Polyelectrolyte Multilayer Capsule (PEMC)-Based Scaffolds for Tissue Engineering
Abstract
:1. Introduction
The Need for Controlled Delivery of Bioactives
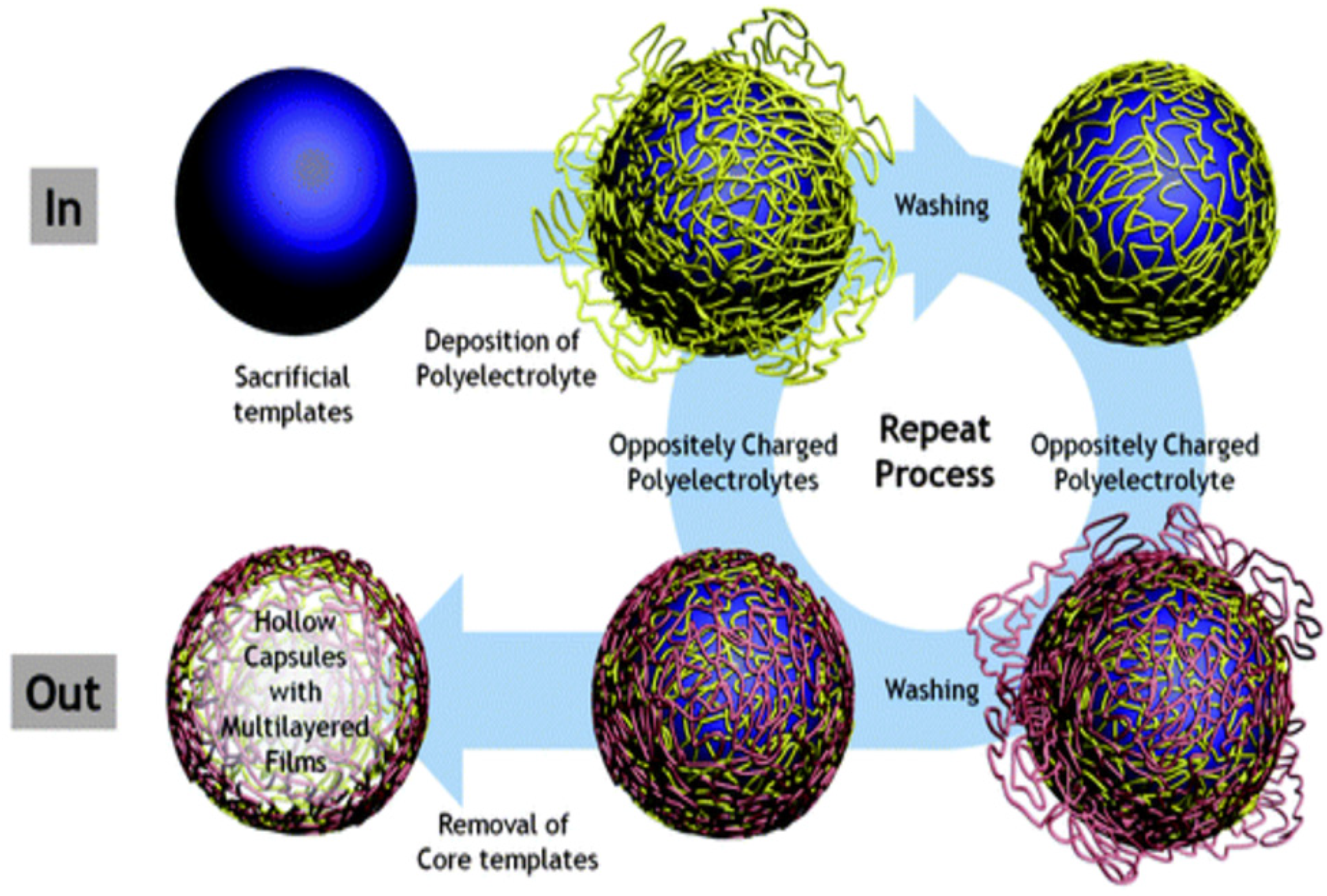
2. The Fabrication of PEMC Structures
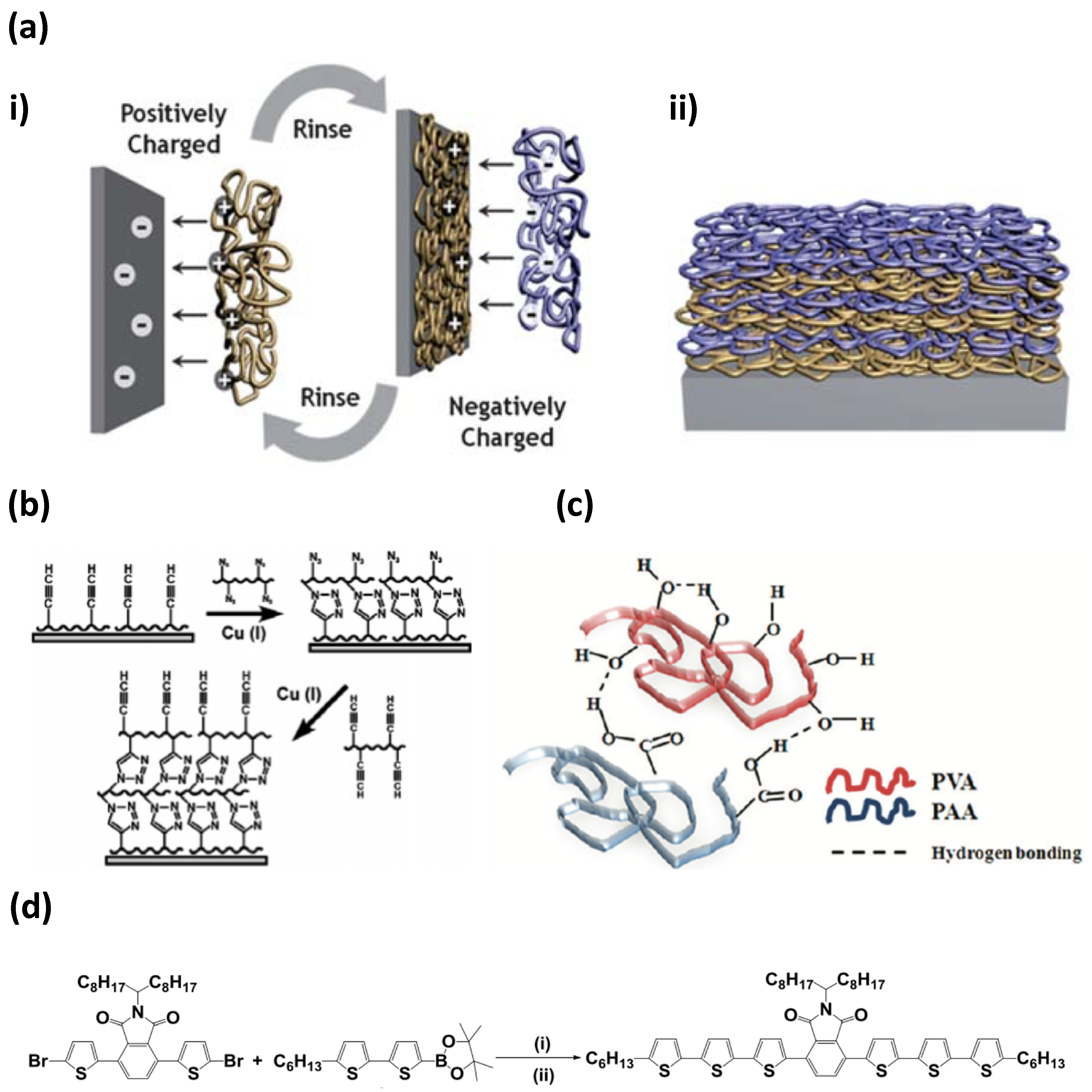
2.1. Multilayer Shell Composition
2.2. Sacrificial Cores
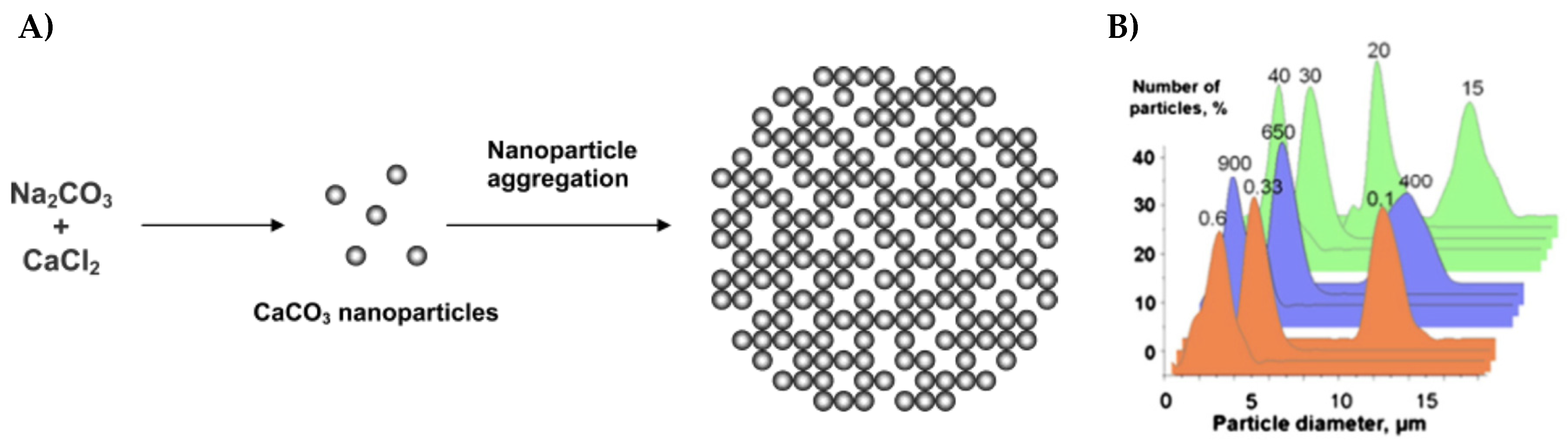
Vaterite CaCO3 Crystals
3. Applications of PEMCs as Delivery Carriers
4. Problems Associated with PEMCs
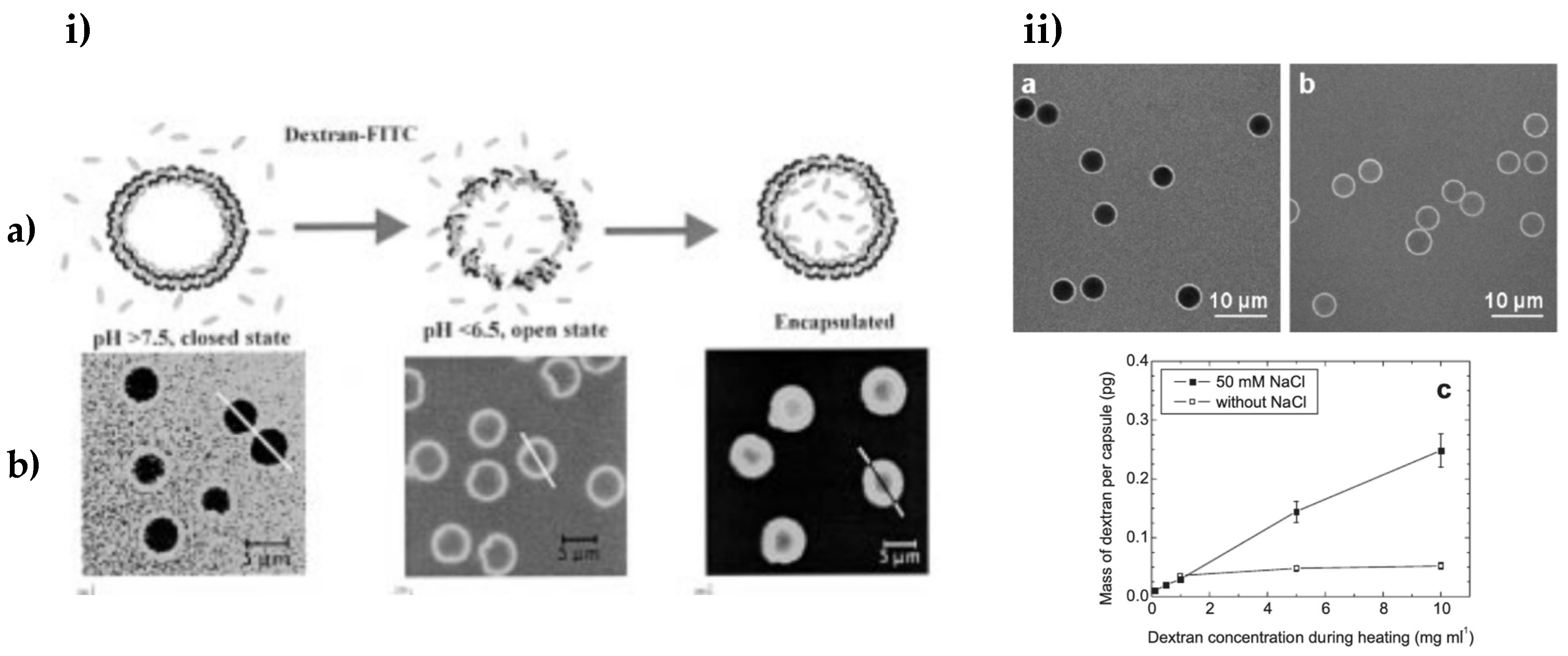
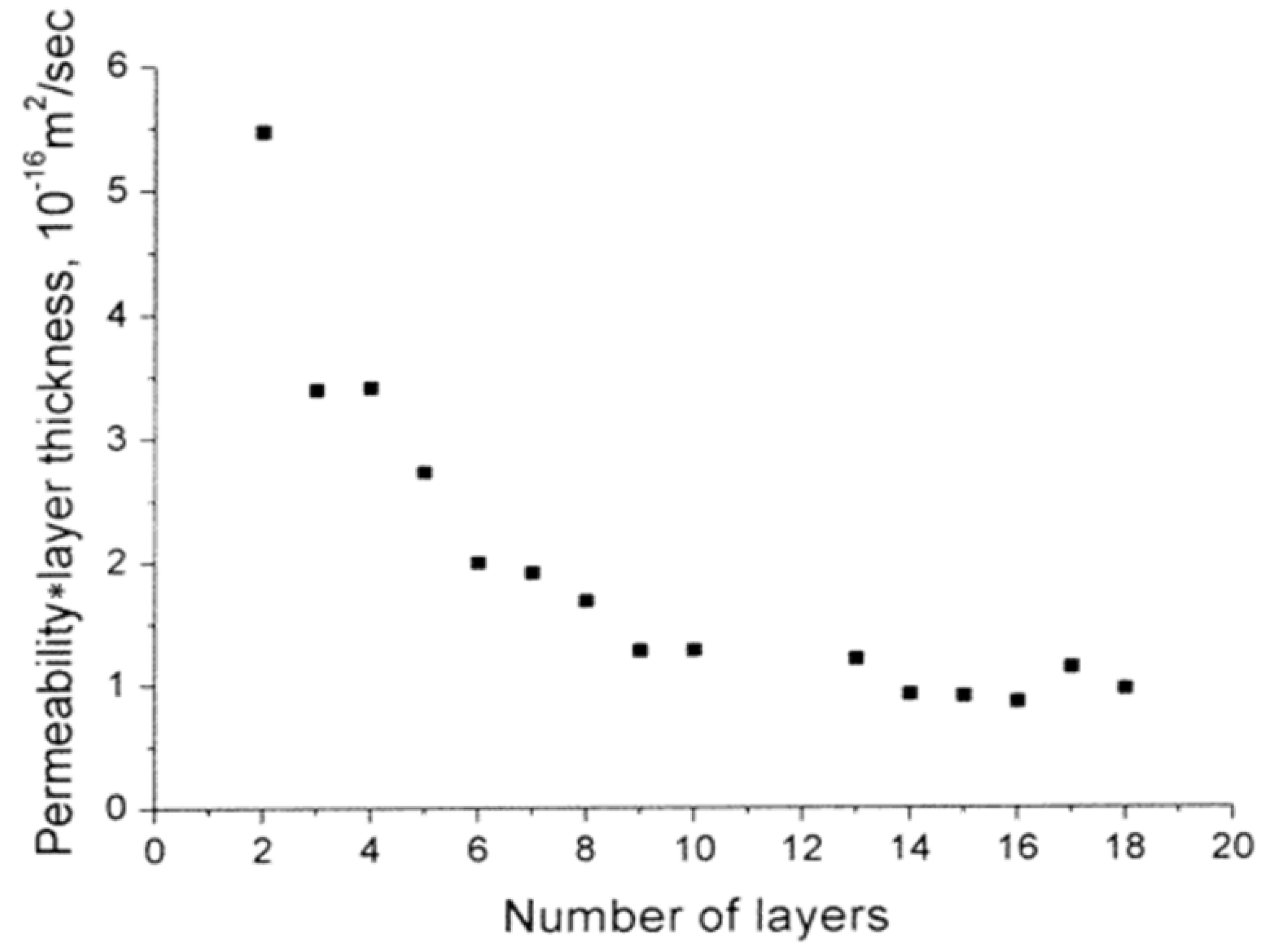
4.1. Factors Influencing Bioactive Retention of PEMCs
4.2. Role of Osmotic Pressure in PEMC Formation
5. PEMC-Based Scaffolds
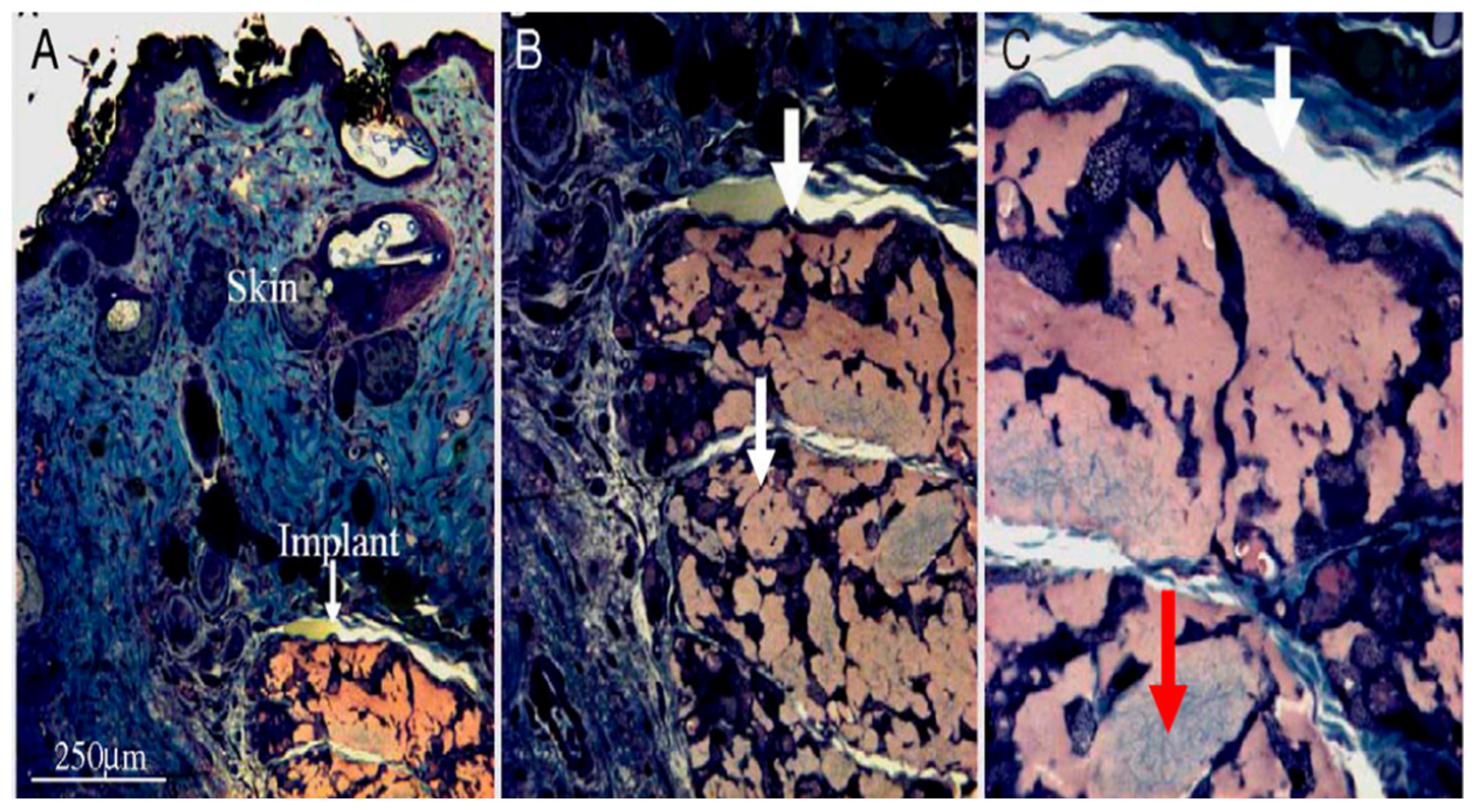
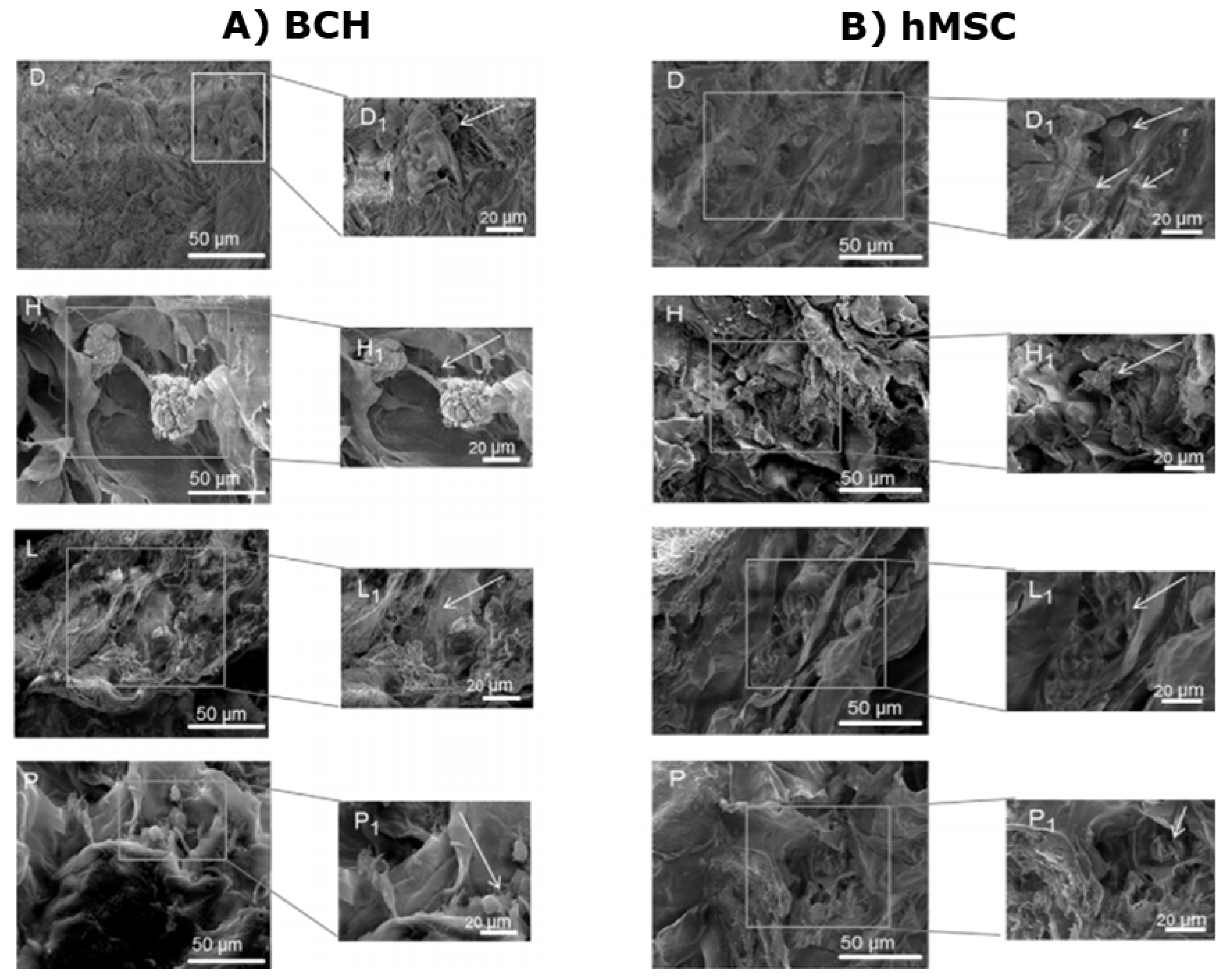
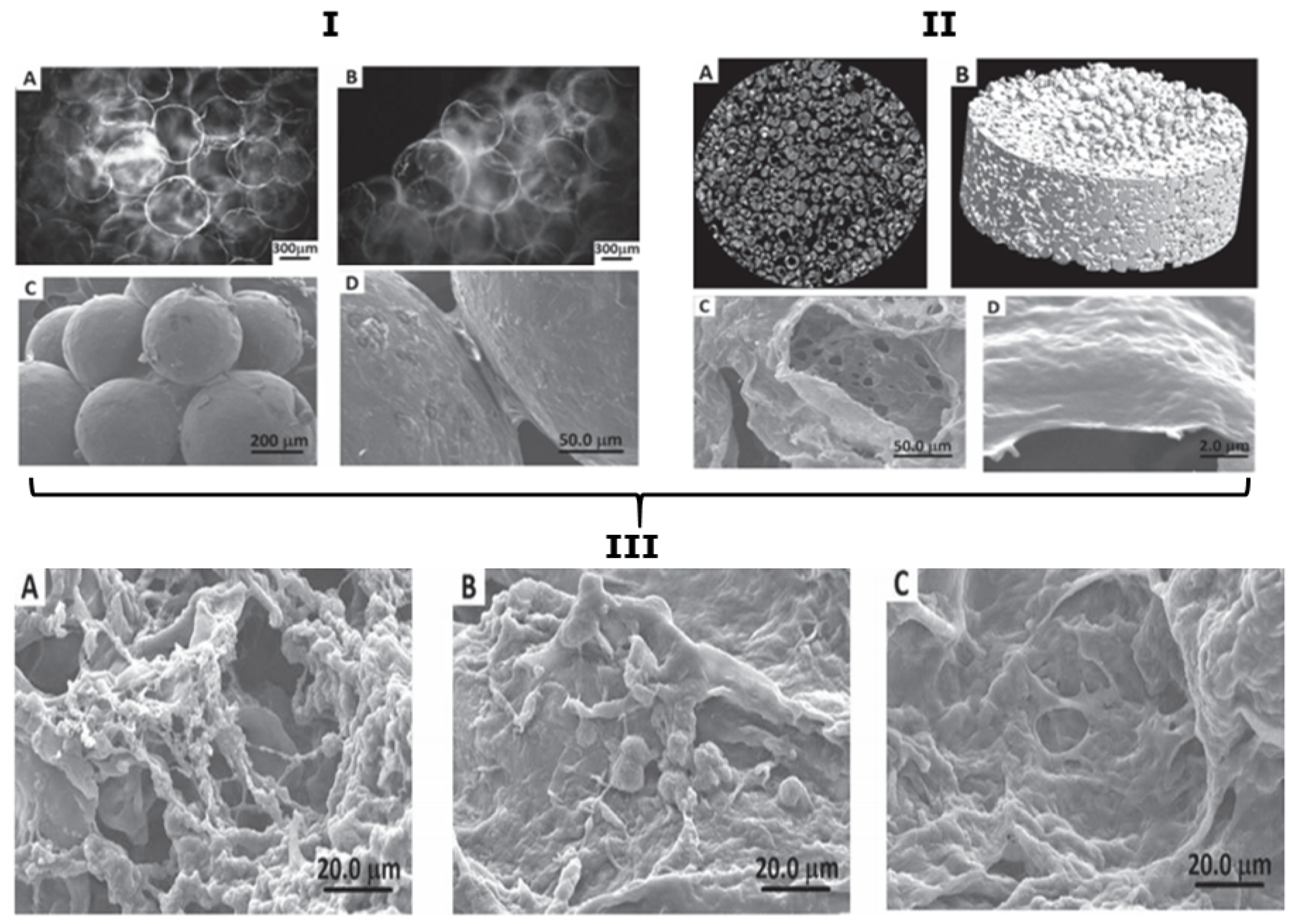
5.1. Scaffolds with Integrated PEMCs
5.2. Scaffolds Consisting Purely of PEMCs
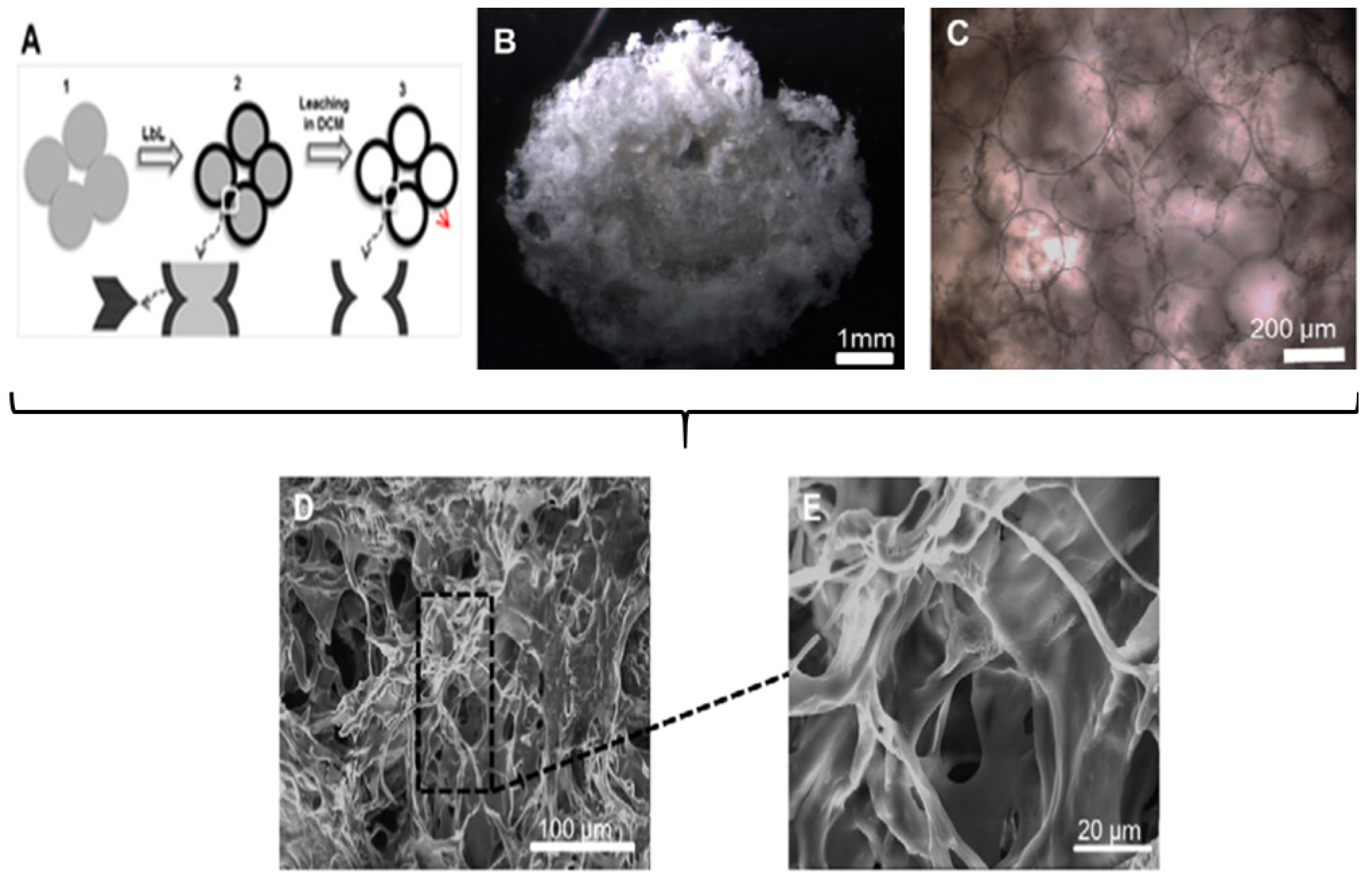
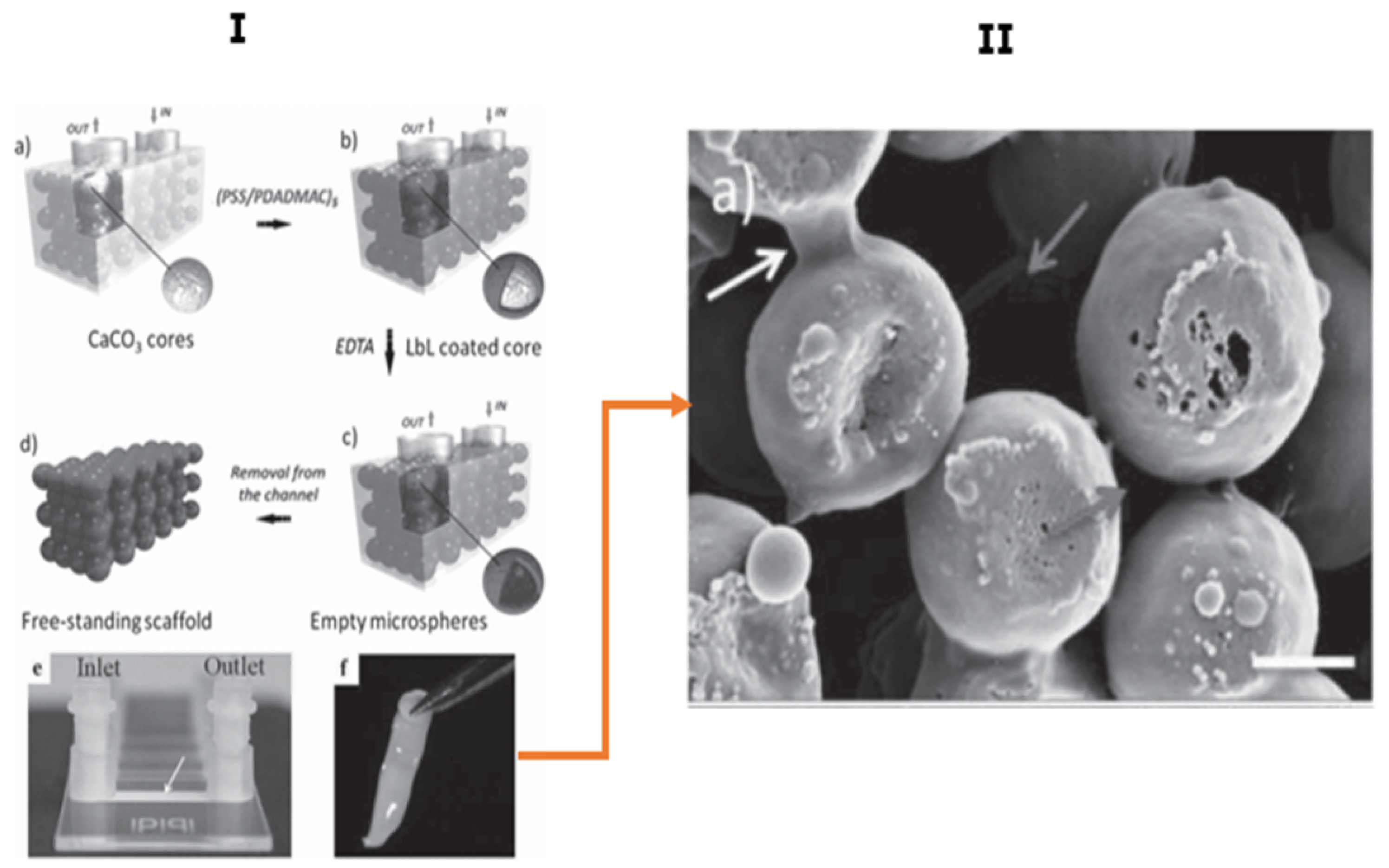
5.3. Microfluidic Assisted Formation of PEMC-Based Scaffolds
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shi, Q.; Qian, Z.; Liu, D.; Liu, H. Surface Modification of Dental Titanium Implant by Layer-by-Layer Electrostatic Self-Assembly. Front. Physiol. 2017, 8, 574. [Google Scholar] [CrossRef] [Green Version]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable Materials and Metallic Implants—A Review. J. Funct. Biomater. 2017, 8, 44. [Google Scholar] [CrossRef] [Green Version]
- Mao, A.S.; Mooney, D.J. Regenerative Medicine: Current Therapies and Future Directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef] [Green Version]
- Nicolas, C.T.; Hickey, R.D.; Chen, H.S.; Mao, S.A.; Lopera Higuita, M.; Wang, Y.; Nyberg, S.L. Concise Review: Liver Regenerative Medicine: From Hepatocyte Transplantation to Bioartificial Livers and Bioengineered Grafts. Stem Cells 2017, 35, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in Biomaterials for Tissue Engineering. Nat. Mater. 2009, 8, 457–470. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone Substitutes: An Update. Injury 2005, 36, 20–27. [Google Scholar] [CrossRef]
- Tirrell, D.A.; Langer, R. Designing Materials for Biology and Medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers. 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, A.R.; Singh, U.S.; Momin, M.; Bhavsar, C. Chitosan: Application in Tissue Engineering and Skin Grafting. J. Polym. Res. 2017, 24, 125. [Google Scholar] [CrossRef]
- Zhu, M.; Li, W.; Dong, X.; Yuan, X.; Midgley, A.C.; Chang, H.; Wang, Y.; Wang, H.; Wang, K.; Ma, P.X.; et al. In Vivo Engineered Extracellular Matrix Scaffolds with Instructive Niches for Oriented Tissue Regeneration. Nat. Commun. 2019, 10, 4620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, S.; Ding, F.; Gong, L.; Gu, X. Extracellular Matrix Scaffolds for Tissue Engineering and Regenerative Medicine. Curr. Stem Cell Res. Ther. 2017, 12, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ko, H.; Kwon, I.K.; Shin, K. Extracellular Matrix Revisited: Roles in Tissue Engineering. Int. Neurourol. J. 2016, 20, S23. [Google Scholar] [CrossRef] [PubMed]
- Bitar, K.N.; Zakhem, E. Design Strategies of Biodegradable Scaffolds for Tissue Regeneration. Biomed. Eng. Comput. Biol. 2014, 6, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Janik, H.; Marzec, M. A Review: Fabrication of Porous Polyurethane Scaffolds. Mater. Sci. Eng. C 2015, 48, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Sart, S.; Yan, Y.; Lochner, E.; Zeng, C.; Ma, T.; Li, Y. Crosslinking of Extracellular Matrix Scaffolds Derived from Pluripotent Stem Cell Aggregates Modulates Neural Differentiation. Acta Biomater. 2016, 30, 222–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The Design of Scaffolds for Use in Tissue Engineering. Part I. Traditional Factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef] [Green Version]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and Bioactive Porous Polymer/Inorganic Composite Scaffolds for Bone Tissue Engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Otoni, C.G.; De France, K.J.; Barud, H.S.; Lona, L.M.F.; Cranston, E.D.; Rojas, O.J. Porous Nanocellulose Gels and Foams: Breakthrough Status in the Development of Scaffolds for Tissue Engineering. Mater. Today 2020, 37, 126–141. [Google Scholar] [CrossRef]
- Osorio, M.; Fernández-Morales, P.; Gañán, P.; Zuluaga, R.; Kerguelen, H.; Ortiz, I.; Castro, C. Development of Novel Three-Dimensional Scaffolds Based on Bacterial Nanocellulose for Tissue Engineering and Regenerative Medicine: Effect of Processing Methods, Pore Size, and Surface Area. J. Biomed. Mater. Res. Part A 2018, 107, 348–359. [Google Scholar] [CrossRef]
- Ai, F.; Chen, L.; Yan, J.; Yang, K.; Li, S.; Duan, H.; Cao, C.; Li, W.; Zhou, K. Hydroxyapatite Scaffolds Containing Copper for Bone Tissue Engineering. J. Sol-Gel Sci. Technol. 2020, 95, 168–179. [Google Scholar] [CrossRef]
- Teixeira, S.; Rodriguez, M.A.; Pena, P.; De Aza, A.H.; De Aza, S.; Ferraz, M.P.; Monteiro, F.J. Physical Characterization of Hydroxyapatite Porous Scaffolds for Tissue Engineering. Mater. Sci. Eng. C 2008, 29, 1510–1514. [Google Scholar] [CrossRef]
- Erisken, C.; Kalyon, D.M.; Wang, H. Functionally Graded Electrospun Polycaprolactone and β-Tricalcium Phosphate Nanocomposites for Tissue Engineering Applications. Biomaterials 2008, 29, 4065–4073. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.N.; Ko, Y.G.; Yeo, T.; Kim, E.J.; Kwon, O.K.; Kwon, O.H. Guided Regeneration of Rabbit Calvarial Defects Using Silk Fibroin Nanofiber-Poly(Glycolic Acid) Hybrid Scaffolds. ACS Biomater. Sci. Eng. 2019, 5, 5266–5272. [Google Scholar] [CrossRef]
- Zhang, J.; Song, C.; Han, Y.; Xi, Z.; Zhao, L.; Cen, L.; Yang, Y. Regulation of Inflammatory Response to Polyglycolic Acid Scaffolds through Incorporation of Sodium Tripolyphosphate. Eur. Polym. J. 2020, 122, 109349. [Google Scholar] [CrossRef]
- Salem, S.A.; Rashidbenam, Z.; Jasman, M.H.; Ho, C.C.K.; Sagap, I.; Singh, R.; Yusof, M.R.; Zainuddin, Z.M.; Haji Idrus, R.B.; Ng, M.H. Incorporation of Smooth Muscle Cells Derived from Human Adipose Stem Cells on Poly(Lactic-Co-Glycolic Acid) Scaffold for the Reconstruction of Subtotally Resected Urinary Bladder in Athymic Rats. Tissue Eng. Regen. Med. 2020, 17, 553–563. [Google Scholar] [CrossRef]
- Yang, L.; Liu, S.; Fang, W.; Chen, J.; Chen, Y. Poly(Lactic-co-glycolic Acid)-bioactive Glass Composites as Nanoporous Scaffolds for Bone Tissue Engineering: In Vitro and in Vivo Studies. Exp. Ther. Med. 2019, 18, 4874–4880. [Google Scholar] [CrossRef] [Green Version]
- Ghalia, M.A.; Dahman, Y. Biodegradable Poly(Lactic Acid)-Based Scaffolds: Synthesis and Biomedical Applications. J. Polym. Res. 2017, 24, 1123. [Google Scholar] [CrossRef]
- Savioli Lopes, M.; Jardini, A.L.; Maciel Filho, R. Poly (Lactic Acid) Production for Tissue Engineering Applications. Procedia Eng. 2012, 42, 1402–1413. [Google Scholar] [CrossRef] [Green Version]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-Lactic Acid Synthesis for Application in Biomedical Devices—A Review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Shuai, C.; Wu, P.; Zhong, Y.; Feng, P.; Gao, C.; Huang, W.; Zhou, Z.; Chen, L.; Shuai, C. Polyetheretherketone/Poly (Glycolic Acid) Blend Scaffolds with Biodegradable Properties. J. Biomater. Sci. Polym. Ed. 2016, 27, 1434–1446. [Google Scholar] [CrossRef] [PubMed]
- Serbezeanu, D.; Vlad-Bubulac, T.; Rusu, D.; Pircalabioru, G.G.; Samoilă, I.; Dinescu, S.; Aflori, M. Functional Polyimide-Based Electrospun Fibers for Biomedical Application. Materials 2019, 12, 3201. [Google Scholar] [CrossRef] [Green Version]
- Kucinska-Lipka, J.; Gubanska, I.; Janik, H.; Sienkiewicz, M. Fabrication of Polyurethane and Polyurethane Based Composite Fibres by the Electrospinning Technique for Soft Tissue Engineering of Cardiovascular System. Mater. Sci. Eng. C 2015, 46, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, L.P.; Rodrigues, A.A.; Macedo, M.; Jardini, A.L.; Maciel Filho, R. Electrospun Polyurethane Membranes for Tissue Engineering Applications. Mater. Sci. Eng. C 2017, 72, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Bax, D.V.; Davidenko, N.; Gullberg, D.; Hamaia, S.W.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Fundamental Insight into the Effect of Carbodiimide Crosslinking on Cellular Recognition of Collagen-Based Scaffolds. Acta Biomater. 2017, 49, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Levingstone, T.J.; Ramesh, A.; Brady, R.T.; Brama, P.A.J.; Kearney, C.; Gleeson, J.P.; O’Brien, F.J. Cell-Free Multi-Layered Collagen-Based Scaffolds Demonstrate Layer Specific Regeneration of Functional Osteochondral Tissue in Caprine Joints. Biomaterials 2016, 87, 69–81. [Google Scholar] [CrossRef]
- Achilli, M.; Mantovani, D. Tailoring Mechanical Properties of Collagen-Based Scaffolds for Vascular Tissue Engineering: The Effects of PH, Temperature and Ionic Strength on Gelation. Polymers 2010, 2, 664–680. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Ikram, S. Chitosan Based Scaffolds and Their Applications in Wound Healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Zakhem, E.; Raghavan, S.; Gilmont, R.R.; Bitar, K.N. Chitosan-Based Scaffolds for the Support of Smooth Muscle Constructs in Intestinal Tissue Engineering. Biomaterials 2012, 33, 4810–4817. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.C.; Yang, K.C.; Lin, K.H.; Liu, H.C.; Lin, F.H. A Highly Organized Three-Dimensional Alginate Scaffold for Cartilage Tissue Engineering Prepared by Microfluidic Technology. Biomaterials 2011, 32, 7118–7126. [Google Scholar] [CrossRef]
- Lee, H.J.; Ahn, S.H.; Kim, G.H. Three-Dimensional Collagen/Alginate Hybrid Scaffolds Functionalized with a Drug Delivery System (DDS) for Bone Tissue Regeneration. Chem. Mater. 2012, 24, 881–891. [Google Scholar] [CrossRef]
- McManus, M.; Boland, E.; Sell, S.; Bowen, W.; Koo, H.; Simpson, D.; Bowlin, G. Electrospun Nanofibre Fibrinogen for Urinary Tract Tissue Reconstruction. Biomed. Mater. 2007, 2, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Stapelfeldt, K.; Stamboroski, S.; Mednikova, P.; Brüggemann, D. Fabrication of 3D-Nanofibrous Fibrinogen Scaffolds Using Salt-Induced Self Assembly. Biofabrication 2019, 11, 025010. [Google Scholar] [CrossRef]
- Buie, T.; McCune, J.; Cosgriff-Hernandez, E. Gelatin Matrices for Growth Factor Sequestration. Trends Biotechnol. 2020, 38, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Contessi Negrini, N.; Bonnetier, M.; Giatsidis, G.; Orgill, D.P.; Farè, S.; Marelli, B. Tissue-Mimicking Gelatin Scaffolds by Alginate Sacrificial Templates for Adipose Tissue Engineering. Acta Biomater. 2019, 87, 61–75. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & Scaffolds for Tissue Engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Chen, F.M.; Liu, X. Advancing Biomaterials of Human Origin for Tissue Engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.D.; Grande, D.A. The Current State of Scaffolds for Musculoskeletal Regenerative Applications. Nat. Rev. Rheumatol. 2015, 11, 213–222. [Google Scholar] [CrossRef]
- Font Tellado, S.; Balmayor, E.R.; Van Griensven, M. Strategies to Engineer Tendon/Ligament-to-Bone Interface: Biomaterials, Cells and Growth Factors. Adv. Drug Deliv. Rev. 2015, 94, 126–140. [Google Scholar] [CrossRef]
- Laidmäe, I.; Ērglis, K.; Cēbers, A.; Janmey, P.A.; Uibo, R. Salmon Fibrinogen and Chitosan Scaffold for Tissue Engineering: In Vitro and in Vivo Evaluation. J. Mater. Sci. Mater. Med. 2018, 29. [Google Scholar] [CrossRef] [Green Version]
- Afjoul, H.; Shamloo, A.; Kamali, A. Freeze-Gelled Alginate/Gelatin Scaffolds for Wound Healing Applications: An in Vitro, in Vivo Study. Mater. Sci. Eng. C 2020, 113, 110957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, Y.; Zhang, N.; Shi, J.; Zhang, X.; Qi, C.; Midgley, A.C.; Wang, S. Potentials of Sandwich-like Chitosan/Polycaprolactone/Gelatin Scaffolds for Guided Tissue Regeneration Membrane. Mater. Sci. Eng. C 2020, 109, 110618. [Google Scholar] [CrossRef] [PubMed]
- Paulraj, T.; Feoktistova, N.; Velk, N.; Uhlig, K.; Duschl, C.; Volodkin, D. Microporous Polymeric 3D Scaffolds Templated by the Layer-by-Layer Self-Assembly. Macromol. Rapid Commun. 2014, 35, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Ghilan, A.; Chiriac, A.P.; Nita, L.E.; Rusu, A.G.; Neamtu, I.; Chiriac, V.M. Trends in 3D Printing Processes for Biomedical Field: Opportunities and Challenges. J. Polym. Environ. 2020, 28, 1345–1367. [Google Scholar] [CrossRef]
- Di Gesù, R.; Acharya, A.P.; Jacobs, I.; Gottardi, R. 3D Printing for Tissue Engineering in Otolaryngology. Connect. Tissue Res. 2019, 61, 117–136. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Sittinger, M.; Risbud, M.V. Scaffold-Based Tissue Engineering: Rationale for Computer-Aided Design and Solid Free-Form Fabrication Systems. Trends Biotechnol. 2004, 22, 354–362. [Google Scholar] [CrossRef]
- Sin, D.C.; Miao, X.; Liu, G.; Wei, F.; Chadwick, G.; Yan, C.; Friis, T. Polyurethane (PU) Scaffolds Prepared by Solvent Casting/Particulate Leaching (SCPL) Combined with Centrifugation. Mater. Sci. Eng. C 2010, 30, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Obayemi, J.D.; Jusu, S.M.; Salifu, A.A.; Ghahremani, S.; Tadesse, M.; Uzonwanne, V.O.; Soboyejo, W.O. Degradable Porous Drug-Loaded Polymer Scaffolds for Localized Cancer Drug Delivery and Breast Cell/Tissue Growth. Mater. Sci. Eng. C 2020, 112, 110794. [Google Scholar] [CrossRef]
- Sultana, N.; Wang, M. Fabrication of HA/PHBV Composite Scaffolds through the Emulsion Freezing/Freeze-Drying Process and Characterisation of the Scaffolds. J. Mater. Sci. Mater. Med. 2008, 19, 2555–2561. [Google Scholar] [CrossRef]
- Shahabi, S.; Rezaei, Y.; Moztarzadeh, F.; Najafi, F. In Vitro Degradation and Bioactivity of Poly(Propylene Fumarate)/Bioactive Glass Sintered Microsphere Scaffolds for Bone Tissue Engineering. Sci. Eng. Compos. Mater. 2016, 23, 245–256. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Hu, T.; Guo, B.; Ma, P.X. Electrospun Conductive Nanofibrous Scaffolds for Engineering Cardiac Tissue and 3D Bioactuators. Acta Biomater. 2017, 59, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S.; Balagangadharan, K.; Selvamurugan, N. Chitosan and Gelatin-Based Electrospun Fibers for Bone Tissue Engineering. Int. J. Biol. Macromol. 2019, 133, 354–364. [Google Scholar] [CrossRef]
- Costantini, M.; Colosi, C.; Mozetic, P.; Jaroszewicz, J.; Tosato, A.; Rainer, A.; Trombetta, M.; Święszkowski, W.; Dentini, M.; Barbetta, A. Correlation between Porous Texture and Cell Seeding Efficiency of Gas Foaming and Microfluidic Foaming Scaffolds. Mater. Sci. Eng. C 2016, 62, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zhou, C.; Fan, H.; Zhang, B.; Pei, X.; Fan, Y.; Jiang, Q.; Bao, R.; Yang, Q.; Dong, Z.; et al. Novel 3D Porous Biocomposite Scaffolds Fabricated by Fused Deposition Modeling and Gas Foaming Combined Technology. Compos. Part B Eng. 2018, 152, 151–159. [Google Scholar] [CrossRef]
- Biswas, D.P.; Tran, P.A.; Tallon, C.; O’Connor, A.J. Combining Mechanical Foaming and Thermally Induced Phase Separation to Generate Chitosan Scaffolds for Soft Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2016, 28, 207–226. [Google Scholar] [CrossRef] [PubMed]
- Jaklenec, A.; Wan, E.; Murray, M.E.; Mathiowitz, E. Novel Scaffolds Fabricated from Protein-Loaded Microspheres for Tissue Engineering. Biomaterials 2008, 29, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Boffito, M.; Sartori, S.; Ciardelli, G. Polymeric Scaffolds for Cardiac Tissue Engineering: Requirements and Fabrication Technologies. Polym. Int. 2014, 63, 2–11. [Google Scholar] [CrossRef]
- Caballero Aguilar, L.M.; Silva, S.M.; Moulton, S.E. Growth Factor Delivery: Defining the next Generation Platforms for Tissue Engineering. J. Control. Release 2019, 306, 40–58. [Google Scholar] [CrossRef]
- Biondi, M.; Ungaro, F.; Quaglia, F.; Netti, P.A. Controlled Drug Delivery in Tissue Engineering. Adv. Drug Deliv. Rev. 2008, 60, 229–242. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel Biomaterial Strategies for Controlled Growth Factor Delivery for Biomedical Applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]
- Nyberg, E.; Holmes, C.; Witham, T.; Grayson, W.L. Growth Factor-Eluting Technologies for Bone Tissue Engineering. Drug Deliv. Transl. Res. 2016, 6, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Trushina, D.B.; Burova, A.S.; Borodina, T.N.; Soldatov, M.A.; Klochko, T.Y.; Bukreeva, T.V. Thermo-Induced Shrinking of “Dextran Sulfate/Polyarginine” Capsules with Magnetic Nanoparticles in the Shell. Colloid J. 2018, 80, 710–715. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Gorin, D.A.; Möhwald, H. Ultrasonically Induced Opening of Polyelectrolyte Microcontainers. Langmuir 2006, 22, 7400–7404. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Chen, W.H.; Zhang, J.; Li, C.; Zhuo, R.X.; Zhang, X.Z. Design of a Photoswitchable Hollow Microcapsular Drug Delivery System by Using a Supramolecular Drug-Loading Approach. J. Phys. Chem. B 2011, 115, 13796–13802. [Google Scholar] [CrossRef]
- Marchenko, I.; Yashchenok, A.; Borodina, T.; Bukreeva, T.; Konrad, M.; Möhwald, H.; Skirtach, A. Controlled Enzyme-Catalyzed Degradation of Polymeric Capsules Templated on CaCO3: Influence of the Number of LbL Layers, Conditions of Degradation, and Disassembly of Multicompartments. J. Control. Release 2012, 162, 599–605. [Google Scholar] [CrossRef]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A Review of Stimuli-Responsive Nanocarriers for Drug and Gene Delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef]
- Iafisco, M.; Sandri, M.; Panseri, S.; Delgado-López, J.M.; Gómez-Morales, J.; Tampieri, A. Magnetic Bioactive and Biodegradable Hollow Fe-Doped Hydroxyapatite Coated Poly(l -Lactic) Acid Micro-Nanospheres. Chem. Mater. 2013, 25, 2610–2617. [Google Scholar] [CrossRef]
- Gentile, P.; Nandagiri, V.K.; Daly, J.; Chiono, V.; Mattu, C.; Tonda-Turo, C.; Ciardelli, G.; Ramtoola, Z. Localised Controlled Release of Simvastatin from Porous Chitosan-Gelatin Scaffolds Engrafted with Simvastatin Loaded PLGA-Microparticles for Bone Tissue Engineering Application. Mater. Sci. Eng. C 2016, 59, 249–257. [Google Scholar] [CrossRef]
- Wang, X.; Wu, X.; Xing, H.; Zhang, G.; Shi, Q.; Lingling, E.; Liu, N.; Yang, T.; Wang, D.; Qi, F.; et al. Porous Nanohydroxyapatite/Collagen Scaffolds Loading Insulin PLGA Particles for Restoration of Critical Size Bone Defect. ACS Appl. Mater. Interfaces 2017, 9, 11380–11391. [Google Scholar] [CrossRef]
- Patel, Z.S.; Ueda, H.; Yamamoto, M.; Tabata, Y.; Mikos, A.G. In Vitro and in Vivo Release of Vascular Endothelial Growth Factor from Gelatin Microparticles and Biodegradable Composite Scaffolds. Pharm. Res. 2008, 25, 2370–2378. [Google Scholar] [CrossRef]
- Quinlan, E.; López-Noriega, A.; Thompson, E.M.; Hibbitts, A.; Cryan, S.A.; O’Brien, F.J. Controlled Release of Vascular Endothelial Growth Factor from Spray-Dried Alginate Microparticles in Collagen–Hydroxyapatite Scaffolds for Promoting Vascularization and Bone Repair. J. Tissue Eng. Regen. Med. 2017, 11, 1097–1109. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Eltohamy, M.; Kim, M.; Perez, R.A.; Kim, J.H.; Yun, Y.R.; Jang, J.H.; Lee, E.J.; Knowles, J.C.; Kim, H.W. Therapeutic Foam Scaffolds Incorporating Biopolymer-Shelled Mesoporous Nanospheres with Growth Factors. Acta Biomater. 2014, 10, 2612–2621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kehr, N.S.; Prasetyanto, E.A.; Benson, K.; Ergün, B.; Galstyan, A.; Galla, H.J. Periodic Mesoporous Organosilica-Based Nanocomposite Hydrogels as Three-Dimensional Scaffolds. Angew. Chemie Int. Ed. 2013, 52, 1156–1160. [Google Scholar] [CrossRef]
- Li, X.; Fan, C.; Xiao, Z.; Zhao, Y.; Zhang, H.; Sun, J.; Zhuang, Y.; Wu, X.; Shi, J.; Chen, Y.; et al. A Collagen Microchannel Scaffold Carrying Paclitaxel-Liposomes Induces Neuronal Differentiation of Neural Stem Cells through Wnt/β-Catenin Signaling for Spinal Cord Injury Repair. Biomaterials 2018, 183, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Mufamadi, M.S.; Kumar, P.; du Toit, L.C.; Choonara, Y.E.; Obulapuram, P.K.; Modi, G.; Naidoo, D.; Iyuke, S.E.; Pillay, V. Liposome-Embedded, Polymeric Scaffold for Extended Delivery of Galantamine. J. Drug Deliv. Sci. Technol. 2019, 50, 255–265. [Google Scholar] [CrossRef]
- Tessmar, J.K.; Göpferich, A.M. Matrices and Scaffolds for Protein Delivery in Tissue Engineering. Adv. Drug Deliv. Rev. 2007, 59, 274–291. [Google Scholar] [CrossRef]
- Simioni, A.R.; de Jesus, P.C.C.; Tedesco, A.C. Layer-by-Layer Hollow Photosensitizer Microcapsule Design via a Manganese Carbonate Hard Template for Photodynamic Therapy in Cells. Photodiagnosis Photodyn. Ther. 2018, 22, 169–177. [Google Scholar] [CrossRef]
- Moya, S.; Dähne, L.; Voigt, A.; Leporatti, S.; Donath, E.; Möhwald, H. Polyelectrolyte Multilayer Capsules Templated on Biological Cells: Core Oxidation Influences Layer Chemistry. Colloids Surfaces A Physicochem. Eng. Asp. 2001, 183, 27–40. [Google Scholar] [CrossRef]
- Peyratout, C.S.; Dähne, L. Tailor-Made Polyelectrolyte Microcapsules: From Multilayers to Smart Containers. Angew. Chem. Int. Ed. 2004, 43, 3762–3783. [Google Scholar] [CrossRef]
- Balabushevich, N.G.; Lopez De Guerenu, A.V.; Feoktistova, N.A.; Skirtach, A.G.; Volodkin, D. Protein-Containing Multilayer Capsules by Templating on Mesoporous CaCO3 Particles: POST- and PRE-Loading Approaches. Macromol. Biosci. 2016, 16, 95–105. [Google Scholar] [CrossRef]
- Del Mercato, L.L.; Ferraro, M.M.; Baldassarre, F.; Mancarella, S.; Greco, V.; Rinaldi, R.; Leporatti, S. Biological Applications of LbL Multilayer Capsules: From Drug Delivery to Sensing. Adv. Colloid Interface Sci. 2014, 207, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Soenen, S.J.; Braeckmans, K.; Skirtach, A.G. Towards Theranostic Multicompartment Microcapsules: In-Situ Diagnostics and Laser-Induced Treatment. Theranostics 2013, 3, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Delcea, M.; Möhwald, H.; Skirtach, A.G. Stimuli-Responsive LbL Capsules and Nanoshells for Drug Delivery. Adv. Drug Deliv. Rev. 2011, 63, 730–747. [Google Scholar] [CrossRef] [PubMed]
- De Cock, L.J.; De Wever, O.; Van Vlierberghe, S.; Vanderleyden, E.; Dubruel, P.; De Vos, F.; Vervaet, C.; Remon, J.P.; De Geest, B.G. Engineered (Hep/PARG) 2 Polyelectrolyte Capsules for Sustained Release of Bioactive TGF-Β1. Soft Matter 2012, 8, 1146–1154. [Google Scholar] [CrossRef]
- Wang, L.M.; Chang, H.; Zhang, H.; Ren, K.F.; Li, H.; Hu, M.; Li, B.C.; Martins, M.C.L.; Barbosa, M.A.; Ji, J. Dynamic Stiffness of Polyelectrolyte Multilayer Films Based on Disulfide Bonds for in Situ Control of Cell Adhesion. J. Mater. Chem. B 2015, 3, 7546–7553. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Crouzier, T.; Roy, C.; Picart, C. Polyelectrolyte Multilayer Films of Controlled Stiffness Modulate Myoblast Cell Differentiation. Adv. Funct. Mater. 2008, 18, 1378–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, A.; Francius, G.; Obeid, R.; Schwinté, P.; Hemmerlé, J.; Frisch, B.; Schaaf, P.; Voegel, J.C.; Senger, B.; Picart, C. Polyelectrolyte Multilayers with a Tunable Young’s Modulus: Influence of Film Stiffness on Cell Adhesion. Langmuir 2006, 22, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Richert, L.; Francius, G.; Voegel, J.C.; Picart, C. Elasticity, Biodegradability and Cell Adhesive Properties of Chitosan/Hyaluronan Multilayer Films. Biomed. Mater. 2007, 2, S45. [Google Scholar] [CrossRef]
- Gaudière, F.; Morin-Grognet, S.; Bidault, L.; Lembré, P.; Pauthe, E.; Vannier, J.P.; Atmani, H.; Ladam, G.; Labat, B. Genipin-Cross-Linked Layer-by-Layer Assemblies: Biocompatible Microenvironments to Direct Bone Cell Fate. Biomacromolecules 2014, 15, 1602–1611. [Google Scholar] [CrossRef]
- Singh, M.N.; Hemant, K.S.Y.; Ram, M.; Shivakumar, H.G. Microencapsulation: A Promising Technique for Controlled Drug Delivery. Res. Pharm. Sci. 2010, 5, 65–77. [Google Scholar]
- Jeannot, L.; Bell, M.; Ashwell, R.; Volodkin, D.; Vikulina, A.S. Internal Structure of Matrix-Type Multilayer Capsules Templated on Porous Vaterite CaCO3 Crystals as Probed by Staining with a Fluorescence Dye. Micromachines 2018, 9, 547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radhakrishnan, K.; Thomas, M.B.; Pulakkat, S.; Gnanadhas, D.P.; Chakravortty, D.; Raichur, A.M. Stimuli-Responsive Protamine-Based Biodegradable Nanocapsules for Enhanced Bioavailability and Intracellular Delivery of Anticancer Agents. J. Nanoparticle Res. 2015, 17, 341. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Zhao, Z.; Li, J.; Zhang, R.; Yao, F. Formation and Characterization of Natural Polysaccharide Hollow Nanocapsules via Template Layer-by-Layer Self-Assembly. J. Colloid Interface Sci. 2012, 379, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Paşcaləu, V.; Soritau, O.; Popa, F.; Pavel, C.; Coman, V.; Perhaita, I.; Borodi, G.; Dirzu, N.; Tabaran, F.; Popa, C.; et al. Curcumin Delivered through Bovine Serum Albumin/Polysaccharides Multilayered Microcapsules. J. Biomater. Appl. 2016, 30, 857–872. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, A.V.; Inozemtseva, O.A.; Gorin, D.A.; Sukhorukov, G.B.; Belyakov, S.; Antipina, M.N. Influence of Heat Treatment on Loading of Polymeric Multilayer Microcapsules with Rhodamine B. Macromol. Rapid Commun. 2018, 40, 1800200. [Google Scholar] [CrossRef]
- Tabata, Y. The Importance of Drug Delivery Systems in Tissue Engineering. Pharm. Sci. Technol. Today 2000, 3, 80–89. [Google Scholar] [CrossRef]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth Factor Delivery-Based Tissue Engineering: General Approaches and a Review of Recent Developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef] [Green Version]
- Babensee, J.E.; McIntire, L.V.; Mikos, A.G. Growth Factor Delivery for Tissue Engineering. Pharm. Res. 2000, 17, 497–504. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, H.; Song, T.; Liu, X.; Gao, Y.; Zhou, J.; Li, Y. Sustainable Dual Release of Antibiotic and Growth Factor from pH-Responsive Uniform Alginate Composite Microparticles to Enhance Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 22730–22744. [Google Scholar] [CrossRef]
- Azizian, S.; Hadjizadeh, A.; Niknejad, H. Chitosan-Gelatin Porous Scaffold Incorporated with Chitosan Nanoparticles for Growth Factor Delivery in Tissue Engineering. Carbohydr. Polym. 2018, 202, 315–322. [Google Scholar] [CrossRef]
- Correia, C.R.; Gil, S.; Reis, R.L.; Mano, J.F. A Closed Chondromimetic Environment within Magnetic-Responsive Liquified Capsules Encapsulating Stem Cells and Collagen II/TGF-Β3 Microparticles. Adv. Healthc. Mater. 2016, 5, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Bellamkonda, R.V. Biomaterials for the Central Nervous System. J. R. Soc. Interface 2008, 5, 957–975. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Xiu, P.; Tan, J.; Jia, Z.; Cai, H.; Liu, Z. Enhanced Angiogenesis and Osteogenesis in Critical Bone Defects by the Controlled Release of BMP-2 and VEGF: Implantation of Electron Beam Melting-Fabricated Porous Ti6Al4V Scaffolds Incorporating Growth Factor-Doped Fibrin Glue. Biomed. Mater. 2015, 10, 35013. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, L.; Shansky, J.; Wang, Z.; Mooney, D.; Vandenburgh, H. Minimally Invasive Approach to the Repair of Injured Skeletal Muscle with a Shape-Memory Scaffold. Mol. Ther. 2014, 22, 1441–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willerth, S.M.; Rader, A.; Sakiyama-Elbert, S.E. The Effect of Controlled Growth Factor Delivery on Embryonic Stem Cell Differentiation inside Fibrin Scaffolds. Stem Cell Res. 2008, 1, 205–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, M.; Jiang, X.; Zhou, X.; Wang, C.; Wu, Q.; Ren, L.; Zhu, J.; Zhu, S.; Tebon, P.; Sun, W.; et al. Stimuli-Responsive Delivery of Growth Factors for Tissue Engineering. Adv. Healthc. Mater. 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Vasita, R.; Katti, D.S. Growth Factor-Delivery Systems for Tissue Engineering: A Materials Perspective. Expert Rev. Med. Devices 2006, 3, 29–47. [Google Scholar] [CrossRef]
- De Cock, L.J.; De Koker, S.; De Geest, B.G.; Grooten, J.; Vervaet, C.; Remon, J.P.; Sukhorukov, G.B.; Antipina, M.N. Polymeric Multilayer Capsules in Drug Delivery. Angew. Chem. Int. Ed. 2010, 49, 6954–6973. [Google Scholar] [CrossRef]
- Hong, J.; Han, J.Y.; Yoon, H.; Joo, P.; Lee, T.; Seo, E.; Char, K.; Kim, B.S. Carbon-Based Layer-by-Layer Nanostructures: From Films to Hollow Capsules. Nanoscale 2011, 3, 4515–4531. [Google Scholar] [CrossRef]
- Volodkin, D.V.; Balabushevitch, N.G.; Sukhorukov, G.B.; Larionova, N.I. Model System for Controlled Protein Release: pH-Sensitive Polyelectrolyte Microparticles. STP Pharma Sci. 2003, 13, 163–170. [Google Scholar]
- Volodkin, D.V.; Balabushevitch, N.G.; Sukhorukov, G.B.; Larionova, N.I. Inclusion of Proteins into Polyelectrolyte Microparticles by Alternative Adsorption of Polyelectrolytes on Protein Aggregates. Biokhimiya 2003, 68, 283–289. [Google Scholar] [CrossRef]
- Madaboosi, N.; Uhlig, K.; Schmidt, S.; Vikulina, A.S.; Möhwald, H.; Duschl, C.; Volodkin, D. A “Cell-Friendly” Window for the Interaction of Cells with Hyaluronic Acid/Poly-l-Lysine Multilayers. Macromol. Biosci. 2017, 18, 1700319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.S.; Hong, J.D.; Kim, C.H.; Kim, K.; Koo, J.P.; Lee, K.B. Layer-by-Layer Deposited Multilayer Assemblies of Ionene-Type Polyelectrolytes Based on the Spin-Coating Method. Macromolecules 2001, 34, 5358–5360. [Google Scholar] [CrossRef]
- Schaaf, P.; Voegel, J.C.; Jierry, L.; Boulmedais, F. Spray-Assisted Polyelectrolyte Multilayer Buildup: From Step-by-Step to Single-Step Polyelectrolyte Film Constructions. Adv. Mater. 2012, 24, 1001–1016. [Google Scholar] [CrossRef] [Green Version]
- Richardson, J.J.; Björnmalm, M.; Caruso, F. Technology-Driven Layer-by-Layer Assembly of Nanofilms. Science 2015, 348, aaa2491. [Google Scholar] [CrossRef] [Green Version]
- Madaboosi, N.; Uhlig, K.; Jäger, M.S.; Möhwald, H.; Duschl, C.; Volodkin, D.V. Microfluidics as a Tool to Understand the Build-up Mechanism of Exponential-like Growing Films. Macromol. Rapid Commun. 2012, 33, 1775–1779. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Zhang, X.; Wu, H.; Shen, J.; Chen, R.; Xiong, Y.; Li, J.; Guo, S. Progress on the Layer-by-Layer Assembly of Multilayered Polymer Composites: Strategy, Structural Control and Applications. Prog. Polym. Sci. 2019, 89, 76–107. [Google Scholar] [CrossRef]
- Such, G.K.; Quinn, J.F.; Quinn, A.; Tjipto, E.; Caruso, F. Assembly of Ultrathin Polymer Multilayer Films by Click Chemistry. J. Am. Chem. Soc. 2006, 128, 9318–9319. [Google Scholar] [CrossRef]
- Lee, H.; Mensire, R.; Cohen, R.E.; Rubner, M.F. Strategies for Hydrogen Bonding Based Layer-by-Layer Assembly of Poly(Vinyl Alcohol) with Weak Polyacids. Macromolecules 2012, 45, 347–355. [Google Scholar] [CrossRef]
- Bathula, C.; Buruga, K.; Lee, S.K.; Khazi, I.A.M.; Kang, Y. Microwave Assisted Synthesis of Bithiophene Based Donor-Acceptor-Donor Oligomers and Their Optoelectronic Performances. J. Mol. Struct. 2017, 1139, 125–129. [Google Scholar] [CrossRef]
- Alkekhia, D.; Shukla, A. Influence of Poly-l-Lysine Molecular Weight on Antibacterial Efficacy in Polymer Multilayer Films. J. Biomed. Mater. Res. Part A 2019, 107, 1324–1339. [Google Scholar] [CrossRef]
- Lvov, Y.; Antipov, A.A.; Mamedov, A.; Möhwald, H.; Sukhorukov, G.B. Urease Encapsulation in Nanoorganized Microshells. Nano Lett. 2001, 1, 125–128. [Google Scholar] [CrossRef]
- Déjugnat, C.; Sukhorukov, G.B. pH-Responsive Properties of Hollow Polyelectrolyte Microcapsules Templated on Various Cores. Langmuir 2004, 20, 7265–7269. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Moya, S.; Lichtenfeld, H.; Casoli, A.; Fiedler, H.; Donath, E.; Möhwald, H. The Decomposition Process of Melamine Formaldehyde Cores: The Key Step in the Fabrication of Ultrathin Polyelectrolyte Multilayer Capsules. Macromol. Mater. Eng. 2001, 286, 355–361. [Google Scholar] [CrossRef]
- Gao, C.; Möhwald, H.; Shen, J. Soluble Microcapsules Assembled Stepwise from Weak Polyelectrolytes Using Acid-Decomposable Cores. Adv. Mater. 2003, 15, 930–933. [Google Scholar] [CrossRef]
- Thomas, M.B.; Radhakrishnan, K.; Gnanadhas, D.P.; Chakravortty, D.; Raichur, A.M. Intracellular Delivery of Doxorubicin Encapsulated in Novel pH-Responsive Chitosan/Heparin Nanocapsules. Int. J. Nanomed. 2013, 8, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Itoh, Y.; Matsusaki, M.; Kida, T.; Akashi, M. Preparation of Biodegradable Hollow Nanocapsules by Silica Template Method. Chem. Lett. 2004, 33, 1552–1553. [Google Scholar] [CrossRef]
- Antipov, A.A.; Sukhorukov, G.B.; Leporatti, S.; Radtchenko, I.L.; Donath, E.; Möhwald, H. Polyelectrolyte Multilayer Capsule Permeability Control. Colloids Surfaces A Physicochem. Eng. Asp. 2002, 198, 535–541. [Google Scholar] [CrossRef]
- Volodkin, D. CaCO3 Templated Micro-Beads and -Capsules for Bioapplications. Adv. Colloid Interface Sci. 2014, 207, 306–324. [Google Scholar] [CrossRef]
- Parakhonskiy, B.V.; Yashchenok, A.M.; Donatan, S.; Volodkin, D.V.; Tessarolo, F.; Antolini, R.; Möhwald, H.; Skirtach, A.G. Macromolecule Loading into Spherical, Elliptical, Star-Like and Cubic Calcium Carbonate Carriers. ChemPhysChem 2014, 15, 2817–2822. [Google Scholar] [CrossRef]
- Konopacka-Łyskawa, D. Synthesis Methods and Favorable Conditions for Spherical Vaterite Precipitation: A Review. Crystals 2019, 9, 223. [Google Scholar] [CrossRef] [Green Version]
- Trushina, D.B.; Bukreeva, T.V.; Antipina, M.N. Size-Controlled Synthesis of Vaterite Calcium Carbonate by the Mixing Method: Aiming for Nanosized Particles. Cryst. Growth Des. 2016, 16, 1311–1319. [Google Scholar] [CrossRef]
- Vagenas, N.V.; Gatsouli, A.; Kontoyannis, C.G. Quantitative Analysis of Synthetic Calcium Carbonate Polymorphs Using FT-IR Spectroscopy. Talanta 2003, 59, 831–836. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Ren, Y.; Yan, H.; Wang, M.; Wang, D.; Lu, X.Y.; Wang, B.; Fan, T.; Guo, H. Controllable Synthesis of All the Anhydrous CaCO3 Polymorphs with Various Morphologies in CaCl2-NH3-CO2 Aqueous System. Powder Technol. 2018, 333, 410–420. [Google Scholar] [CrossRef]
- Christy, A.G. A Review of the Structures of Vaterite: The Impossible, the Possible, and the Likely. Cryst. Growth Des. 2017, 17, 3567–3578. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, A.; Mahapatra, S. Synthesis of All Crystalline Phases of Anhydrous Calcium Carbonate. Cryst. Growth Des. 2010, 10, 2129–2135. [Google Scholar] [CrossRef]
- Naka, K. Delayed Action of Synthetic Polymers for Controlled Mineralization of Calcium Carbonate. In Biomineralization II. Topics in Current Chemistry; Naka, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 119–154. [Google Scholar] [CrossRef]
- Feoktistova, N.A.; Balabushevich, N.G.; Skirtach, A.G.; Volodkin, D.; Vikulina, A.S. Inter-Protein Interactions Govern Protein Loading into Porous Vaterite CaCO3 Crystals. Phys. Chem. Chem. Phys. 2020, 22, 9713–9722. [Google Scholar] [CrossRef] [Green Version]
- Feoktistova, N.; Rose, J.; Prokopović, V.Z.; Vikulina, A.S.; Skirtach, A.; Volodkin, D. Controlling the Vaterite CaCO3 Crystal Pores. Design of Tailor-Made Polymer Based Microcapsules by Hard Templating. Langmuir 2016, 32, 4229–4238. [Google Scholar] [CrossRef] [Green Version]
- Mori, Y.; Enomae, T.; Isogai, A. Preparation of Pure Vaterite by Simple Mechanical Mixing of Two Aqueous Salt Solutions. Mater. Sci. Eng. C 2009, 29, 1409–1414. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Cao, Z.; Xu, Y.; Gong, Y.; Shi, X. Fabrication of Uniform Casein/CaCO3 Vaterite Microspheres and Investigation of Its Formation Mechanism. Cryst. Growth Des. 2017, 17, 6178–6188. [Google Scholar] [CrossRef]
- Volodkin, D.V.; Schmidt, S.; Fernandes, P.; Larionova, N.I.; Sukhorukov, G.B.; Duschl, C.; Möhwald, H.; Von Klitzing, R. One-Step Formulation of Protein Microparticles with Tailored Properties: Hard Templating at Soft Conditions. Adv. Funct. Mater. 2012, 22, 1914–1922. [Google Scholar] [CrossRef]
- Elbaz, N.M.; Owen, A.; Rannard, S.; McDonald, T.O. Controlled Synthesis of Calcium Carbonate Nanoparticles and Stimuli-Responsive Multi-Layered Nanocapsules for Oral Drug Delivery. Int. J. Pharm. 2020, 574, 118866. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, J.P. Formation Mechanism and Morphology in Precipitation of Vaterite—Nano-Aggregation or Crystal Growth? J. Cryst. Growth 2005, 274, 256–264. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The Kinetics and Mechanisms of Amorphous Calcium Carbonate (ACC) Crystallization to Calcite, via Vaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.; Persson, M.; Feng, C.; Parkin, S.J.; Nieminen, T.A.; Wood, B.; Heckenberg, N.R.; Rubinsztein-Dunlop, H. Synthesis and Surface Modification of Birefringent Vaterite Microspheres. Langmuir 2009, 25, 11672–11679. [Google Scholar] [CrossRef]
- Sergeeva, A.; Sergeev, R.; Lengert, E.; Zakharevich, A.; Parakhonskiy, B.; Gorin, D.; Sergeev, S.; Volodkin, D. Composite Magnetite and Protein Containing CaCO3 Crystals. External Manipulation and Vaterite → Calcite Recrystallization-Mediated Release Performance. ACS Appl. Mater. Interfaces 2015, 7, 21315–21325. [Google Scholar] [CrossRef] [PubMed]
- Saraya, M.E.-S.I.; Rokbaa, H.H.A.E.-L. Formation and Stabilization of Vaterite Calcium Carbonate by Using Natural Polysaccharide. Adv. Nanoparticles 2017, 6, 158–182. [Google Scholar] [CrossRef] [Green Version]
- Kontrec, J.; Kralj, D.; Brečević, L.; Falini, G. Influence of Some Polysaccharides on the Production of Calcium Carbonate Filler Particles. J. Cryst. Growth 2008, 310, 4554–4560. [Google Scholar] [CrossRef]
- Volodkin, D.V.; Larionova, N.I.; Sukhorukov, G.B. Protein Encapsulation via Porous CaCO3 Microparticles Templating. Biomacromolecules 2004, 5, 1962–1972. [Google Scholar] [CrossRef]
- Pechenkin, M.A.; Möhwald, H.; Volodkin, D.V. pH- and Salt-Mediated Response of Layer-by-Layer Assembled PSS/PAH Microcapsules: Fusion and Polymer Exchange. Soft Matter 2012, 8, 8659–8665. [Google Scholar] [CrossRef]
- Volodkin, D.V.; Petrov, A.I.; Prevot, M.; Sukhorukov, G.B. Matrix Polyelectrolyte Microcapsules: New System for Macromolecule Encapsulation. Langmuir 2004, 20, 3398–3406. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.P.R.; Cortez, C.; Angelatos, A.S.; Caruso, F. Layer-by-Layer Engineered Capsules and Their Applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 203–209. [Google Scholar] [CrossRef]
- Vikulina, A.S.; Anissimov, Y.G.; Singh, P.; Prokopović, V.Z.; Uhlig, K.; Jaeger, M.S.; Von Klitzing, R.; Duschl, C.; Volodkin, D. Temperature Effect on the Build-up of Exponentially Growing Polyelectrolyte Multilayers. An Exponential-to-Linear Transition Point. Phys. Chem. Chem. Phys. 2016, 18, 7866–7874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, J.M.; Caridade, S.G.; Costa, R.R.; Alves, N.M.; Groth, T.; Picart, C.; Reis, R.L.; Mano, J.F. pH Responsiveness of Multilayered Films and Membranes Made of Polysaccharides. Langmuir 2015, 31, 11318–11328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boddohi, S.; Killingsworth, C.E.; Kipper, M.J. Polyelectrolyte Multilayer Assembly as a Function of pH and Ionic Strength Using the Polysaccharides Chitosan and Heparin. Biomacromolecules 2008, 9, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.E.; Barrett, C.J. pH-Responsive Properties of Multilayered Poly(L-Lysine)/Hyaluronic Acid Surfaces. Biomacromolecules 2003, 4, 1773–1783. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Besseling, N.A.M. Formation of Polyelectrolyte Multilayers: Ionic Strengths and Growth Regimes. Soft Matter 2016, 12, 1032–1040. [Google Scholar] [CrossRef] [Green Version]
- She, Z.; Antipina, M.N.; Li, J.; Sukhorukov, G.B. Mechanism of Protein Release from Polyelectrolyte Multilayer Microcapsules. Biomacromolecules 2010, 11, 1241–1247. [Google Scholar] [CrossRef]
- Kumorek, M.; Minisy, I.M.; Krunclová, T.; Voršiláková, M.; Venclíková, K.; Chánová, E.M.; Janoušková, O.; Kubies, D. pH-Responsive and Antibacterial Properties of Self-Assembled Multilayer Films Based on Chitosan and Tannic Acid. Mater. Sci. Eng. C 2020, 109, 110493. [Google Scholar] [CrossRef]
- Westwood, M.; Gunning, A.P.; Parker, R. Temperature-Dependent Growth of Gelatin-Poly(Galacturonic Acid) Multilayer Films and Their Responsiveness to Temperature, pH, and NaCl. Macromolecules 2010, 43, 10582–10593. [Google Scholar] [CrossRef]
- Sukhorukov, G.B.; Antipov, A.A.; Voigt, A.; Donath, E.; Möhwald, H. pH-Controlled Macromolecule Encapsulation in and Release from Polyelectrolyte Multilayer Nanocapsules. Macromol. Rapid Commun. 2001, 22, 44–46. [Google Scholar] [CrossRef]
- Köhler, K.; Sukhorukov, G.B. Heat Treatment of Polyelectrolyte Multilayer Capsules: A Versatile Method for Encapsulation. Adv. Funct. Mater. 2007, 17, 2053–2061. [Google Scholar] [CrossRef]
- Vergaro, V.; Papadia, P.; Leporatti, S.; De Pascali, S.A.; Fanizzi, F.P.; Ciccarella, G. Synthesis of Biocompatible Polymeric Nano-Capsules Based on Calcium Carbonate: A Potential Cisplatin Delivery System. J. Inorg. Biochem. 2015, 153, 284–292. [Google Scholar] [CrossRef]
- Parakhonskiy, B.V.; Parak, W.J.; Volodkin, D.; Skirtach, A.G. Hybrids of Polymeric Capsules, Lipids, and Nanoparticles: Thermodynamics and Temperature Rise at the Nanoscale and Emerging Applications. Langmuir 2019, 35, 8574–8583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Koker, S.; De Cock, L.J.; Rivera-Gil, P.; Parak, W.J.; Auzély Velty, R.; Vervaet, C.; Remon, J.P.; Grooten, J.; De Geest, B.G. Polymeric Multilayer Capsules Delivering Biotherapeutics. Adv. Drug Deliv. Rev. 2011, 63, 748–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jewell, C.M.; Lynn, D.M. Multilayered Polyelectrolyte Assemblies as Platforms for the Delivery of DNA and Other Nucleic Acid-Based Therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 979–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mzyk, A.; Lackner, J.M.; Wilczek, P.; Lipińska, L.; Niemiec-Cyganek, A.; Samotus, A.; Morenc, M. Polyelectrolyte Multilayer Film Modification for Chemo-Mechano-Regulation of Endothelial Cell Response. RSC Adv. 2016, 6, 8811–8828. [Google Scholar] [CrossRef]
- Schmidt, S.; Madaboosi, N.; Uhlig, K.; Köhler, D.; Skirtach, A.; Duschl, C.; Möhwald, H.; Volodkin, D.V. Control of Cell Adhesion by Mechanical Reinforcement of Soft Polyelectrolyte Films with Nanoparticles. Langmuir 2012, 28, 7249–7257. [Google Scholar] [CrossRef]
- Tong, W.; She, S.; Xie, L.; Gao, C. High Efficient Loading and Controlled Release of Low-Molecular-Weight Drugs by Combination of Spontaneous Deposition and Heat-Induced Shrinkage of Multilayer Capsules. Soft Matter 2011, 7, 8258–8265. [Google Scholar] [CrossRef]
- Balabushevich, N.G.; Sholina, E.A.; Mikhalchik, E.V.; Filatova, L.Y.; Vikulina, A.S.; Volodkin, D. Self-Assembled Mucin-Containing Microcarriers via Hard Templating on CaCO3 Crystals. Micromachines 2018, 9, 307. [Google Scholar] [CrossRef] [Green Version]
- Vikulina, A.S.; Feoktistova, N.A.; Balabushevich, N.G.; Skirtach, A.G.; Volodkin, D. The Mechanism of Catalase Loading into Porous Vaterite CaCO3 Crystals by Co-Synthesis. Phys. Chem. Chem. Phys. 2018, 20, 8822–8831. [Google Scholar] [CrossRef] [Green Version]
- Balabushevich, N.G.; Kovalenko, E.A.; Le-Deygen, I.M.; Filatova, L.Y.; Volodkin, D.; Vikulina, A.S. Hybrid CaCO3-Mucin Crystals: Effective Approach for Loading and Controlled Release of Cationic Drugs. Mater. Des. 2019, 182, 108020. [Google Scholar] [CrossRef]
- Balabushevich, N.G.; Kovalenko, E.A.; Mikhalchik, E.V.; Filatova, L.Y.; Volodkin, D.; Vikulina, A.S. Mucin Adsorption on Vaterite CaCO 3 Microcrystals for the Prediction of Mucoadhesive Properties. J. Colloid Interface Sci. 2019, 545, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Binevski, P.V.; Balabushevich, N.G.; Uvarova, V.I.; Vikulina, A.S.; Volodkin, D. Bio-Friendly Encapsulation of Superoxide Dismutase into Vaterite CaCO3 Crystals. Enzyme Activity, Release Mechanism, and Perspectives for Ophthalmology. Colloids Surfaces B Biointerfaces 2019, 181, 437–449. [Google Scholar] [CrossRef]
- Feoktistova, N.A.; Vikulina, A.S.; Balabushevich, N.G.; Skirtach, A.G.; Volodkin, D. Bioactivity of Catalase Loaded into Vaterite CaCO3 Crystals via Adsorption and Co-Synthesis. Mater. Des. 2020, 185, 108223. [Google Scholar] [CrossRef]
- Prokopovic, V.Z.; Vikulina, A.S.; Sustr, D.; Shchukina, E.M.; Shchukin, D.G.; Volodkin, D.V. Binding Mechanism of the Model Charged Dye Carboxyfluorescein to Hyaluronan/Polylysine Multilayers. ACS Appl. Mater. Interfaces 2017, 9, 38908–38918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhlig, K.; Madaboosi, N.; Schmidt, S.; Jäger, M.S.; Rose, J.; Duschl, C.; Volodkin, D.V. 3D Localization and Diffusion of Proteins in Polyelectrolyte Multilayers. Soft Matter 2012, 8, 11786–11789. [Google Scholar] [CrossRef]
- Radt, B.; Smith, T.A.; Caruso, F. Optically Addressable Nanostructured Capsules. Adv. Mater. 2004, 16, 2184–2189. [Google Scholar] [CrossRef]
- Vikulina, A.S.; Skirtach, A.G.; Volodkin, D. Hybrids of Polymer Multilayers, Lipids, and Nanoparticles: Mimicking the Cellular Microenvironment. Langmuir 2019, 35, 8565–8573. [Google Scholar] [CrossRef] [Green Version]
- Volodkin, D.; von Klitzing, R.; Moehwald, H. Polyelectrolyte Multilayers: Towards Single Cell Studies. Polymers 2014, 6, 1502–1527. [Google Scholar] [CrossRef]
- Novoselova, M.V.; Loh, H.M.; Trushina, D.B.; Ketkar, A.; Abakumova, T.O.; Zatsepin, T.S.; Kakran, M.; Brzozowska, A.M.; Lau, H.H.; Gorin, D.A.; et al. Biodegradable Polymeric Multilayer Capsules for Therapy of Lung Cancer. ACS Appl. Mater. Interfaces 2020, 12, 5610–5623. [Google Scholar] [CrossRef] [PubMed]
- Leopold, L.F.; Marişca, O.; Oprea, I.; Rugină, D.; Suciu, M.; Nistor, M.; Tofană, M.; Leopold, N.; Coman, C. Cellular Internalization of Beta-Carotene Loaded Polyelectrolyte Multilayer Capsules by Raman Mapping. Molecules 2020, 25, 1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gribova, V.; Auzely-Velty, R.; Picart, C. Polyelectrolyte Multilayer Assemblies on Materials Surfaces: From Cell Adhesion to Tissue Engineering. Chem. Mater. 2012, 24, 854–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, S.; Feilen, P.J.; Slotty, V.; Kampfner, D.; Preuss, S.; Berger, S.; Beyer, J.; Pommersheim, R. Multilayer Capsules: A Promising Microencapsulation System for Transplantation of Pancreatic Islets. Biomaterials 2001, 22, 1961–1970. [Google Scholar] [CrossRef]
- Mano, J.F. Designing Biomaterials for Tissue Engineering Based on the Deconstruction of the Native Cellular Environment. Mater. Lett. 2015, 141, 198–202. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Xiong, X.; Zou, Q.; Ouyang, P.; Krastev, R. Controlled Heparinase-Catalyzed Degradation of Polyelectrolyte Multilayer Capsules with Heparin as Responsive Layer. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Lomova, M.V.; Brichkina, A.I.; Kiryukhin, M.V.; Vasina, E.N.; Pavlov, A.M.; Gorin, D.A.; Sukhorukov, G.B.; Antipina, M.N. Multilayer Capsules of Bovine Serum Albumin and Tannic Acid for Controlled Release by Enzymatic Degradation. ACS Appl. Mater. Interfaces 2015, 7, 11732–11740. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Tripathy, J.; Raichur, A.M. Dual Enzyme Responsive Microcapsules Simulating an “oR” Logic Gate for Biologically Triggered Drug Delivery Applications. Chem. Commun. 2013, 49, 5390–5392. [Google Scholar] [CrossRef] [Green Version]
- Antipov, A.A.; Sukhorukov, G.B. Polyelectrolyte Multilayer Capsules as Vehicles with Tunable Permeability. Adv. Colloid Interface Sci. 2004, 111, 49–61. [Google Scholar] [CrossRef]
- Diamanti, E.; Muzzio, N.; Gregurec, D.; Irigoyen, J.; Pasquale, M.; Azzaroni, O.; Brinkmann, M.; Moya, S.E. Impact of Thermal Annealing on Wettability and Antifouling Characteristics of Alginate Poly-l-Lysine Polyelectrolyte Multilayer Films. Colloids Surfaces B Biointerfaces 2016, 145, 328–337. [Google Scholar] [CrossRef]
- Trushina, D.B.; Bukreeva, T.V.; Borodina, T.N.; Belova, D.D.; Belyakov, S.; Antipina, M.N. Heat-Driven Size Reduction of Biodegradable Polyelectrolyte Multilayer Hollow Capsules Assembled on CaCO3 Template. Colloids Surfaces B Biointerfaces 2018, 170, 312–321. [Google Scholar] [CrossRef]
- Antipov, A.A.; Sukhorukov, G.B.; Donath, E.; Möhwald, H. Sustained Release Properties of Polyelectrolyte Multilayer Capsules. J. Phys. Chem. B 2001, 105, 2281–2284. [Google Scholar] [CrossRef]
- Leporatti, S.; Gao, C.; Voigt, A.; Donath, E.; Mohwald, H. Shrinking of Ultrathin Polyelectrolyte Multilayer Capsules upon Annealing: A Confocal Laser Scanning Microscopy and Scanning Force Microscopy Study. Eur. Phys. J. E 2001, 5, 13–20. [Google Scholar] [CrossRef]
- Van der Meeren, L.; Li, J.; Konrad, M.; Skirtach, A.G.; Volodkin, D.; Parakhonskiy, B.V. Temperature Window for Encapsulation of an Enzyme into Thermally Shrunk, CaCO3 Templated Polyelectrolyte Multilayer Capsules. Macromol. Biosci. 2020, 20, 2000081. [Google Scholar] [CrossRef] [PubMed]
- Di Vona, M.L. Annealing of Polymer Membranes. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 80–81. [Google Scholar]
- Mehnath, S.; Arjama, M.; Rajan, M.; Annamalai, G.; Jeyaraj, M. Co-Encapsulation of Dual Drug Loaded in MLNPs: Implication on Sustained Drug Release and Effectively Inducing Apoptosis in Oral Carcinoma Cells. Biomed. Pharmacother. 2018, 104, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Jensen, A.W.; Mohanty, D.K.; Erndt, J.; Bruening, M.L. Controlling the Permeability of Multilayered Polyelectrolyte Films through Derivatization, Cross-Linking, and Hydrolysis. Langmuir 2001, 17, 931–937. [Google Scholar] [CrossRef]
- Ho, C.S.; Kim, J.W.; Weitz, D.A. Microfluidic Fabrication of Monodisperse Biocompatible and Biodegradable Polymersomes with Controlled Permeability. J. Am. Chem. Soc. 2008, 130, 9543–9549. [Google Scholar] [CrossRef]
- Kaoui, B.; Lauricella, M.; Pontrelli, G. Mechanistic Modelling of Drug Release from Multi-Layer Capsules. Comput. Biol. Med. 2018, 93, 149–157. [Google Scholar] [CrossRef]
- Behzadi, S.; Rosenauer, C.; Kappl, M.; Mohr, K.; Landfester, K.; Crespy, D. Osmotic Pressure-Dependent Release Profiles of Payloads from Nanocontainers by Co-Encapsulation of Simple Salts. Nanoscale 2016, 8, 12998–13005. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Donath, E.; Moya, S.; Dudnik, V.; Möhwald, H. Elasticity of Hollow Polyelectrolyte Capsules Prepared by the Layer-by-Layer Technique. Eur. Phys. J. E 2001, 5, 21–27. [Google Scholar] [CrossRef]
- De Geest, B.G.; McShane, M.J.; Demeester, J.; De Smedt, S.C.; Hennink, W.E. Microcapsules Ejecting Nanosized Species into the Environment. J. Am. Chem. Soc. 2008, 130, 14480–14482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, E.J.; Pontrelli, G. Drug Delivery from Microcapsules: How Can We Estimate the Release Time? Math. Biosci. 2019, 315, 108216. [Google Scholar] [CrossRef] [Green Version]
- She, S.; Xu, C.; Yin, X.; Tong, W.; Gao, C. Shape Deformation and Recovery of Multilayer Microcapsules after Being Squeezed through a Microchannel. Langmuir 2012, 28, 5010–5016. [Google Scholar] [CrossRef] [PubMed]
- Facca, S.; Cortez, C.; Mendoza-Palomares, C.; Messadeq, N.; Dierich, A.; Johnston, A.P.R.; Mainard, D.; Voegel, J.C.; Caruso, F.; Benkirane-Jessel, N. Active Multilayered Capsules for in Vivo Bone Formation. Proc. Natl. Acad. Sci. USA 2010, 107, 3406–3411. [Google Scholar] [CrossRef] [Green Version]
- Del Mercato, L.L.; Passione, L.G.; Izzo, D.; Rinaldi, R.; Sannino, A.; Gervaso, F. Design and Characterization of Microcapsules-Integrated Collagen Matrixes as Multifunctional Three-Dimensional Scaffolds for Soft Tissue Engineering. J. Mech. Behav. Biomed. Mater. 2016, 62, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, A.; Vikulina, A.S.; Volodkin, D. Porous Alginate Scaffolds Assembled Using Vaterite CaCO3 Crystals. Micromachines 2019, 10, 357. [Google Scholar] [CrossRef] [Green Version]
- Sergeeva, A.; Feoktistova, N.; Prokopovic, V.; Gorin, D.; Volodkin, D. Design of Porous Alginate Hydrogels by Sacrificial CaCO3 Templates: Pore Formation Mechanism. Adv. Mater. Interfaces 2015, 2, 1500386. [Google Scholar] [CrossRef]
- Silva, J.M.; Georgi, N.; Costa, R.; Sher, P.; Reis, R.L.; van Blitterswijk, C.A.; Karperien, M.; Mano, J.F. Nanostructured 3D Constructs Based on Chitosan and Chondroitin Sulphate Multilayers for Cartilage Tissue Engineering. PLoS ONE 2013, 8, e55451. [Google Scholar] [CrossRef] [Green Version]
- Sher, P.; Custódio, C.A.; Mano, J.F. Layer-by-Layer Technique for Producing Porous Nanostructured 3D Constructs Using Moldable Freeform Assembly of Spherical Templates. Small 2010, 6, 2644–2648. [Google Scholar] [CrossRef]
- Minnikanti, S.; Gangopadhyay, A.; Reyes, D.R. Polyelectrolyte Multilayers in Microfluidic Systems for Biological Applications. Polymers 2014, 6, 2100–2115. [Google Scholar] [CrossRef]
- Castleberry, S.A.; Li, W.; Deng, D.; Mayner, S.; Hammond, P.T. Capillary Flow Layer-by-Layer: A Microfluidic Platform for the High-Throughput Assembly and Screening of Nanolayered Film Libraries. ACS Nano 2014, 8, 6580–6589. [Google Scholar] [CrossRef]
- Vikulina, A.S.; Aleed, S.T.; Paulraj, T.; Vladimirov, Y.A.; Duschl, C.; Von Klitzing, R.; Volodkin, D. Temperature-Induced Molecular Transport through Polymer Multilayers Coated with PNIPAM Microgels. Phys. Chem. Chem. Phys. 2015, 17, 12771–12777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prokopović, V.Z.; Vikulina, A.S.; Sustr, D.; Duschl, C.; Volodkin, D. Biodegradation-Resistant Multilayers Coated with Gold Nanoparticles. Toward a Tailor-Made Artificial Extracellular Matrix. ACS Appl. Mater. Interfaces 2016, 8, 24345–24349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vikulina, A.; Voronin, D.; Fakhrullin, R.; Vinokurov, V.; Volodkin, D. Naturally Derived Nano- And Micro-Drug Delivery Vehicles: Halloysite, Vaterite and Nanocellulose. New J. Chem. 2020, 44, 5638–5655. [Google Scholar] [CrossRef] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kastania, G.; Campbell, J.; Mitford, J.; Volodkin, D. Polyelectrolyte Multilayer Capsule (PEMC)-Based Scaffolds for Tissue Engineering. Micromachines 2020, 11, 797. https://doi.org/10.3390/mi11090797
Kastania G, Campbell J, Mitford J, Volodkin D. Polyelectrolyte Multilayer Capsule (PEMC)-Based Scaffolds for Tissue Engineering. Micromachines. 2020; 11(9):797. https://doi.org/10.3390/mi11090797
Chicago/Turabian StyleKastania, Georgia, Jack Campbell, Jacob Mitford, and Dmitry Volodkin. 2020. "Polyelectrolyte Multilayer Capsule (PEMC)-Based Scaffolds for Tissue Engineering" Micromachines 11, no. 9: 797. https://doi.org/10.3390/mi11090797




