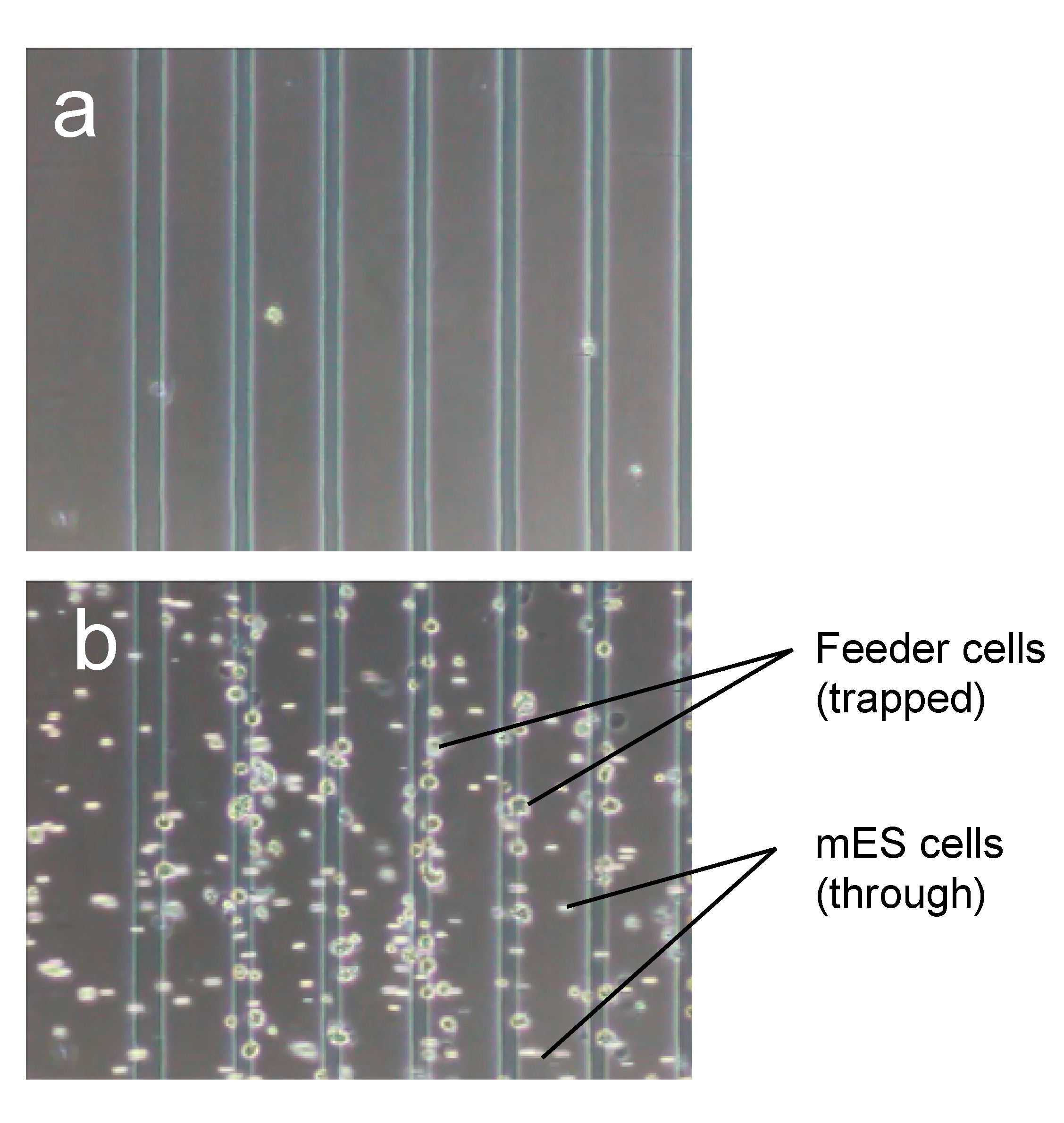
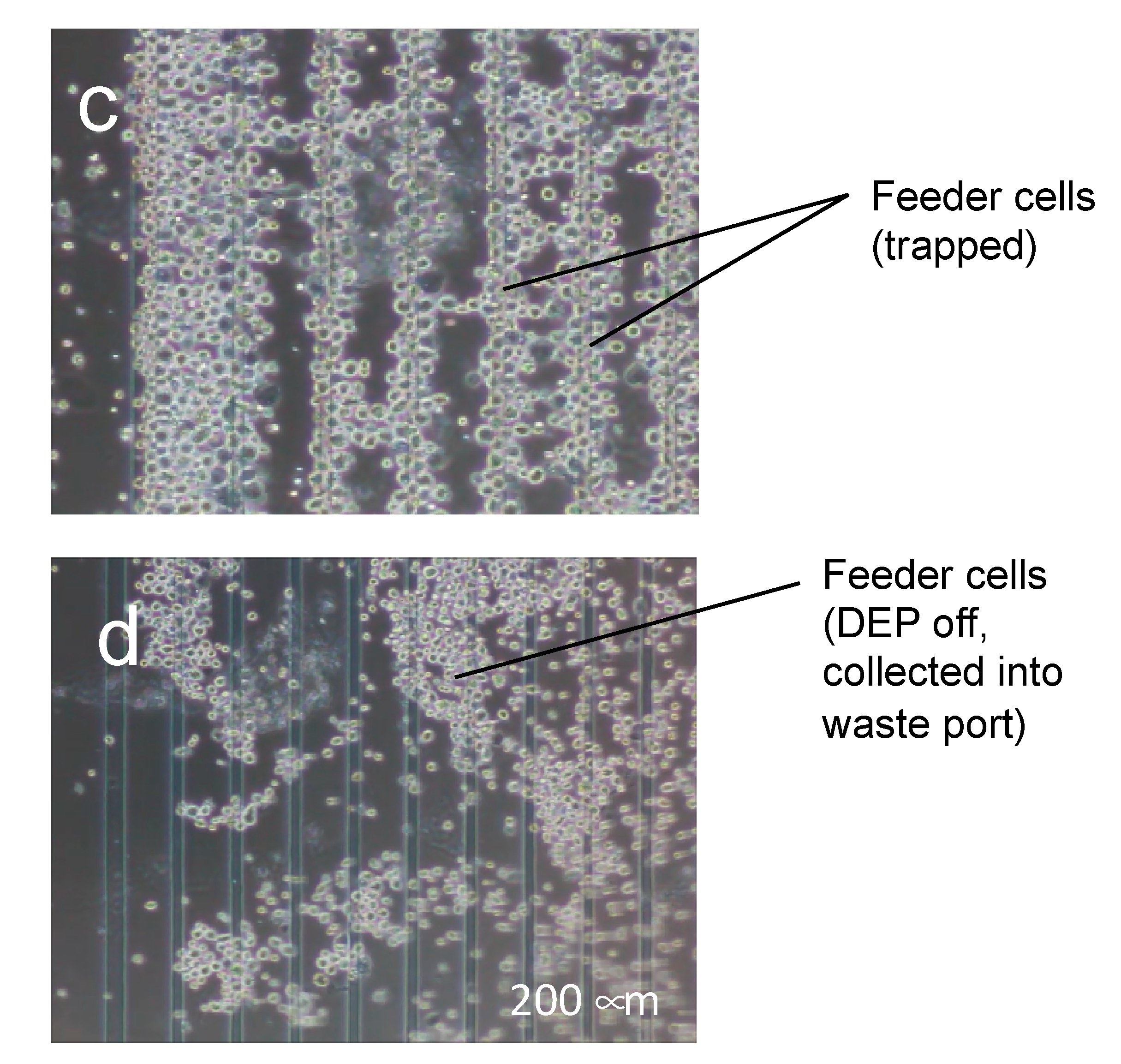
3.2. Purification Efficacy of DEP Cell-Sorting Device
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Okita, K.; Ichisaka, T.; Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 2007, 448, 313–317. [Google Scholar] [CrossRef]
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Braam, S.R.; Zeinstra, L.; Litjens, S.; van Oostwaard, D.W.; van den Brink, S.; van Laake, L.; Lebrin, F.; Kats, P.; Hochstenbach, R.; Passier, R.; et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells 2008, 26, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Inokuma, M.S.; Denham, J.; Golds, K.; Kundu, P.; Gold, J.D.; Carpenter, M.K. Feeder-free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 2001, 19, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Stover, A.E.; Schwartz, P.H. Adaptation of human pluripotent stem cells to feeder-free conditions in chemically defined medium with enzymatic single-cell passaging. Methods Mol. Biol. 2011, 767, 137–146. [Google Scholar] [PubMed] [Green Version]
- Totonchi, M.; Taei, A.; Seifinejad, A.; Tabebordbar, M.; Rassouli, H.; Farrokhi, A.; Gourabi, H.; Aghdami, N.; Hosseini-Salekdeh, G.; Baharvand, H. Feeder- and serum-free establishment and expansion of human induced pluripotent stem cells. Int. J. Dev. Biol. 2010, 54, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Kasai, K.; Kimura, Y.; Miyata, S. Improvement of adhesion and proliferation of mouse embryonic stem cells cultured on ozone/UV surface-modified substrates. Mater. Sci. Eng. C 2017, 78, 354–361. [Google Scholar] [CrossRef]
- Kimura, Y.; Kasai, K.; Miyata, S. Feeder-free culture for mouse induced pluripotent stem cells by using UV/ozone surface-modified substrates. Mater. Sci. Eng. C 2018, 92, 280–286. [Google Scholar] [CrossRef]
- Kasai, K.; Tohyama, S.; Suzuki, H.; Tanosaki, S.; Fukuda, K.; Fujita, J.; Miyata, S. Cost-effective culture of human induced pluripotent stem cells using UV/ozone-modified culture plastics with reduction of cell-adhesive matrix coating. Mater. Sci. Eng. C 2020, 111, 110788. [Google Scholar] [CrossRef]
- Rodin, S.; Domogatskaya, A.; Ström, S.; Hansson, E.M.; Chien, K.R.; Inzunza, J.; Hovatta, O.; Tryggvason, K. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat. Biotechnol. 2010, 28, 611–615. [Google Scholar] [CrossRef] [Green Version]
- Pouliot, N.; Kusuma, N. Laminin-511: A multi-functional adhesion protein regulating cell migration, tumor invasion and metastasis. Cell Adhes. Migr. 2013, 7, 142–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, T.; Futaki, S.; Hasegawa, K.; Kawasaki, M.; Sanzen, N.; Hayashi, M.; Kawase, E.; Sekiguchi, K.; Nakatsuji, N.; Suemori, H. Recombinant human laminin isoforms can support the undifferentiated growth of human embryonic stem cells. Biochem. Biophys. Res. Commun. 2008, 375, 27–32. [Google Scholar] [CrossRef]
- Miyazaki, T.; Futaki, S.; Suemori, H.; Taniguchi, Y.; Yamada, M.; Kawasaki, M.; Hayashi, M.; Kumagai, H.; Nakatsuji, N.; Sekiguchi, K.; et al. Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nat. Commun. 2012, 3, 1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herzenberg, L.A. The History and Future of the Fliorescence Activated Cell Sorter and Flow Cytometry: A View from Stanford. Clin. Chem. 2002, 48, 1819–1827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, S.F.; Van Den Engh, G. High-speed cell sorting: Fundamentals and recent advances. Curr. Opin. Biotechnol. 2003, 14, 5–12. [Google Scholar] [CrossRef]
- Makker, K.; Agarwal, A. Magnetic activated cell sorting (MACS): Utility in assisted reproduction. Indian, J. Exp. Biol. 2008, 46, 491–497. [Google Scholar]
- Parks, D.R.; Herzenberg, L.A. Fluorescence-activated cell sorting: Theory, experimental optimization, and applications in lymphoid cell biology. Method Enzymol. 1984, 108, 197–241. [Google Scholar]
- Krüger, J.; Singh, K. Development of a microfluidic device for fluorescence activated cell sorting. J. Micromech. Microeng. 2002, 12, 486–494. [Google Scholar]
- Rosenthal, A.; Voldman, J. Dielectrophoretic traps for single-particle patterning. Biophys. J. 2005, 88, 2193–2205. [Google Scholar] [CrossRef] [Green Version]
- Punjiya, M.; Nejad, H.R.; Mathews, J.; Levin, M.; Sonkusale, S. A flow through device for simultaneous dielectrophoretic cell trapping and AC electroporation. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lapizco-Encinas, B.H.; Simmons, B.A.; Cummings, E.B.; Fintschenko, Y. Insulator-based dielectrophoresis for the selective concentration and separation of live bacteria in water. Electrophoresis 2004, 25, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Kuczenski, R.S.; Chang, H.C.; Revzin, A. Dielectrophoretic microfluidic device for the continuous sorting of Escherichia coli from blood cells. Biomicrofluidics 2011, 5, 032005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.Y.; Kwak, H.Y. Separation of microparticles and biological cells inside an evaporating droplet using dielectrophoresis. Anal. Chem. 2007, 79, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- Lewpiriyawong, N.; Yang, C.; Lam, Y.C. Continuous sorting and separation of microparticles by size using AC dielectrophoresis in a PDMS microfluidic device with 3-D conducting PDMS composite electrodes. Electrophoreis 2010, 31, 2622–2631. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, H.; Sano, M.B.; Henslee, E.A.; Caldwell, J.L.; Davalos, R.V. Selective isolation of live/dead cells using contactless dielectrophoresis (cDEP). Lab Chip 2010, 10, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Jubery, T.Z.; Dutta, P. A new Design for Efficient Dielectrophoretic Separation of Cells in a Microdevice. Electrophoresis 2013, 35, 643–650. [Google Scholar] [CrossRef]
- Adams, T.N.G.; Jiang, A.Y.L.; Vyas, P.D.; Flanagan, L.A. Separation of neural stem cells by whole cell membrane capacitance using dielectrophoresis. Methods 2018, 133, 91–103. [Google Scholar] [CrossRef]
- Wang, L.; Lu, J.; Marchenko, S.A.; Monuki, E.S.; Flanagan, L.A.; Lee, A.P. Dual frequency dielectrophoresis with interdigitated sidewall electrodes for microfluidic flow-through separation of beads and cells. Electrophoresis 2009, 30, 782–791. [Google Scholar] [CrossRef]
- Waheed, W.; Alazzam, A.; Mathew, B.; Christoforou, N.; Abu-Nada, E. Lateral fluid flow fractionation using dielectrophoresis (LFFF-DEP) for size-independent, label-free isolation of circulating tumor cells. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1087–1088, 133–137. [Google Scholar] [CrossRef]
- Choi, S.; Park, J.K. Microfluidic system for dielectrophoretic separation based on a trapezoidal electrode array. Lab Chip 2005, 5, 1161–1167. [Google Scholar] [CrossRef]
- Gascoyne, P.R.C.; Vykoukal, J. Particle separation by dielectrophoresis. Electrophoresis 2002, 23, 1973–1983. [Google Scholar] [CrossRef]
- Morgan, H.; Hughes, M.P. Separation of submicron bioparticles by dielectrophoresis. Biophis. J. 1995, 77, 516–525. [Google Scholar] [CrossRef] [Green Version]
- Piacentini, N.; Mernier, G. Separation of platelets from other blood cells in continuous-flow by dielectrophoresis field-flow-fractionation. Biomicrofluidics 2011, 5, 034122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamloo, A.; Kamali, A. Numerical analysis of a dielectrophoresis field-flow fractionation device for the separation of multiple cell types. J. Sep. Sci. 2017, 40, 4067–4075. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Kim, J. Dielectrophoretic oocyte selection chip for in vitro fertilization. Biomed. Microdevice 2008, 10, 337–345. [Google Scholar] [CrossRef]
- Ding, J.; Woolley, C.; Hayes, M.A. Biofluid pretreatment using gradient insulator-based dielectrophoresis: Separating cells from biomarkers. Anal. Bioanal. Chem. 2017, 409, 6405–6414. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Miyata, S. Micro cell patterning technology by dielectrophoresis and application to regenerated cartilage. Trans. JSME 2010, 76, 3015–3020. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, Y.; Miyata, S. Multi-layered cell assembling technology using dielectrophoresis and construction of skin tissue microelement. Trans. JSME 2017, 83, 16–00387. [Google Scholar]
- Ojima, Y.; Miyata, S. Discrimination methodology of living-cells and microbeads using dielectrophoresis and fluid-induced shear force. J. Biorheol. 2015, 29, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Jones, T.B. Electromechanics of Particles; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Zhu, J.; Xuan, X. Curvature-induced dielectrophoresis for continuous separation of particles by charge in spiral microchannels. Biomicrofluidics 2011, 5, 024111. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Ren, Y.; Tao, Y.; Hou, L.; Jiang, H. High-Throughput Separation, Trapping, and Manipulation of Single Cells and Particles by Combined Dielectrophoresis at a Bipolar Electrode Array. Anal. Chem. 2018, 90, 11461–11469. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.; Mohammadi, A.; Kalhor, H.R. A continuous flow microfluidic device based on contactless dielectrophoresis for bioparticles enrichment. Electrophoresis 2018, 39, 445–455. [Google Scholar] [CrossRef]
- Cetin, B.; Li, D. Lab-on-a-chip device for continuous particle and cell separation based on electrical properties via alternating current dielectrophoresis. Electrophoresis 2010, 31, 3035–3043. [Google Scholar] [CrossRef] [PubMed]
- Braschler, T.; Demierre, N.; Nascimento, E.; Silva, T.; Oliva, A.G.; Renaud, P. Continuous separation of cells by balanced dielectrophoretic forces at multiple frequencies. Lab Chip 2008, 8, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.Y.L.; Yale, A.R.; Aghaamoo, M.; Lee, D.H.; Lee, A.P.; Adams, T.N.G.; Flanagan, L.A. High-throughput continuous dielectrophoretic separation of neural stem cells. Biomicrofluidics 2019, 13, 064111. [Google Scholar] [CrossRef] [PubMed] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, Y.; Miyata, S. Continuous ES/Feeder Cell-Sorting Device Using Dielectrophoresis and Controlled Fluid Flow. Micromachines 2020, 11, 734. https://doi.org/10.3390/mi11080734
Takahashi Y, Miyata S. Continuous ES/Feeder Cell-Sorting Device Using Dielectrophoresis and Controlled Fluid Flow. Micromachines. 2020; 11(8):734. https://doi.org/10.3390/mi11080734
Chicago/Turabian StyleTakahashi, Yuuwa, and Shogo Miyata. 2020. "Continuous ES/Feeder Cell-Sorting Device Using Dielectrophoresis and Controlled Fluid Flow" Micromachines 11, no. 8: 734. https://doi.org/10.3390/mi11080734




