Fabrication of Planar Microelectrode Array Using Laser-Patterned ITO and SU-8
Abstract
:1. Introduction
2. Materials and Methods
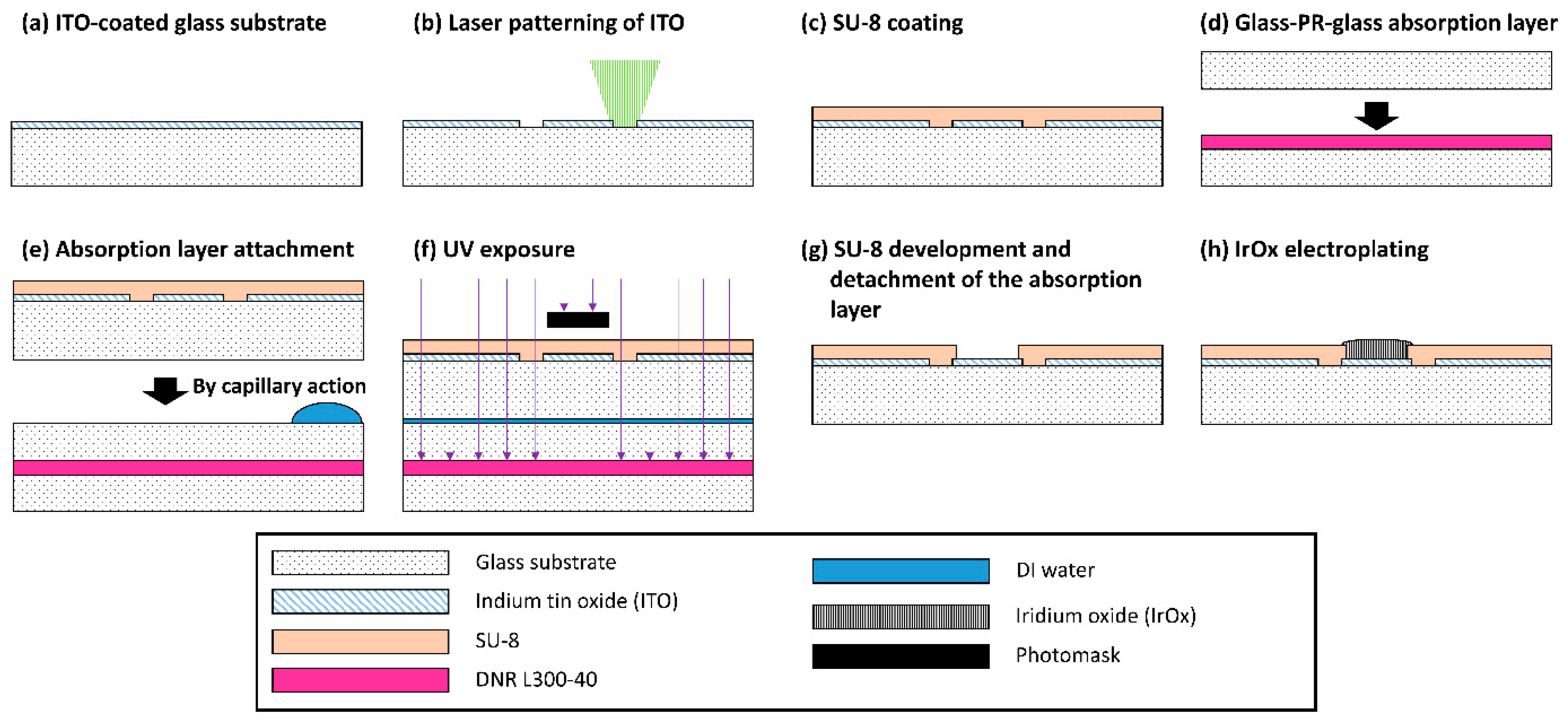
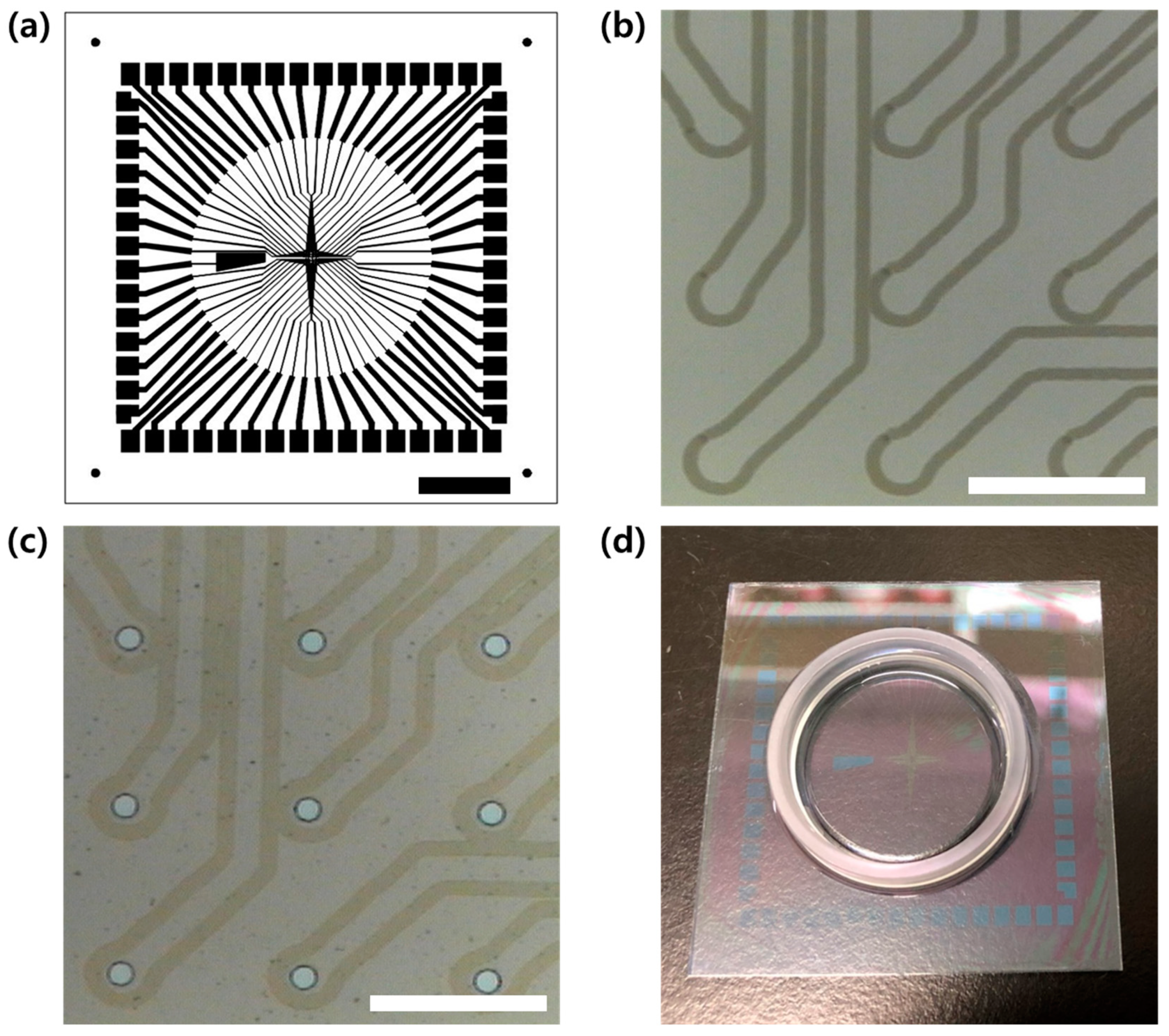
2.1. Microelectrode Fabrication
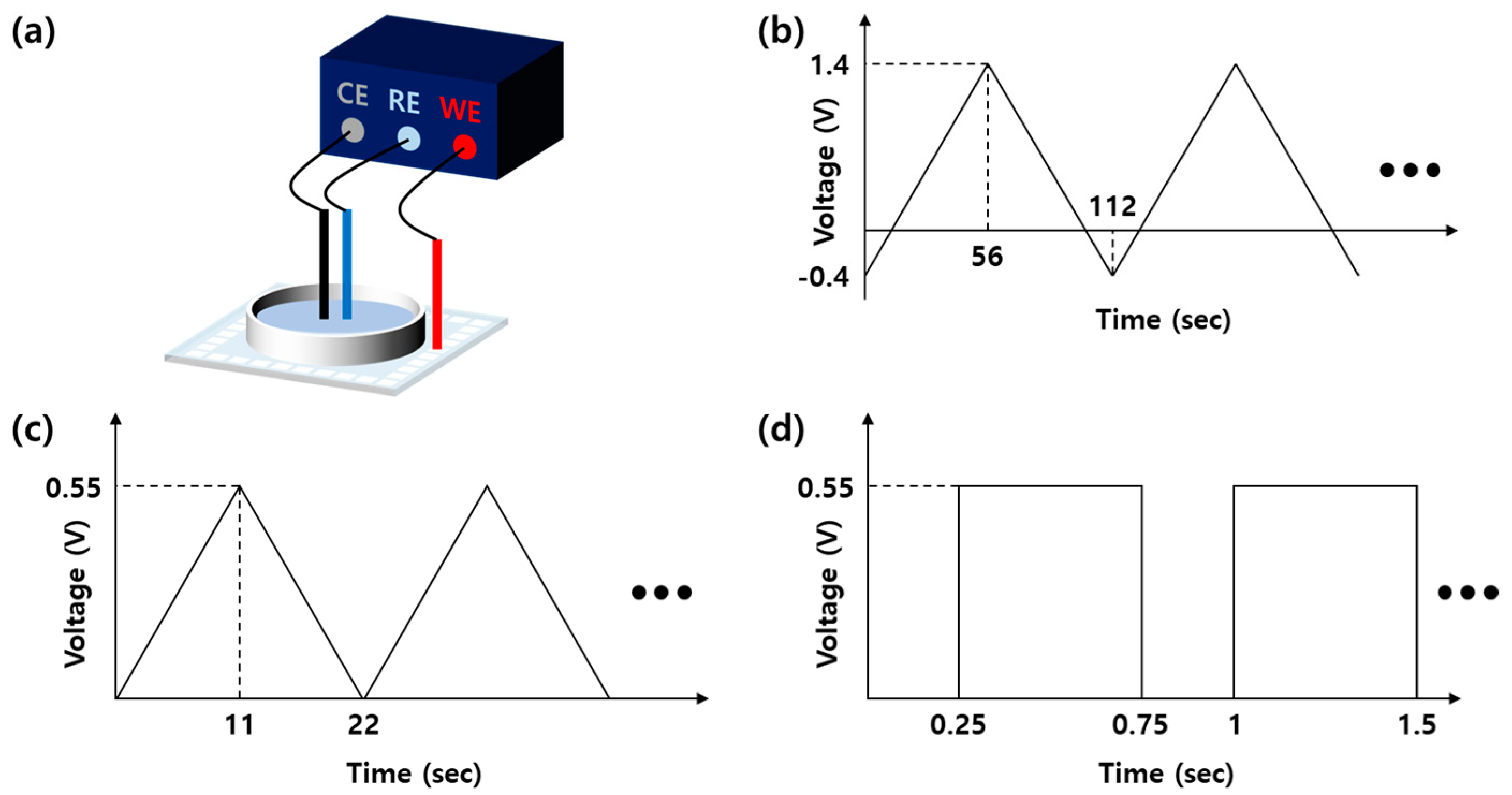
2.2. Iridium Oxide Electroplating
2.3. Electrochemical Measurements
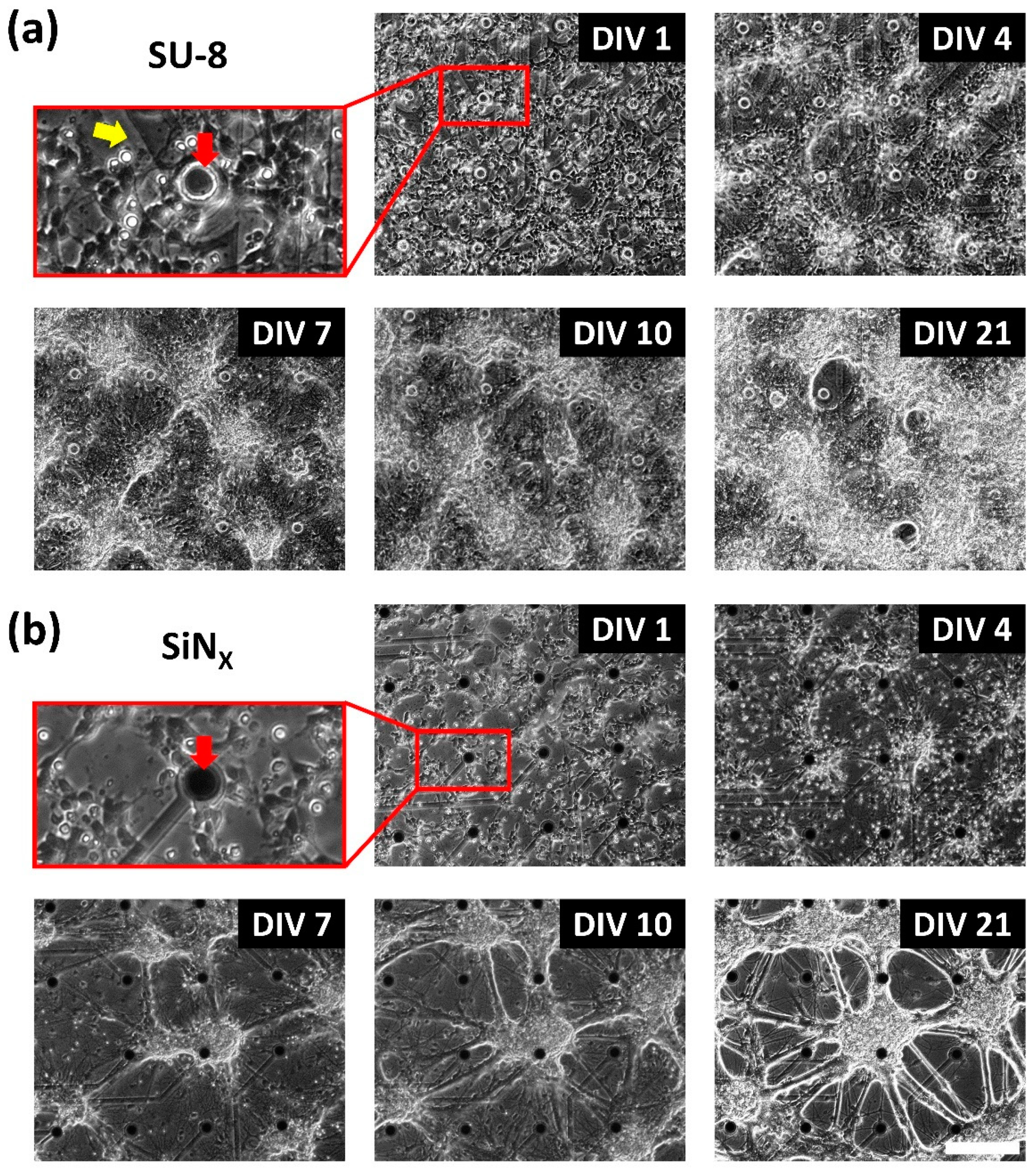
2.4. Cell Culture
2.5. Electrophysiological Recording of Neurons
2.6. Statistical Analysis
3. Results
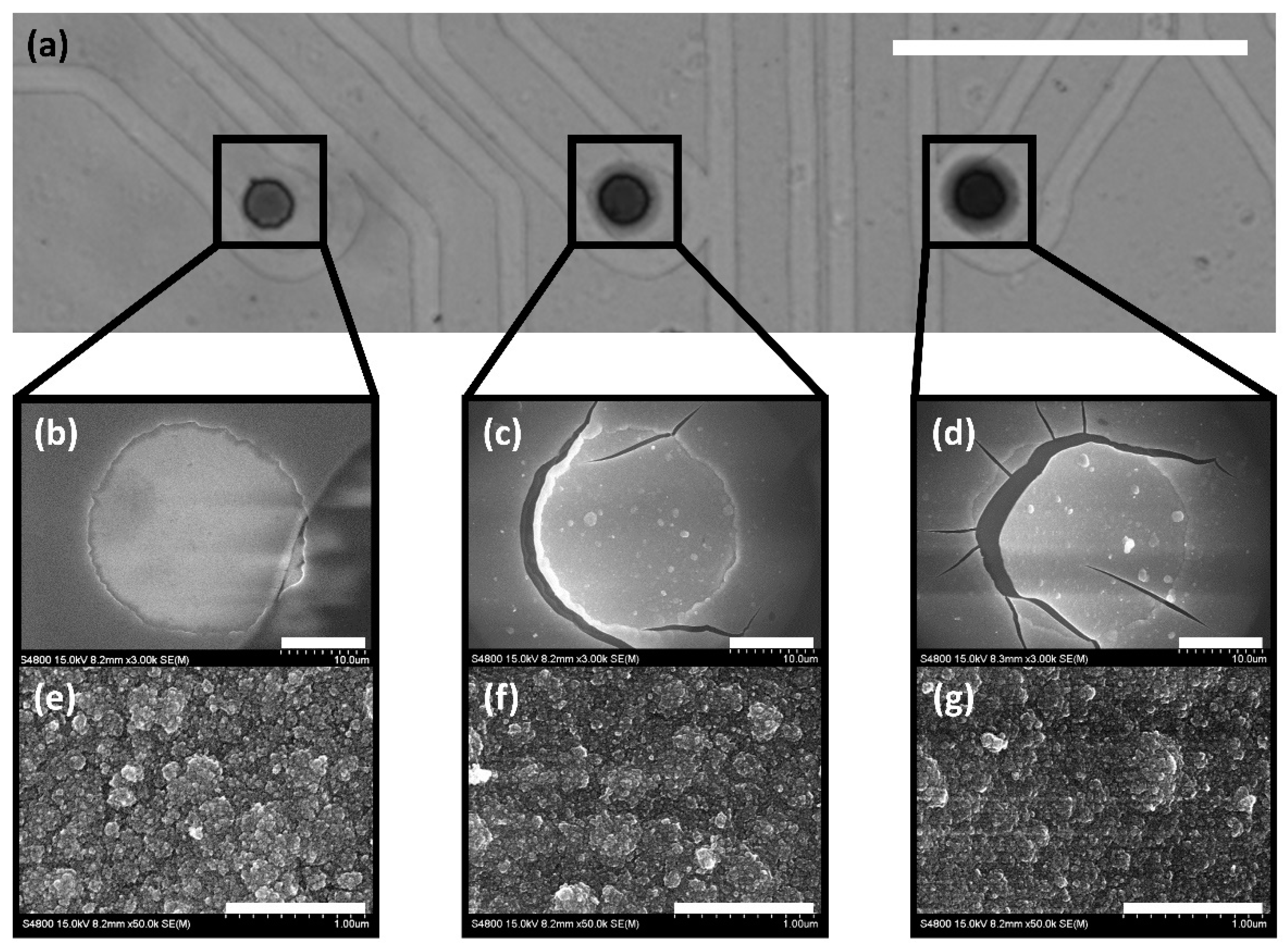
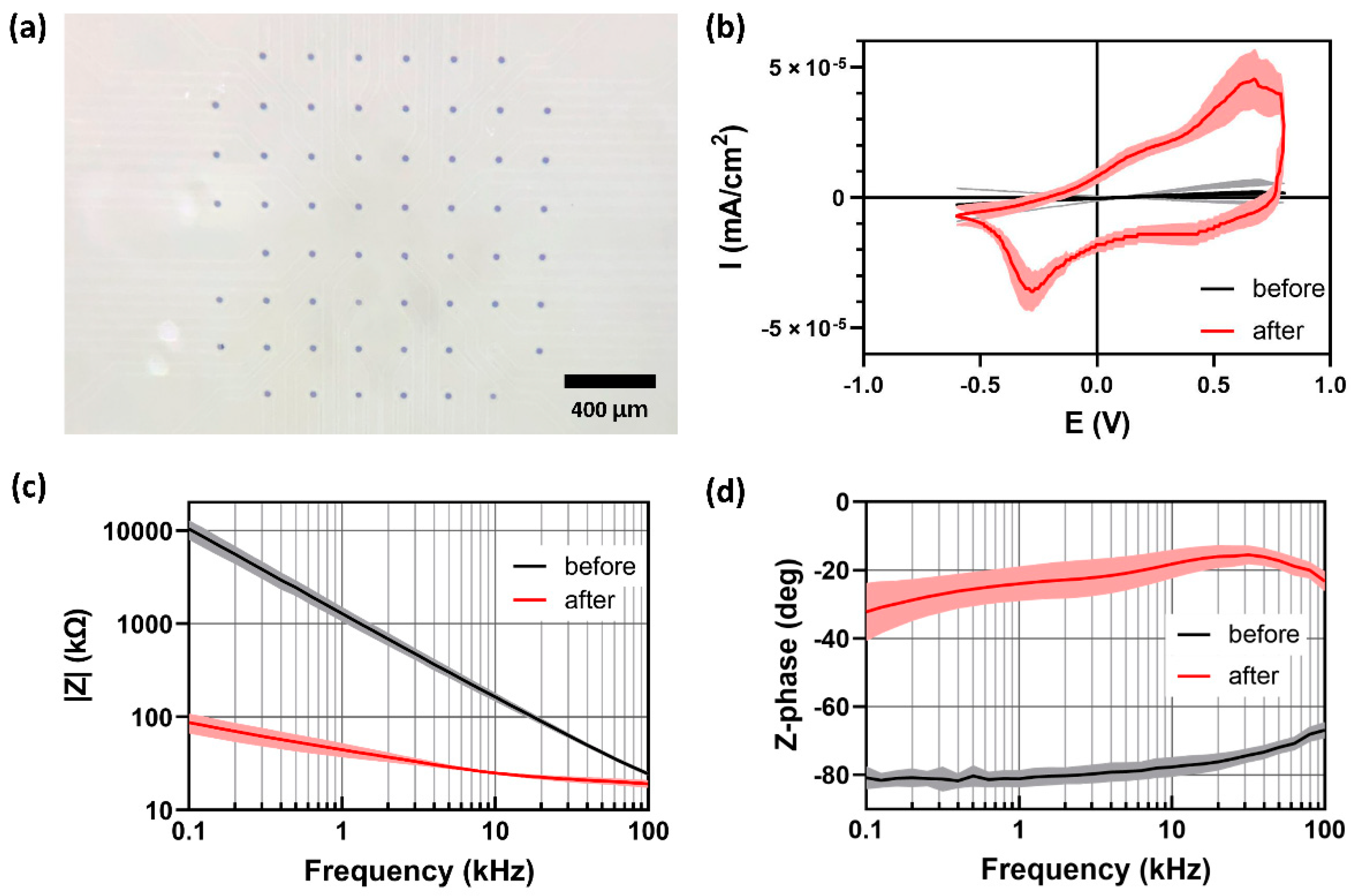
3.1. MEA Fabrication
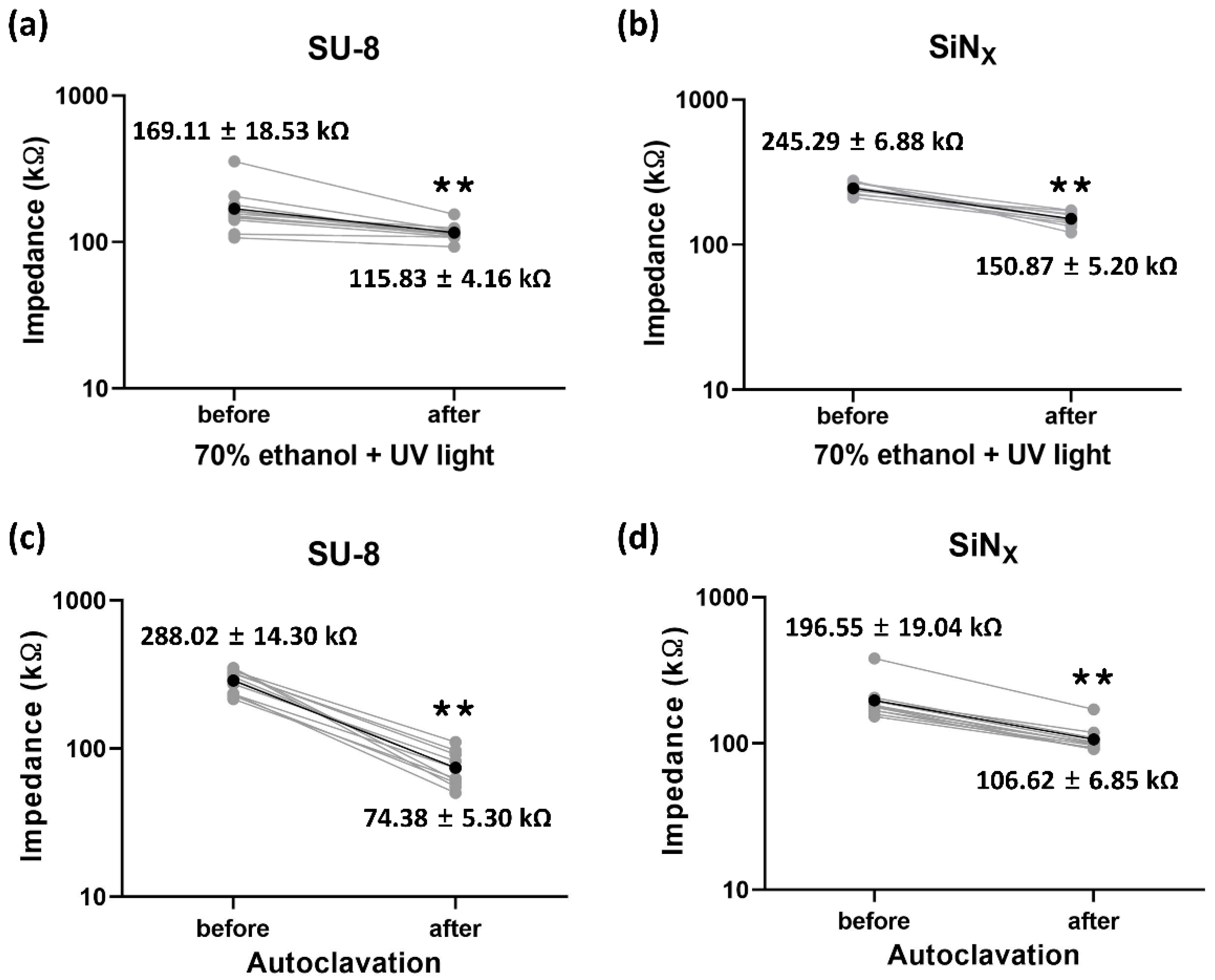
3.2. Electrochemical Characterization
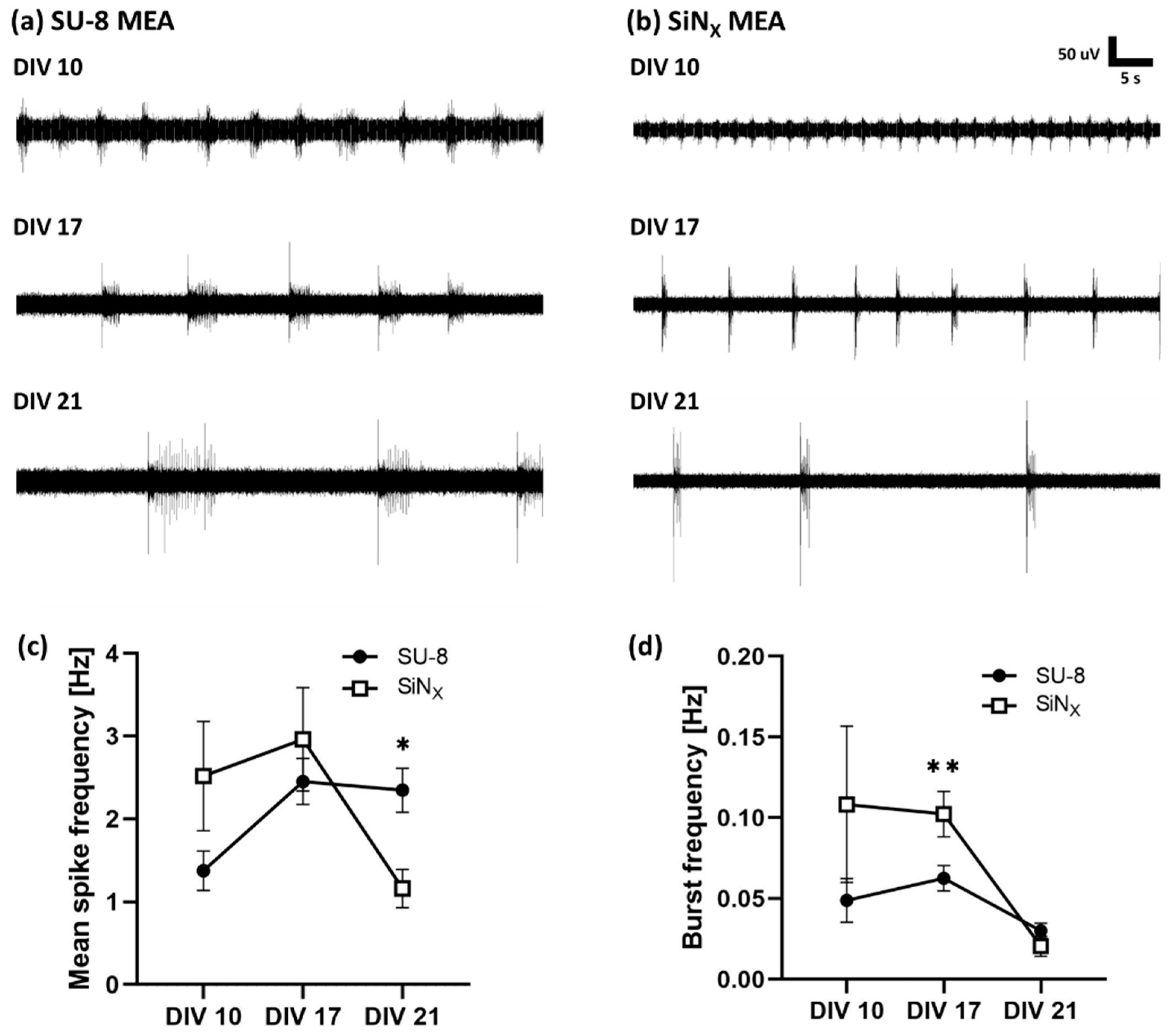
3.3. Electrophysiology
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morin, F.O.; Takamura, Y.; Tamiya, E. Investigating neuronal activity with planar microelectrode arrays: Achievements and new perspectives. J. Biosci. Bioeng. 2005, 100, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.L.; Livi, P.; Lewandowska, M.K.; Fiscella, M.; Roscic, B.; Hierlemann, A. The potential of microelectrode arrays and microelectronics for biomedical research and diagnostics. Anal. Bioanal. Chem. 2011, 399, 2313–2329. [Google Scholar] [CrossRef] [PubMed]
- Hayes, H.B.; Nicolini, A.M.; Arrowood, C.A.; Chvatal, S.A.; Wolfson, D.W.; Cho, H.C.; Sullivan, D.D.; Chal, J.; Fermini, B.; Clements, M.; et al. Novel method for action potential measurements from intact cardiac monolayers with multiwell microelectrode array technology. Sci. Rep. 2019, 9, 11893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nisch, W.; Böck, J.; Egert, U.; Hämmerle, H.; Mohr, A. A thin film microelectrode array for monitoring extracellular neuronal activity in vitro. Biosens. Bioelectron. 1994, 9, 737–741. [Google Scholar] [CrossRef]
- Rutten, W.L. Selective electrical interfaces with the nervous system. Annu. Rev. Biomed. Eng. 2002, 4, 407–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Ali, J.; Sorger, P.K.; Jensen, K.F. Cells on chips. Nature 2006, 442, 403–411. [Google Scholar] [CrossRef]
- Pearce, T.M.; Williams, J.C. Microtechnology: Meet neurobiology. Lab Chip 2007, 7, 30–40. [Google Scholar] [CrossRef]
- Yeung, C.K.; Sommerhage, F.; Wrobel, G.; Offenhäusser, A.; Chan, M.; Ingebrandt, S. Drug profiling using planar microelectrode arrays. Anal. Bioanal. Chem. 2007, 387, 2673–2680. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, S.B.; Ko, H.J.; Kim, S.J.; Park, T.H. Cell-based olfactory biosensor using microfabricated planar electrode. Biosens. Bioelectron. 2009, 24, 2659–2664. [Google Scholar] [CrossRef]
- Gautam, V.; Rand, D.; Hanein, Y.; Narayan, K.S. A polymer optoelectronic interface provides visual cues to a blind retina. Adv. Mater. 2014, 26, 1751–1756. [Google Scholar] [CrossRef]
- Thomas, C.A., Jr.; Springer, P.A.; Loeb, G.E.; Berwald-Netter, Y.; Okun, L.M. A miniature microelectrode array to monitor the bioelectric activity of cultured cells. Exp. Cell Res. 1972, 74, 61–66. [Google Scholar] [CrossRef]
- Pine, J. Recording action potentials from cultured neurons with extracellular microcircuit electrodes. J. Neurosci. Methods 1980, 2, 19–31. [Google Scholar] [CrossRef]
- Gross, G.W.; Williams, A.N.; Lucas, J.H. Recording of spontaneous activity with photoetched microelectrode surfaces from mouse spinal neurons in culture. J. Neurosci. Methods 1982, 5, 13–22. [Google Scholar] [CrossRef]
- Lambacher, A.; Jenkner, M.; Merz, M.; Eversmann, B.; Kaul, R.; Hofmann, F.; Thewes, R.; Fromherz, P. Electrical imaging of neuronal activity by multi-transistor-array (MTA) recording at 7.8 μm resolution. Appl. Phys. A 2004, 79, 1607–1611. [Google Scholar] [CrossRef]
- Boehler, M.D.; Leondopulos, S.S.; Wheeler, B.C.; Brewer, G.J. Hippocampal networks on reliable patterned substrates. J. Neurosci. Methods 2012, 203, 344–353. [Google Scholar] [CrossRef] [Green Version]
- Brewer, G.J.; Boehler, M.D.; Leondopulos, S.; Pan, L.; Alagapan, S.; DeMarse, T.B.; Wheeler, B.C. Toward a self-wired active reconstruction of the hippocampal trisynaptic loop: DG-CA3. Front. Neural Circuits 2013, 7, 165. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.; Joo, S.; Jung, H.; Hong, N.; Nam, Y. Recent trends in microelectrode array technology for in vitro neural interface platform. Biomed. Eng. Lett. 2014, 4, 129–141. [Google Scholar] [CrossRef]
- Soscia, D.A.; Lam, D.; Tooker, A.C.; Enright, H.A.; Triplett, M.; Karande, P.; Peters, S.K.G.; Sales, A.P.; Wheeler, E.K.; Fischer, N.O. A flexible 3-dimensional microelectrode array for in vitro brain models. Lab Chip 2020, 20, 901–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stotter, J.; Show, Y.; Wang, S.; Swain, G. Comparison of the electrical, optical, and electrochemical properties of diamond and indium tin oxide thin-film electrodes. Chem. Mater. 2005, 17, 4880–4888. [Google Scholar] [CrossRef]
- Tran, D.-P.; Lu, H.-I.; Lin, C.-K. Conductive characteristics of indium tin oxide thin film on polymeric substrate under long-term static deformation. Coatings 2018, 8, 212. [Google Scholar] [CrossRef] [Green Version]
- Jun, S.B.; Hynd, M.R.; Dowell-Mesfin, N.; Smith, K.L.; Turner, J.N.; Shain, W.; Kim, S.J. Low-density neuronal networks cultured using patterned poly-l-lysine on microelectrode arrays. J. Neurosci. Methods 2007, 160, 317–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metallo, C.; White, R.D.; Trimmer, B.A. Flexible parylene-based microelectrode arrays for high resolution EMG recordings in freely moving small animals. J. Neurosci. Methods 2011, 195, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Im, C.; Lee, W.R. Plateau-Shaped Flexible Polymer Microelectrode Array for Neural Recording. Polymers 2017, 9, 690. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.K.; English, A.E.; Jun, S.I.; Kihm, K.D.; Rack, P.D. An endothelial cell compatible biosensor fabricated using optically thin indium tin oxide silicon nitride electrodes. Biosens. Bioelectron. 2007, 22, 2585–2590. [Google Scholar] [CrossRef] [PubMed]
- Koester, P.J.; Buehler, S.M.; Stubbe, M.; Tautorat, C.; Niendorf, M.; Baumann, W.; Gimsa, J. Modular glass chip system measuring the electric activity and adhesion of neuronal cells--application and drug testing with sodium valproic acid. Lab Chip 2010, 10, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, G.; Schultze, J.W.; Faßbender, F.; Buß, G.; Lüth, H.; Schöning, M.J. Passivation and corrosion of microelectrode arrays. Electrochim. Acta 1999, 44, 3865–3883. [Google Scholar] [CrossRef]
- Barrese, J.C.; Aceros, J.; Donoghue, J.P. Scanning electron microscopy of chronically implanted intracortical microelectrode arrays in non-human primates. J. Neural Eng. 2016, 13, 026003. [Google Scholar] [CrossRef] [Green Version]
- Barrese, J.C.; Rao, N.; Paroo, K.; Triebwasser, C.; Vargas-Irwin, C.; Franquemont, L.; Donoghue, J.P. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J. Neural Eng. 2013, 10, 066014. [Google Scholar] [CrossRef]
- Ravula, S.K.; McClain, M.A.; Wang, M.S.; Glass, J.D.; Frazier, A.B. A multielectrode microcompartment culture platform for studying signal transduction in the nervous system. Lab Chip 2006, 6, 1530–1536. [Google Scholar] [CrossRef]
- Mao, X.; Yang, J.; Ji, A.; Yang, F. Two new methods to improve the lithography precision for SU-8 photoresist on glass substrate. J. Microelectromech. Syst. 2012, 22, 124–130. [Google Scholar] [CrossRef]
- Meyer, R.D.; Cogan, S.F.; Nguyen, T.H.; Rauh, R.D. Electrodeposited iridium oxide for neural stimulation and recording electrodes. IEEE Trans. Neural Syst. Rehabil. Eng. 2001, 9, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Kim, J.; Jeong, J.; Gwon, T.M.; Choi, G.J.; Lee, S.E.; Kim, J.; Jun, S.B.; Chang, J.W.; Kim, S.J. High charge storage capacity electrodeposited iridium oxide film on liquid crystal polymer-based neural electrodes. Sens. Mater. 2016, 28, 243–260. [Google Scholar]
- Geissler, M.; Faissner, A. A new indirect co-culture set up of mouse hippocampal neurons and cortical astrocytes on microelectrode arrays. J. Neurosci. Methods 2012, 204, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Maeng, J.; Chakraborty, B.; Geramifard, N.; Kang, T.; Rihani, R.T.; Joshi-Imre, A.; Cogan, S.F. High-charge-capacity sputtered iridium oxide neural stimulation electrodes deposited. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Cogan, S.F.; Troyk, P.R.; Ehrlich, J.; Plante, T.D. In vitro comparison of the charge-injection limits of activated iridium oxide (AIROF) and platinum-iridium microelectrodes. IEEE Trans. Biomed. Eng. 2005, 52, 1612–1614. [Google Scholar] [CrossRef]
- Ha, Y.; Yoo, H.J.; Shin, S.; Jun, S.B. Hemispherical Microelectrode Array for Ex Vivo Retinal Neural Recording. Micromachines 2020, 11, 538. [Google Scholar] [CrossRef]
- Shin, S.; Ha, Y.; Choi, G.; Hyun, J.; Kim, S.; Oh, S.H.; Min, K.S. Manufacturable 32-Channel Cochlear Electrode Array and Preliminary Assessment of Its Feasibility for Clinical Use. Micromachines 2021, 12, 778. [Google Scholar] [CrossRef] [PubMed]
- Weiland, J.D.; Anderson, D.J.; Humayun, M.S. In vitro electrical properties for iridium oxide versus titanium nitride stimulating electrodes. IEEE Trans. Biomed. Eng. 2002, 49, 1574–1579. [Google Scholar] [CrossRef]
- Weiland, J.D.; Anderson, D.J. Chronic neural stimulation with thin-film, iridium oxide electrodes. IEEE Trans. Biomed. Eng. 2000, 47, 911–918. [Google Scholar] [CrossRef]
- Chen, Z.; Lee, J.B. Biocompatibility of SU-8 and Its Biomedical Device Applications. Micromachines 2021, 12, 794. [Google Scholar] [CrossRef]
- Abdolahi, M.; Jiang, H.; Patel, D.; Kaminska, B. Nickel stamp origination from generic SU-8 nanostructure arrays patterned with improved thermal development and reshaping. Nanotechnology 2018, 29, 405303. [Google Scholar] [CrossRef] [PubMed]
- Mojena-Medina, D.; Hubl, M.; Bäuscher, M.; Jorcano, J.L.; Ngo, H.D.; Acedo, P. Real-Time Impedance Monitoring of Epithelial Cultures with Inkjet-Printed Interdigitated-Electrode Sensors. Sensors 2020, 20, 5711. [Google Scholar] [CrossRef] [PubMed]
- Lotfi Marchoubeh, M.; Cobb, S.J.; Abrego Tello, M.; Hu, M.; Jaquins-Gerstl, A.; Robbins, E.M.; Macpherson, J.V.; Michael, A.C.; Fritsch, I. Miniaturized probe on polymer SU-8 with array of individually addressable microelectrodes for electrochemical analysis in neural and other biological tissues. Anal. Bioanal. Chem. 2021, 413, 6777–6791. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Lu, Y.; Zhao, R.; Liu, X.; De-Eknamkul, C.; Ren, C.; Mehrsa, A.; Komiyama, T.; Kuzum, D. Evaluation of Durability of Transparent Graphene Electrodes Fabricated on Different Flexible Substrates for Chronic In Vivo Experiments. IEEE Trans. Biomed. Eng. 2020, 67, 3203–3210. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Sokuler, M.; Fell, D.; Keller, S.; Boisen, A.; Butt, H.J.; Auernhammer, G.K.; Bonaccurso, E. Diffusion of water into SU-8 microcantilevers. Phys. Chem. Chem. Phys. 2010, 12, 10577–10583. [Google Scholar] [CrossRef] [PubMed]
- Vogt, M.; Hauptmann, R. Plasma-deposited passivation layers for moisture and water protection. Surf. Coat. Technol. 1995, 74–75, 676–681. [Google Scholar] [CrossRef]
- Kaloyeros, A.E.; Pan, Y.; Goff, J.; Arkles, B. Review—Silicon Nitride and Silicon Nitride-Rich Thin Film Technologies: State-of-the-Art Processing Technologies, Properties, and Applications. ECS J. Solid State Sci. Technol. 2020, 9, 063006. [Google Scholar] [CrossRef]
- Pan, Y.; Lv, X.; Zhang, H.; Chen, J.; Han, B.; Shen, Z.; Lu, J.; Ni, X. Millisecond laser machining of transparent materials assisted by a nanosecond laser with different delays. Opt. Lett. 2016, 41, 2807–2810. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, H.S.; Hwang, S.; Min, K.S.; Jun, S.B. Fabrication of Planar Microelectrode Array Using Laser-Patterned ITO and SU-8. Micromachines 2021, 12, 1347. https://doi.org/10.3390/mi12111347
Jeong HS, Hwang S, Min KS, Jun SB. Fabrication of Planar Microelectrode Array Using Laser-Patterned ITO and SU-8. Micromachines. 2021; 12(11):1347. https://doi.org/10.3390/mi12111347
Chicago/Turabian StyleJeong, Hee Soo, Seoyoung Hwang, Kyou Sik Min, and Sang Beom Jun. 2021. "Fabrication of Planar Microelectrode Array Using Laser-Patterned ITO and SU-8" Micromachines 12, no. 11: 1347. https://doi.org/10.3390/mi12111347
APA StyleJeong, H. S., Hwang, S., Min, K. S., & Jun, S. B. (2021). Fabrication of Planar Microelectrode Array Using Laser-Patterned ITO and SU-8. Micromachines, 12(11), 1347. https://doi.org/10.3390/mi12111347





