Low-Temperature Flexible Micro Hydrogen Sensor Embedded in a Proton Battery for Real-Time Microscopic Diagnosis
Abstract
:1. Introduction
2. Sensing Principle of Flexible Micro Hydrogen Sensor
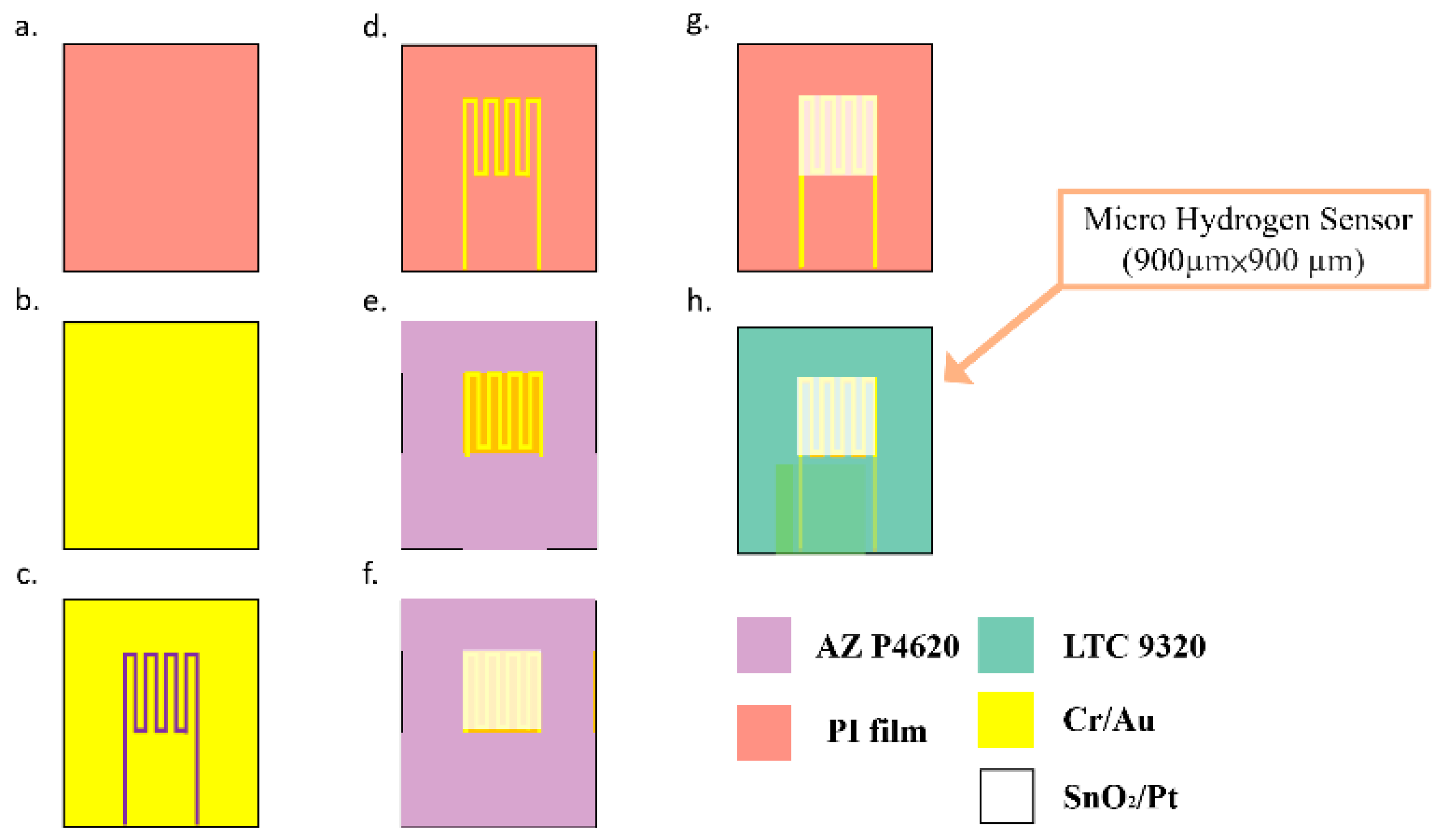
3. Process Development of Flexible Micro Hydrogen Sensor
- The substrate of PI film is cleaned with organic solutions acetone and methanol, the residual methanol is removed by deionized (DI) water and the surface dust and residual oil and fat are removed, so as to enhance the adhesive ability of thin film metal.
- The Cr is evaporated by E-beam evaporator (Junsun, Taipei, Taiwan) as the adhesion layer of Au and PI film, the adhesion of Au and PI film is enhanced, and the deposition of 1000 Å thick Au is completed at a deposition rate of 0.1 Å/s.
- The AZ P4620 (positive photoresist) is spin coated; the electrode pattern of the micro hydrogen sensor is defined by exposure and development.
- The Au is etched by Au etching solution (Type-TFA, AppliChem Technology, Miaoli, Taiwan), and the Cr is etched by Cr etching solution (Cr-7T, AppliChem Technology, Miaoli, Taiwan); the photoresist as etching mask is removed by Remove 1165.
- The AZ P4620 is spin coated again as the mask for evaporation, and the pattern of micro hydrogen sensor is defined by exposure, and then the required pattern is developed by a developer.
- The SnO2 is evaporated by E-beam evaporator on the surface of the defined pattern as a gas sensing layer, and the Pt is evaporated as a catalyst layer.
- The sample is put in the photoresist remover (Remove 1165, MicroChemicals, Hsinchu, Taiwan) at 80 °C, kept still for about 20 min, then the photoresist mask is lifted off and cleaned with acetone and methanol (Minyung, Taoyuan, Taiwan).
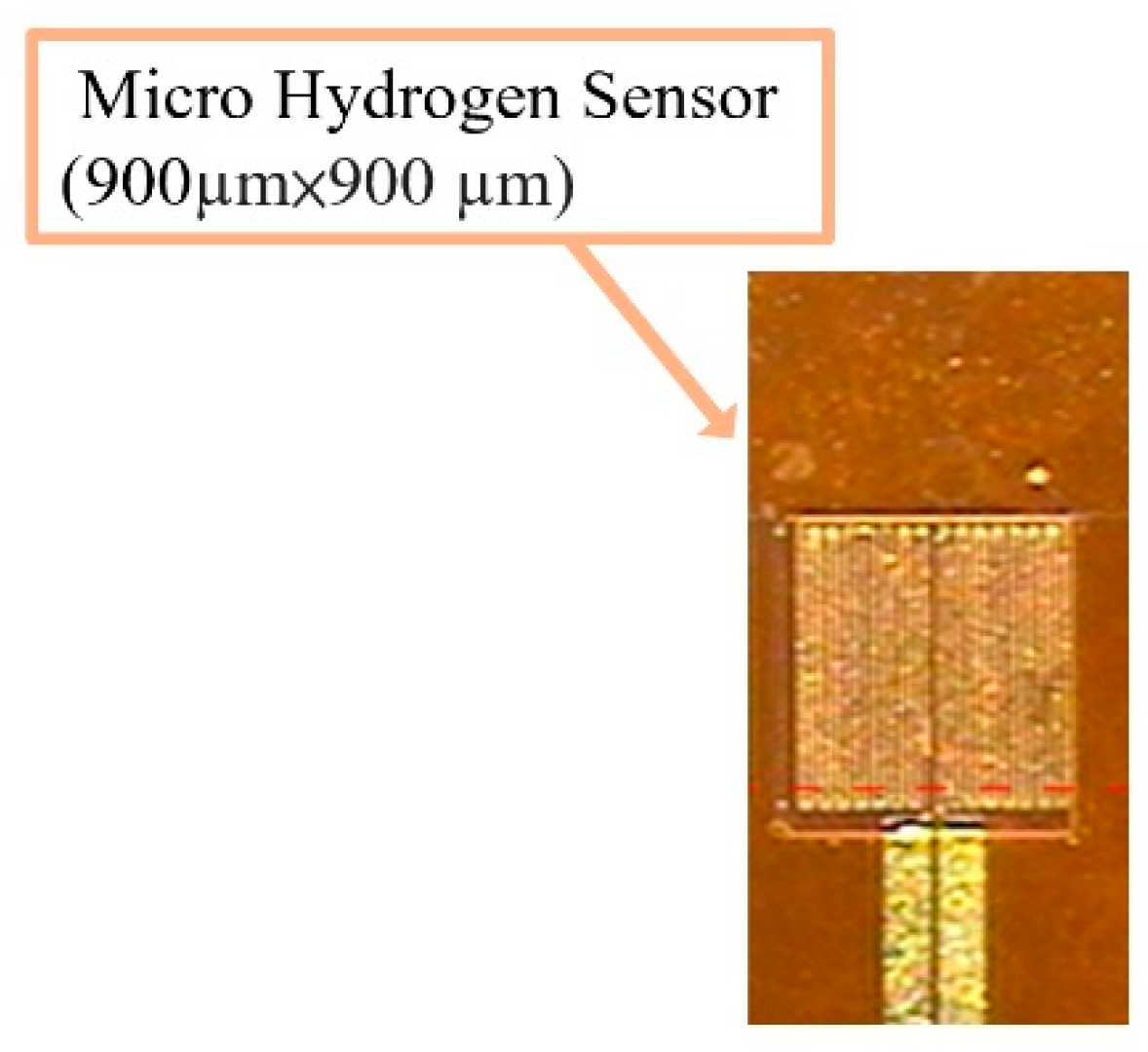
- Finally, LTC 9320 (negative photoresist)( MicroChemicals, Hsinchu, Taiwan) is spin coated on the flexible micro hydrogen sensor to complete the protection layer. The optical microphotograph is shown in Figure 2.
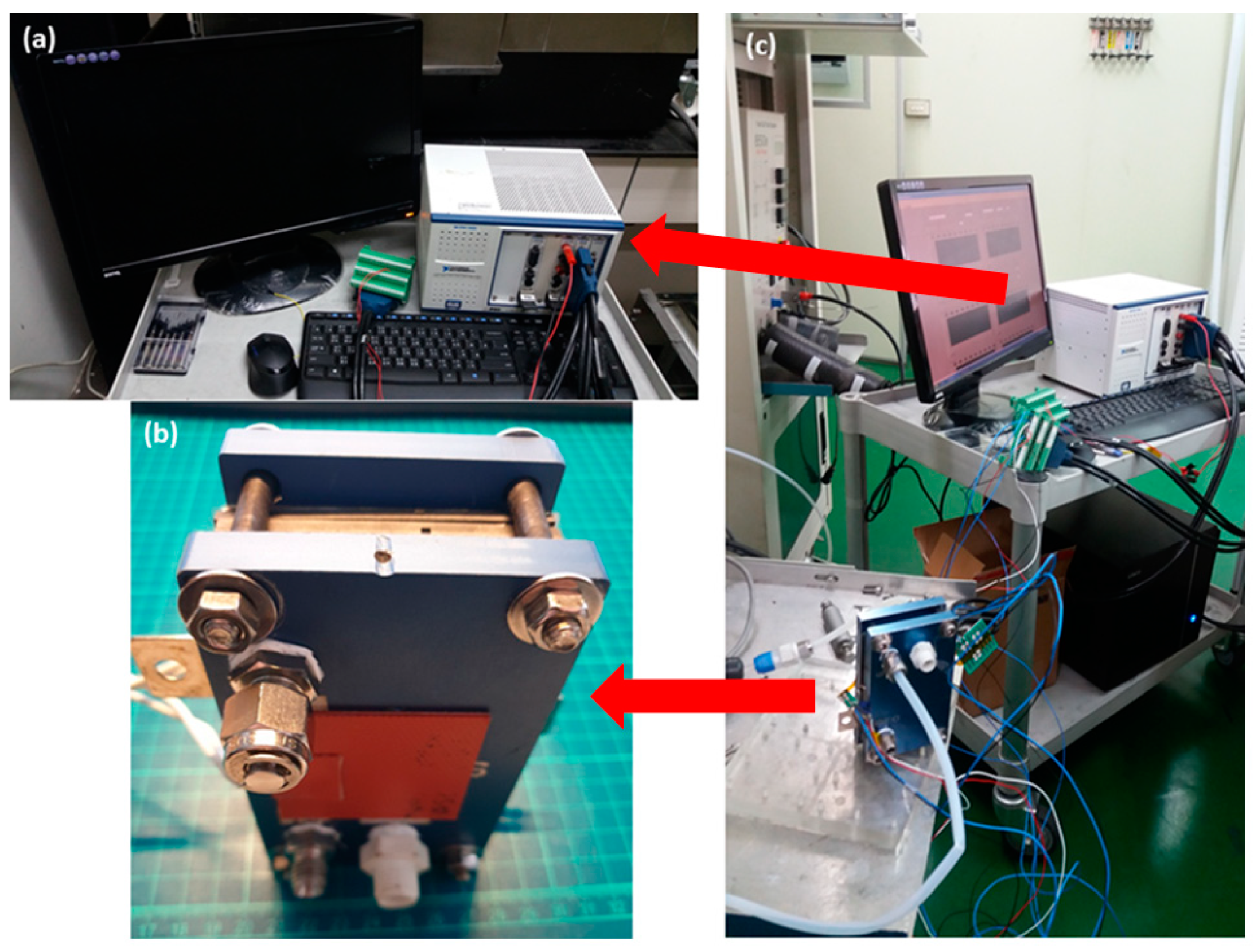
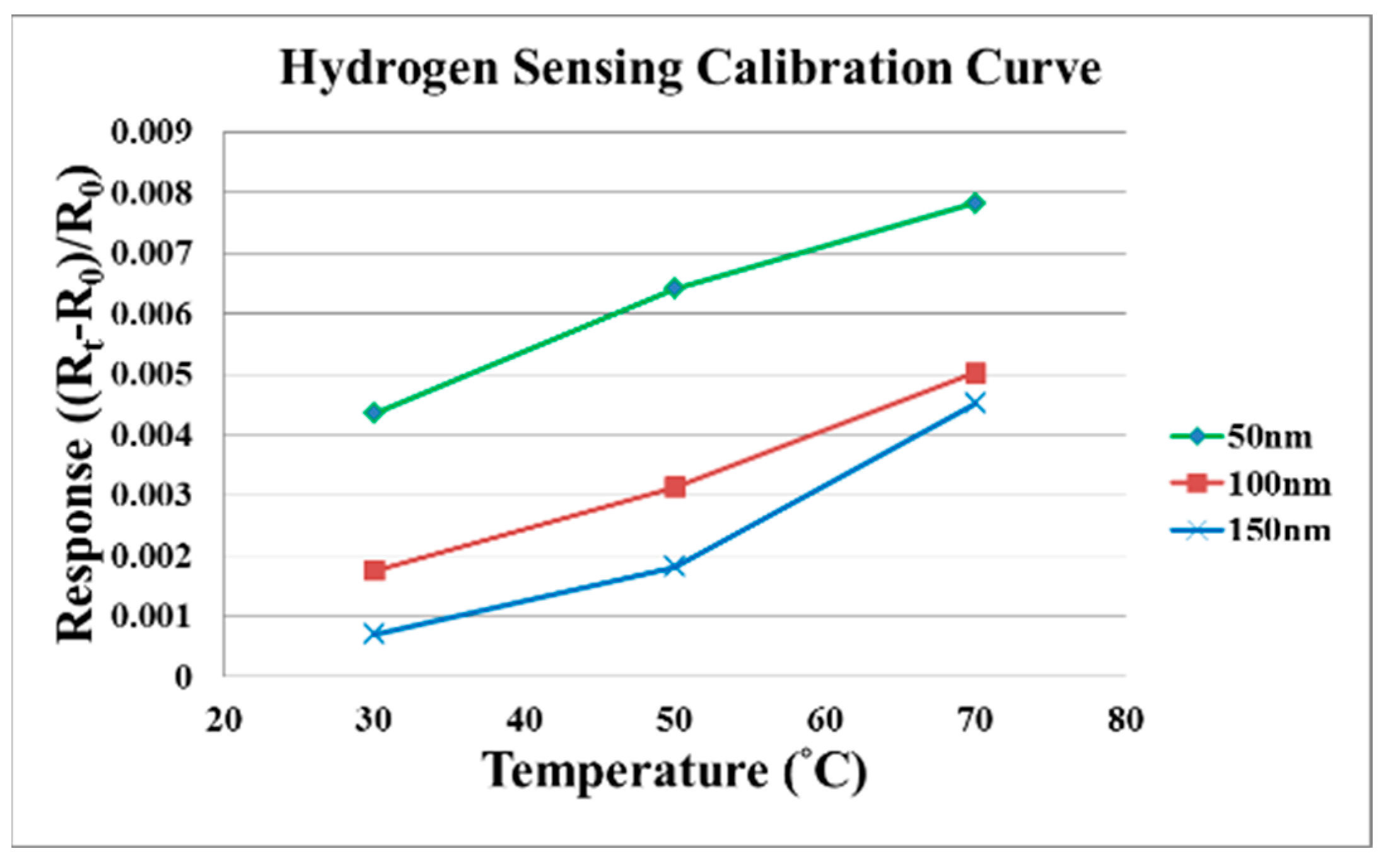
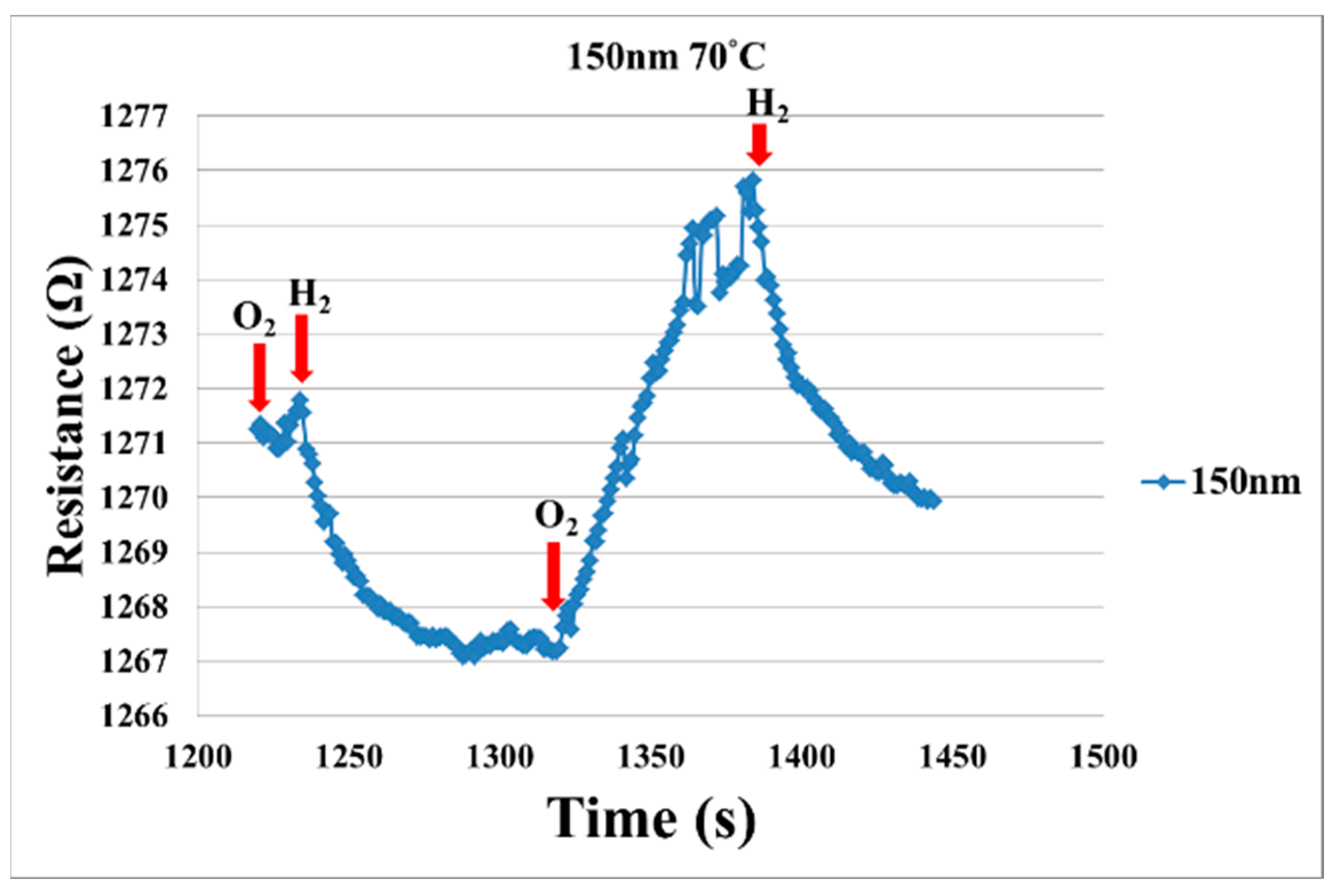
4. Test and Correction of Flexible Micro Hydrogen Sensor
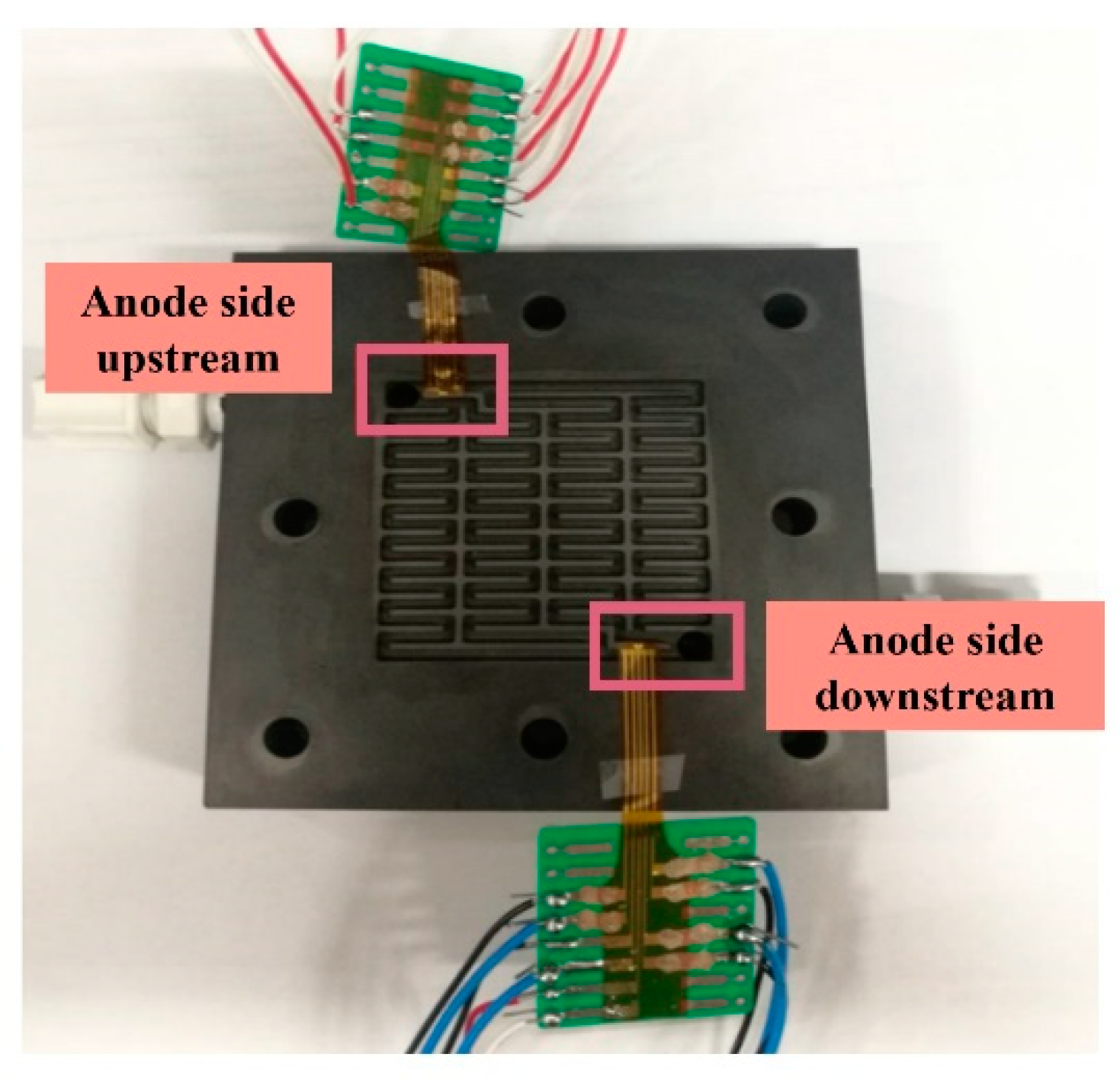
5. Hydrogen Sensing Inside Proton Battery
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heidari, S.; Mohammadi, S.S.; Oberoi, A.S.; Andrews, J. Technical feasibility of a proton battery with an activated carbon electrode. Int. J. Hydrog. Energy 2018, 43, 6197–6209. [Google Scholar] [CrossRef]
- Folonari, C.; Iemmi, G.; Manfredi, F.; Rolle, A. Metal hydride fuel cells: A feasibility study and perspectives for vehicular applications. J. Less Common Met. 1980, 74, 371–378. [Google Scholar] [CrossRef]
- Condon, J.B.; Schober, T. Proton conductors and metal hydrides. Solid State Ion. 1995, 77, 299–304. [Google Scholar] [CrossRef]
- Selembo, P.A.; Merrill, M.D.; Logan, B.E. Hydrogen production with nickel powder cathode catalysts in microbial electrolysis cells. Int. J. Hydrog. Energy 2010, 35, 428–437. [Google Scholar] [CrossRef]
- Jurewicz, K.; Frackowiak, E.; Béguin, F. Enhancement of reversible hydrogen capacity into activated carbon through water electrolysis. Electrochem. Solid State Lett. 2001, 4, A27–A29. [Google Scholar] [CrossRef]
- Jurewicz, K.; Frackowiak, E.; Béguin, F. Electrochemical storage of hydrogen in activated carbons. Fuel Process. Technol. 2002, 77–78, 415–421. [Google Scholar] [CrossRef]
- Jurewicz, K.; Frackowiak, E.; Béguin, F. Towards the mechanism of electrochemical hydrogen storage in nanostructured carbon materials. Appl. Phys. A 2004, 78, 981–987. [Google Scholar] [CrossRef]
- Babel, K.; Jurewicz, K. KOH activated lignin based nanostructured carbon exhibiting high hydrogen electrosorption. Carbon 2008, 46, 1948–1956. [Google Scholar] [CrossRef]
- Bosch, M.; Zhou, H.C. Porous carbons for hydrogen storage. In Nanostructured Materials for Next-Generation Energy Storage and Conversion; Springer: Berlin/Heidelberg, Germany, 2016; pp. 171–202. [Google Scholar]
- Paul, B.; Andrews, J. PEM unitised reversible/regenerative hydrogen fuel cell systems: State of the art and technical challenges. Renew. Sustain. Energy Rev. 2017, 79, 585–599. [Google Scholar] [CrossRef]
- Doddathimmaiah, A.; Andrews, J. Theory, modelling and performance measurement of unitised regenerative fuel cells. Int. J. Hydrog. Energy 2009, 34, 8157–8170. [Google Scholar] [CrossRef]
- Wang, Y.; Leung, D.Y.C.; Xuana, J.; Wang, H. A review on unitized regenerative fuel cell technologies, part A: Unitized regenerative proton exchange membrane fuel cells. Renew. Sustain. Energy Rev. 2016, 65, 961–977. [Google Scholar] [CrossRef]
- Chen, S.C.; Chao, H.; Fan, L.; Lv, M.; Tian, C.; Shu, G.; Shu, K. Tuning the electrochemical behavior of CoxMn3−x sulfides by varying different Co/Mn ratios in supercapacitor. J. Mater. Sci. 2017, 52, 6687–6696. [Google Scholar] [CrossRef]
- Chen, H.; Fan, M.; Chao, L.; Tian, G.; Lv, C.; Da, C.; Shu, K.; Jiang, J. One-pot synthesis of hollow NiSe–CoSe nanoparticles with improved performance for hybrid supercapacitors. J. Power Sources 2016, 329, 314–322. [Google Scholar] [CrossRef]
- Bamsaoud, S.F.; Rane, S.B.; Karekar, R.N.; Aiyer, R.C. SnO2 film with bimodal distribution of nano-particles for low concentration hydrogen sensor: Effect of firing temperature on sensing properties. Mater. Chem. Phys. 2012, 133, 681–687. [Google Scholar] [CrossRef]
- Lőrinczi, A.; Cosma, E.F.; Socol, G.; Mihăilescu, A.; Matei, E.; Sava, F.; Ştefan, M. SnSe2-Zn-porphyrin Nanocomposite thin films for threshold methane concentration detection at room temperature. Chemosensors 2020, 8, 134. [Google Scholar] [CrossRef]
- Fahad, H.M.; Shiraki, H.; Amani, M.; Zhang, C.; Hebbar, V.S.; Gao, W.; Ota, H.; Hettick, M.; Kiriya, D.; Chen, Y.Z.; et al. Room temperature multiplexed gas sensing using chemical-sensitive 3.5-nm-thin silicon transistors. Sci. Adv. 2017, 3, e1602557–e1602564. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Laboratory Sample | Specific Surface Area |
|---|---|
| Original activated carbon of Company A | 618.2029 m2/g |
| KOH concentration ratio 3:1 of Company A | 1326.9233 m2/g |
| KOH concentration ratio 4.5:1 of Company A | 1285.9408 m2/g |
| Original activated carbon of Company B | 822.8134 m2/g |
| Company B after cleaning | 846.6372 m2/g |
| KOH concentration ratio 4.5:1 of Company B | 1025.9340 m2/g |
| KOH concentration ratio 7:1 of Company B | 1173.1066 m2/g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-Y.; Chen, C.-H.; Yang, C.-Y.; Cheong, J.-S.; Chien, Y.-H.; Lin, Y.-C. Low-Temperature Flexible Micro Hydrogen Sensor Embedded in a Proton Battery for Real-Time Microscopic Diagnosis. Micromachines 2021, 12, 1215. https://doi.org/10.3390/mi12101215
Lee C-Y, Chen C-H, Yang C-Y, Cheong J-S, Chien Y-H, Lin Y-C. Low-Temperature Flexible Micro Hydrogen Sensor Embedded in a Proton Battery for Real-Time Microscopic Diagnosis. Micromachines. 2021; 12(10):1215. https://doi.org/10.3390/mi12101215
Chicago/Turabian StyleLee, Chi-Yuan, Chia-Hung Chen, Chin-Yuan Yang, John-Shong Cheong, Yun-Hsiu Chien, and Yi-Chuan Lin. 2021. "Low-Temperature Flexible Micro Hydrogen Sensor Embedded in a Proton Battery for Real-Time Microscopic Diagnosis" Micromachines 12, no. 10: 1215. https://doi.org/10.3390/mi12101215
APA StyleLee, C.-Y., Chen, C.-H., Yang, C.-Y., Cheong, J.-S., Chien, Y.-H., & Lin, Y.-C. (2021). Low-Temperature Flexible Micro Hydrogen Sensor Embedded in a Proton Battery for Real-Time Microscopic Diagnosis. Micromachines, 12(10), 1215. https://doi.org/10.3390/mi12101215






