Synthesis of Carbon-Supported PdIrNi Catalysts and Their Performance towards Ethanol Electrooxidation
Abstract
:1. Introduction
2. Materials and Methods
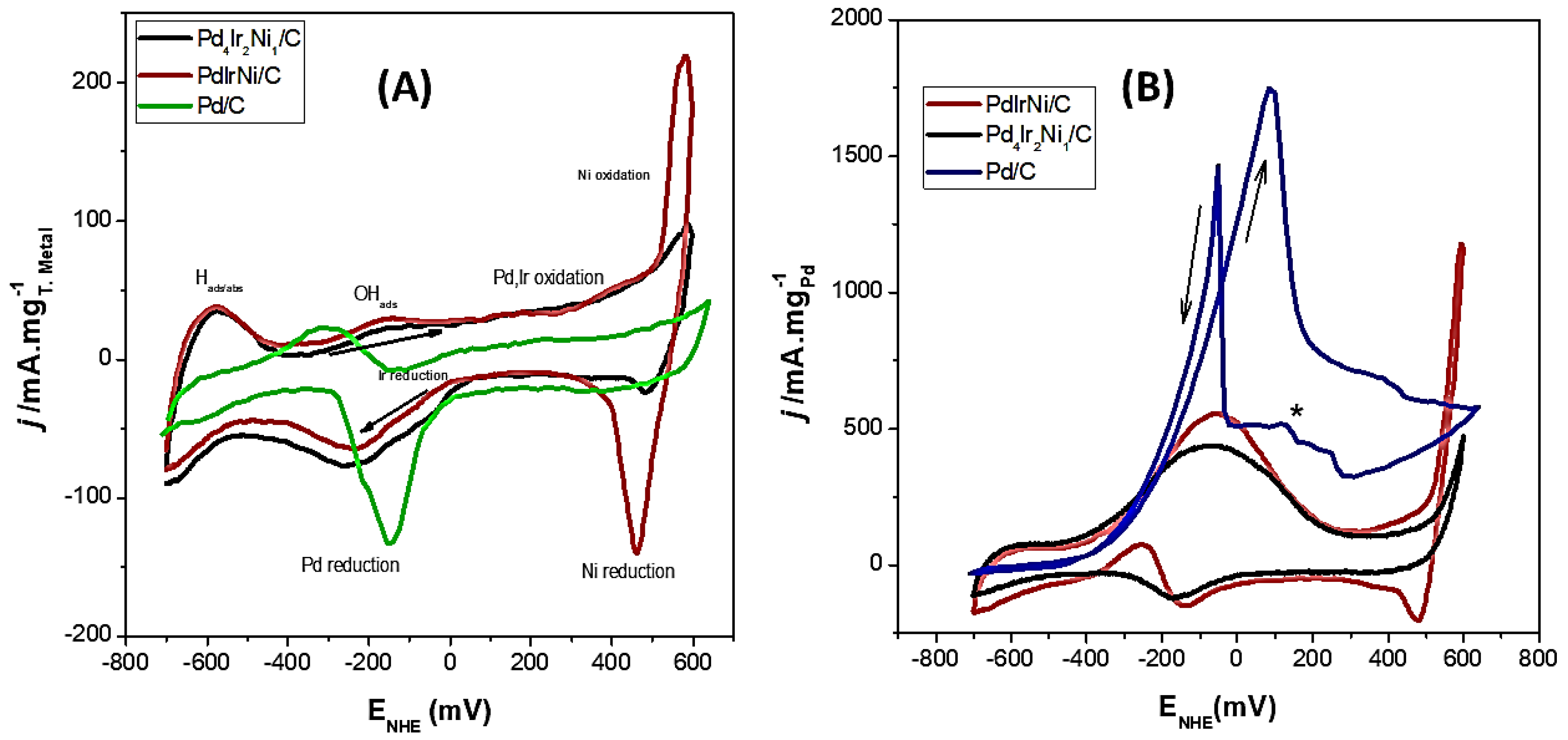
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Monyoncho, E.A.; Steinmann, S.N.; Michel, C.; Baranova, E.A.; Woo, T.K.; Sautet, P. Ethanol Electro-oxidation on Palladium Revisited Using Polarization Modulation Infrared Reflection Absorption Spectroscopy (PM-IRRAS) and Density Functional Theory (DFT): Why Is It Difficult To Break the C–C Bond? ACS Catal. 2016, 6, 4894–4906. [Google Scholar] [CrossRef]
- Shen, S.Y.; Guo, Y.G.; Wei, G.H.; Luo, L.X.; Li, F.; Zhang, J.L. A perspective on the promoting effect of Ir and Au on Pd toward the ethanol oxidation reaction in alkaline media. Front. Energy 2018, 12, 501–508. [Google Scholar] [CrossRef]
- Mohamed, I.M.A.; Khalil, K.A.; Mousa, H.M.; Barakat, N.A.M. Ni/Pd-Decorated Carbon NFs as an Efficient Electrocatalyst for Methanol Oxidation in Alkaline Medium. J. Electron. Mater. 2017, 46, 265–273. [Google Scholar] [CrossRef]
- Shen, S.; Zhao, T.S.; Xu, J.; Li, Y. High performance of a carbon supported ternary PdIrNi catalyst for ethanol electro-oxidation in anion-exchange membrane direct ethanol fuel cells. Energy Environ. Sci. 2011, 4, 1428. [Google Scholar] [CrossRef]
- Moraes, L.P.R.; Matos, B.R.; Radtke, C.; Santiago, E.I.; Fonseca, F.C.; Amico, S.C.; Malfatti, C.F. Synthesis and performance of palladium-based electrocatalysts in alkaline direct ethanol fuel cell. Int. J. Hydrogen Energy 2016, 41, 6457–6468. [Google Scholar] [CrossRef]
- Neto, A.O.; Da Silva, S.G.; Buzzo, G.S.; De Souza, R.F.B.; Assumpção, M.H.M.T.; Spinacé, E.V.; Silva, J.C.M. Ethanol electrooxidation on PdIr/C electrocatalysts in alkaline media: Electrochemical and fuel cell studies. Ionics 2015, 21, 487–495. [Google Scholar] [CrossRef]
- Cronin, J.; Anandarajah, G.; Dessens, O. Climate change impacts on the energy system: A review of trends and gaps. Clim. Chang. 2018, 151, 79–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamarudin, M.Z.F.; Kamarudin, S.K.; Masdar, M.S.; Daud, W.R.W. Review: Direct ethanol fuel cells. Int. J. Hydrogen Energy 2013, 38, 9438–9453. [Google Scholar] [CrossRef]
- Brouzgou, A.; Podias, A.; Tsiakaras, P. PEMFCs and AEMFCs directly fed with ethanol: A current status comparative review. J. Appl. Electrochem. 2013, 43, 119–136. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Y.; Wei, X. Catalytic performances of PdNi/MWCNT for electrooxidations of methanol and ethanol in alkaline media. Int. J. Hydrogen Energy 2015, 40, 1154–1162. [Google Scholar] [CrossRef]
- Calvillo, L.; Celorrio, V.; Moliner, R.; Garcia, A.B.; Caméan, I.; Lazaro, M.J. Comparative study of Pt catalysts supported on different high conductive carbon materials for methanol and ethanol oxidation. Electrochim. Acta 2013, 102, 19–27. [Google Scholar] [CrossRef]
- Chen, L.Y.; Chen, N.; Hou, Y.; Wang, Z.C.; Lv, S.H.; Fujita, T.; Jiang, J.H.; Hirata, A.; Chen, M.W. Geometrically Controlled Nanoporous PdAu Bimetallic Catalysts with Tunable Pd/Au Ratio for Direct Ethanol Fuel Cells. ACS Catal. 2013, 3, 1220–1230. [Google Scholar] [CrossRef]
- Ma, L.; Chu, D.; Chen, R. Comparison of ethanol electro-oxidation on Pt/C and Pd/C catalysts in alkaline media. Int. J. Hydrogen Energy 2012, 37, 11185–11194. [Google Scholar] [CrossRef]
- O’Hayre, R.; Cha, S.W.; Colella, W.; Prinz, F.B. Chapter 14: Environmental Impact of Fuel Cells. In Fuel Cell Fundamentals; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 481–516. [Google Scholar] [CrossRef]
- Antolini, E.; Gonzalez, E.R. Alkaline direct alcohol fuel cells. J. Power Sources 2010, 195, 3431–3450. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, S.; Pastor, E.; Lázaro, M.J. Electrochemical behavior of the carbon black Vulcan XC-72R: Influence of the surface chemistry. Int. J. Hydrogen Energy 2018, 43, 7911–7922. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, E.L.; Ortega Vega, M.R.; Correa, P.D.S.; Cuña, A.; Tancredi, N.; Malfatti, C.D.F. Influence of activated carbon porous texture on catalyst activity for ethanol electro-oxidation. Int. J. Hydrogen Energy 2014, 39, 14760–14767. [Google Scholar] [CrossRef]
- Elsheikh, A.; Martins, V.L.V.L.; McGregor, J. Influence of physicochemical characteristics of carbon supports on Pd ethanol oxidation catalysts. Energy Procedia 2018, 151, 79–83. [Google Scholar] [CrossRef]
- Antolini, E. Carbon supports for low-temperature fuel cell catalysts. Appl. Catal. B Environ. 2009, 88, 1–24. [Google Scholar] [CrossRef]
- Carmo, M.; Brandalise, M.; Neto, A.O.; Spinacé, E.V.; Taylor, A.D.; Linardi, M.; Rocha Poço, J.G.; Rocha Poo, J.G. Enhanced activity observed for sulfuric acid and chlorosulfuric acid functionalized carbon black as PtRu and PtSn electrocatalyst support for DMFC and DEFC applications. Int. J. Hydrogen Energy 2011, 36, 14659–14667. [Google Scholar] [CrossRef]
- Jurzinsky, T.; Gomez-Villa, E.D.; Kübler, M.; Bruns, M.; Elsässer, P.; Melke, J.; Scheiba, F.; Cremers, C. Functionalization of multi-walled carbon nanotubes with indazole. Electrochim. Acta 2019, 298, 884–892. [Google Scholar] [CrossRef]
- Yang, C.; Hu, X.; Wang, D.; Dai, C.; Zhang, L.; Jin, H.; Agathopoulos, S. Ultrasonically treated multi-walled carbon nanotubes (MWCNTs) as PtRu catalyst supports for methanol electrooxidation. J. Power Sources 2006, 160, 187–193. [Google Scholar] [CrossRef]
- Kanninen, P.; Borghei, M.; Hakanpää, J.; Kauppinen, E.I.; Ruiz, V.; Kallio, T. Temperature dependent performance and catalyst layer properties of PtRu supported on modified few-walled carbon nanotubes for the alkaline direct ethanol fuel cell. J. Electroanal. Chem. 2017, 793, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.J.; Fang, B.; Li, H.; Bi, X.T.; Wang, H. Progress in modified carbon support materials for Pt and Pt-alloy cathode catalysts in polymer electrolyte membrane fuel cells. Prog. Mater. Sci. 2016, 82, 445–498. [Google Scholar] [CrossRef]
- Sheikh, A.M.; Correa, P.S.; Leal da Silva, E.; Savaris, I.D.; Amico, S.C.; Malfatti, C.F.; Silva, E.L.; Savaris, I.D.; Amico, S.C.; Malfatti, C.F. Energy conversion using pd-based catalysts in direct ethanol fuel cell. Renew. Energy Power Qual. J. 2013, 1, 342–345. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, A.M.; Silva, E.L.; Correa, P.S.; Menezes, T.L. The Effect of Adding Ni and Sn to Pd Catalysts on the Direct Ethanol Fuel Cell Performance. In Proceedings of the International Conference on Energy, Environment and Materials Engineering (EEME 2014), Shenzhen, China, 22–23 February 2014. [Google Scholar]
- Sheikh, A.M.; Silva, E.L.; Moares, L.; Antonini, L.M.; Abellah, M.Y.; Malfatti, C.F. Pd-based Catalysts for Ethanol Oxidation in Alkaline Electrolyte. Am. J. Min. Metall. 2014, 2, 64–69. [Google Scholar] [CrossRef]
- Martins, M.; Šljukić, B.; Metin, Ö.; Sevim, M.; Sequeira, C.A.C.; Şener, T.; Santos, D.M.F. Bimetallic PdM (M = Fe, Ag, Au) alloy nanoparticles assembled on reduced graphene oxide as catalysts for direct borohydride fuel cells. J. Alloys Compd. 2017, 718, 204–214. [Google Scholar] [CrossRef]
- Chen, T.; Rodionov, V.O. Controllable Catalysis with Nanoparticles: Bimetallic Alloy Systems and Surface Adsorbates. ACS Catal. 2016, 6, 4025–4033. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Wen, D.; Oschatz, M.; Holzschuh, M.; Liu, W.; Herrmann, A.-K.K.; Simon, F.; Kaskel, S.; Eychmüller, A. Kinetically controlled synthesis of PdNi bimetallic porous nanostructures with enhanced electrocatalytic activity. Small 2015, 11, 1430–1434. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Zeng, Y.; Guo, Y. Copper@palladium-copper core-shell nanospheres as a highly effective electrocatalyst for ethanol electro-oxidation in alkaline media. J. Power Sources 2014, 270. [Google Scholar] [CrossRef]
- Su, P.P.-C.; Chen, H.-S.H.; Chen, T.T.-Y.; Liu, C.-W.C.; Lee, C.-H.; Lee, J.-F.; Chan, T.-S.; Wang, K.-W. Enhancement of electrochemical properties of Pd/C catalysts toward ethanol oxidation reaction in alkaline solution through Ni and Au alloying. Int. J. Hydrogen Energy 2013, 38, 4474–4482. [Google Scholar] [CrossRef]
- Assumpção, M.H.M.T.; Da Silva, S.G.; De Souza, R.F.B.; Buzzo, G.S.; Spinacé, E.V.; Santos, M.C.; Neto, A.O.; Silva, J.C.M. Investigation of PdIr/C electrocatalysts as anode on the performance of direct ammonia fuel cell. J. Power Sources 2014, 268, 129–136. [Google Scholar] [CrossRef]
- Geraldes, A.N.; da Silva, D.F.; e Silva, L.G.D.A.; Spinacé, E.V.; Neto, A.O.; dos Santos, M.C. Binary and ternary palladium based electrocatalysts for alkaline direct glycerol fuel cell. J. Power Sources 2015, 293, 823–830. [Google Scholar] [CrossRef]
- Ulas, B.; Caglar, A.; Sahin, O.; Kivrak, H. Composition dependent activity of PdAgNi alloy catalysts for formic acid electrooxidation. J. Colloid Interface Sci. 2018, 532, 47–57. [Google Scholar] [CrossRef]
- Dutta, A.; Datta, J. Outstanding Catalyst Performance of PdAuNi Nanoparticles for the Anodic Reaction in an Alkaline Direct Ethanol (with Anion-Exchange Membrane) Fuel Cell. J. Phys. Chem. C 2012, 116, 25677–25688. [Google Scholar] [CrossRef]
- Shang, C.; Hong, W.; Wang, J.; Wang, E. Carbon supported trimetallic nickel-palladium-gold hollow nanoparticles with superior catalytic activity for methanol electrooxidation electrooxidation. J. Power Sources 2015, 285, 12–15. Available online: https://www-sciencedirect-com.sheffield.idm.oclc.org/science/article/pii/S037877531500508X (accessed on 11 May 2019). [CrossRef]
- Sharma, G.; Kumar, D.; Kumar, A.; Al-Muhtaseb, H.; Pathania, D.; Naushad, M.; Tessema Mola, G. Revolution from monometallic to trimetallic nanoparticle composites, various synthesis methods and their applications: A review. Mater. Sci. Eng. C 2017, 71, 1216–1230. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ke, J.; Wang, S.-B.; Ren, J.; Wang, H.-H.; Zhou, Z.-Y.; Si, R.; Zhang, Y.-W.; Yan, C.-H. Shaping Single-Crystalline Trimetallic Pt–Pd–Rh Nanocrystals toward High-Efficiency C–C Splitting of Ethanol in Conversion to CO2. ACS Catal. 2015, 5, 1995–2008. [Google Scholar] [CrossRef]
- Beyhan, S.; Léger, J.-M.; Kadırgan, F. Understanding the influence of Ni, Co, Rh and Pd addition to PtSn/C catalyst for the oxidation of ethanol by in situ Fourier transform infrared spectroscopy. Appl. Catal. B Environ. 2014, 144, 66–74. [Google Scholar] [CrossRef]
- Feng, Y.; Bin, D.; Yan, B.; Du, Y.; Majima, T.; Zhou, W. Porous bimetallic PdNi catalyst with high electrocatalytic activity for ethanol electrooxidation. J. Colloid Interface Sci. 2017, 493, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Datta, J. Energy efficient role of Ni/NiO in PdNi nano catalyst used in alkaline DEFC. J. Mater. Chem. A 2014, 2, 3237. [Google Scholar] [CrossRef]
- Henrique, R.S.; Ayoub, J.M.S.; Piasentin, R.M.; Linardi, M.; Santos, M.C. Preparation of Pt/C-In2O3. SnO2 Electrocatalysts by Borohydride Reduction Process for Ethanol Electro-Oxidation. Int. J. Electrochem. Sci. 2012, 7, 2036–2046. [Google Scholar] [CrossRef]
- Neto, A.O.; Tusi, M.M.; De Oliveira Polanco, N.S.; Da Silva, S.G.; Coelho Dos Santos, M.; Spinacé, E.V. PdBi/C electrocatalysts for ethanol electro-oxidation in alkaline medium. Int. J. Hydrogen Energy 2011, 36, 10522–10526. [Google Scholar] [CrossRef]
- Leng, Y. X-Ray Spectroscopy for Elemental Analysis. In Materials Characterization; John Wiley & Sons, Ltd.: Chichester, UK; pp. 171–196. [CrossRef]
- Amin, R.S.; Hameed, R.M.A.; El-Khatib, K.M.; Youssef, M.E.; Abdel Hameed, R.M.; El-Khatib, K.M.; Elsayed Youssef, M.; Hameed, R.M.A.; El-Khatib, K.M.; Youssef, M.E. Electrocatalytic activity of nanostructured Ni and Pd-Ni on Vulcan XC-72R carbon black for methanol oxidation in alkaline medium. Int. J. Hydrogen Energy 2014, 39, 2026–2041. [Google Scholar] [CrossRef]
- Hermann, K. Appendix E: Parameter Tables of Crystals. Crystallogr. Surf. Struct. 2011, 265–266. [Google Scholar] [CrossRef]
- Zhu, F.; Ma, G.; Bai, Z.; Hang, R.; Tang, B.; Zhang, Z.; Wang, X. High activity of carbon nanotubes supported binary and ternary Pd-based catalysts for methanol, ethanol and formic acid electro-oxidation. J. Power Sources 2013, 242, 610–620. [Google Scholar] [CrossRef]
- Suo, Y.; Hsing, I. Synthesis of bimetallic PdAu nanoparticles for formic acid oxidation. Electrochim. Acta 2011, 56, 2174–2183. [Google Scholar] [CrossRef]
- Chen, A.; Ostrom, C. Palladium-Based Nanomaterials: Synthesis and Electrochemical Applications. Chem. Rev. 2015, 115, 11999–12044. [Google Scholar] [CrossRef] [Green Version]
- Ramulifho, T.; Ozoemena, K.I.; Modibedi, R.M.; Jafta, C.J.; Mathe, M.K. Fast microwave-assisted solvothermal synthesis of metal nanoparticles (Pd, Ni, Sn) supported on sulfonated MWCNTs: Pd-based bimetallic catalysts for ethanol oxidation in alkaline medium. Electrochim. Acta 2012, 59, 310–320. [Google Scholar] [CrossRef] [Green Version]
- Obradović, M.D.; Stančić, Z.M.; Lačnjevac, U.Č.; Radmilović, V.V.; Gavrilović-Wohlmuther, A.; Radmilović, V.R.; Gojković, S.L. Electrochemical oxidation of ethanol on palladium-nickel nanocatalyst in alkaline media. Appl. Catal. B Environ. 2016, 189, 110–118. [Google Scholar] [CrossRef]
- Shen, S.Y.; Zhao, T.S.; Xu, J.B. Carbon-supported bimetallic PdIr catalysts for ethanol oxidation in alkaline media. Electrochim. Acta 2010, 55, 9179–9184. [Google Scholar] [CrossRef]
- Barbosa, A.F.B.; Oliveira, V.L.; Van Drunen, J.; Tremiliosi-Filho, G. Ethanol electro-oxidation reaction using a polycrystalline nickel electrode in alkaline media: Temperature influence and reaction mechanism. J. Electroanal. Chem. 2015, 746, 31–38. [Google Scholar] [CrossRef]
- Lidasan, J.J.B.; Del Rosario, J.A.D.; Ocon, J.D. Ethanol electrooxidation on phase-and morphology-controlled ni(Oh)2 microspheres. Catalysts 2020, 10, 740. [Google Scholar] [CrossRef]
- Amin, S.; Tahira, A.; Solangi, A.R.; Mazzaro, R.; Ibupoto, Z.H.; Fatima, A.; Vomiero, A. Functional Nickel Oxide Nanostructures for Ethanol Oxidation in Alkaline Media. Electroanalysis 2020, 32, 1052–1059. [Google Scholar] [CrossRef]
- Elsheikh, A.; McGregor, J. Synthesis and characterization of pdagni/c trimetallic nanoparticles for ethanol electrooxidation. Nanomaterials 2021, 11, 2244. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, A.M.A.; Backović, G.; Oliveira, R.C.P.; Sequeira, C.A.C.; McGregor, J.; Šljukić, B.; Santos, D.M.F. Carbon-supported trimetallic catalysts (Pdauni/c) for borohydride oxidation reaction. Nanomaterials 2021, 11, 1441. [Google Scholar] [CrossRef]







| Catalyst | C (mg) | PdCl2 (mg) | NiCl2 (mg) | IrCl3 (mg) |
|---|---|---|---|---|
| Pd/C | 132 | 29.4 | - | - |
| PdIrNi/C | 132 | 8.83 | 6.46 | 14.94 |
| Pd4Ir2Ni1/C | 132 | 14.19 | 2.58 | 11.94 |
| Catalyst | XRD * Size (nm) | Lattice Constant (Å) | TEM ** Size (nm) | % Dispersion |
|---|---|---|---|---|
| Pd/C | 4 | 4 | 5 | 25 |
| PdIrNi/C | 1.4 | 3.7 | 1.9 | 52 |
| Pd4Ir2Ni1/C | 1.8 | 3.8 | 2.3 | 44 |
| Catalyst | Acc. Voltage | Pd | Ni | Ir | Metal Load (wt.%) | |||
|---|---|---|---|---|---|---|---|---|
| wt.% | at.% | wt.% | at.% | wt.% | at.% | |||
| PdIrNi/C | 10 kV | 5.75 | 0.41 | 4.86 | 1.14 | 7.40 | 0.41 | 18 |
| 20 kV | 5.36 | 0.62 | 2.51 | 0.53 | 6.62 | 0.39 | 14.5 | |
| Pd4Ir2Ni1/C | 10 kV | 7.15 | 0.97 | 1.15 | 0.28 | 6.95 | 0.52 | 15.3 |
| 20 kV | 6.64 | 0.90 | 0.56 | 0.14 | 6.09 | 0.46 | 13.3 | |
| Catalyst | Pd at. % | Pd 3d5/2 (eV) | C at. % | Ir at. % | Ir 4f7/2 (eV) | Ni at. % | Ni 2p3/2 (eV) * | ||
|---|---|---|---|---|---|---|---|---|---|
| Pd0 | Pd2+ | Ni0 | Ni2+ | ||||||
| Pd/C | 1.63 | 0.45 | 335.43 | 96.13 | x | X | X | x | x |
| PdIrNi/C | 1.18 | 0.13 | 335.54 | 91.09 | 1.48 | 60.88 | 0.32 | 0.93 | 852.74 |
| Pd4Ir2Ni1/C | 1.39 | 0.10 | 335.51 | 93.62 | 1.16 | 60.98 | 0.12 | 0.18 | 852.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsheikh, A.; Mousa, H.M.; McGregor, J. Synthesis of Carbon-Supported PdIrNi Catalysts and Their Performance towards Ethanol Electrooxidation. Micromachines 2021, 12, 1327. https://doi.org/10.3390/mi12111327
Elsheikh A, Mousa HM, McGregor J. Synthesis of Carbon-Supported PdIrNi Catalysts and Their Performance towards Ethanol Electrooxidation. Micromachines. 2021; 12(11):1327. https://doi.org/10.3390/mi12111327
Chicago/Turabian StyleElsheikh, Ahmed, Hamouda M. Mousa, and James McGregor. 2021. "Synthesis of Carbon-Supported PdIrNi Catalysts and Their Performance towards Ethanol Electrooxidation" Micromachines 12, no. 11: 1327. https://doi.org/10.3390/mi12111327







