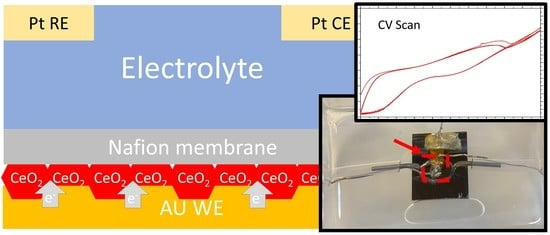
Studying Corrosion Using Miniaturized Particle Attached Working Electrodes and the Nafion Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Agents
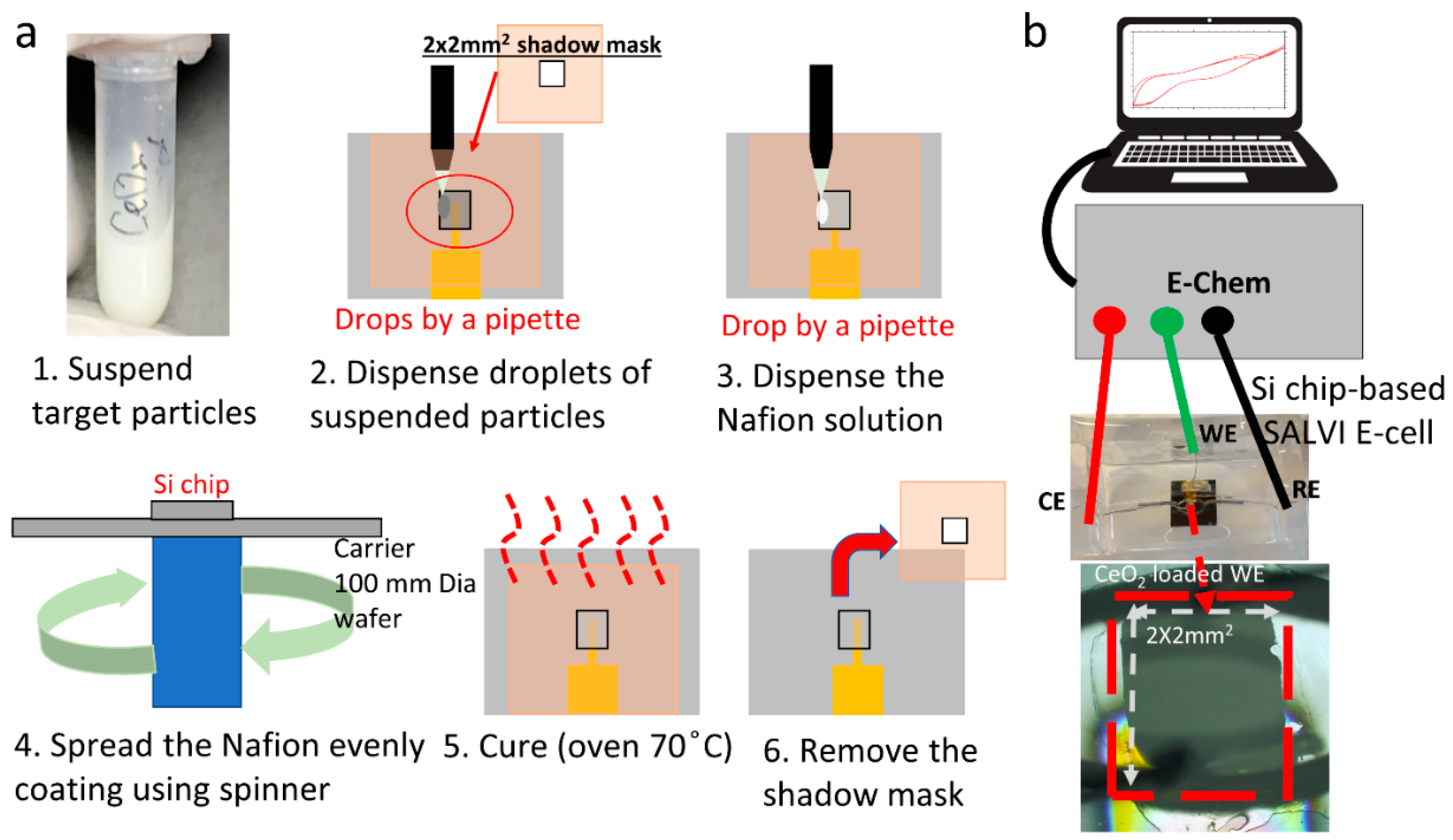
2.2. Microfluidic Electrochemical Cell Fabrication
2.2.1. WE Fabrication
2.2.2. Device Assembly
2.3. Membrane Thickness Mesurements
2.4. Cyclic Voltammetry
2.5. AFM Characterization
2.6. ToF-SIMS Characterization
2.7. SEM-FIB
2.8. XPS
3. Results and Discussion
3.1. WE Fabrication Optimization
3.2. SEM-FIB Characterization
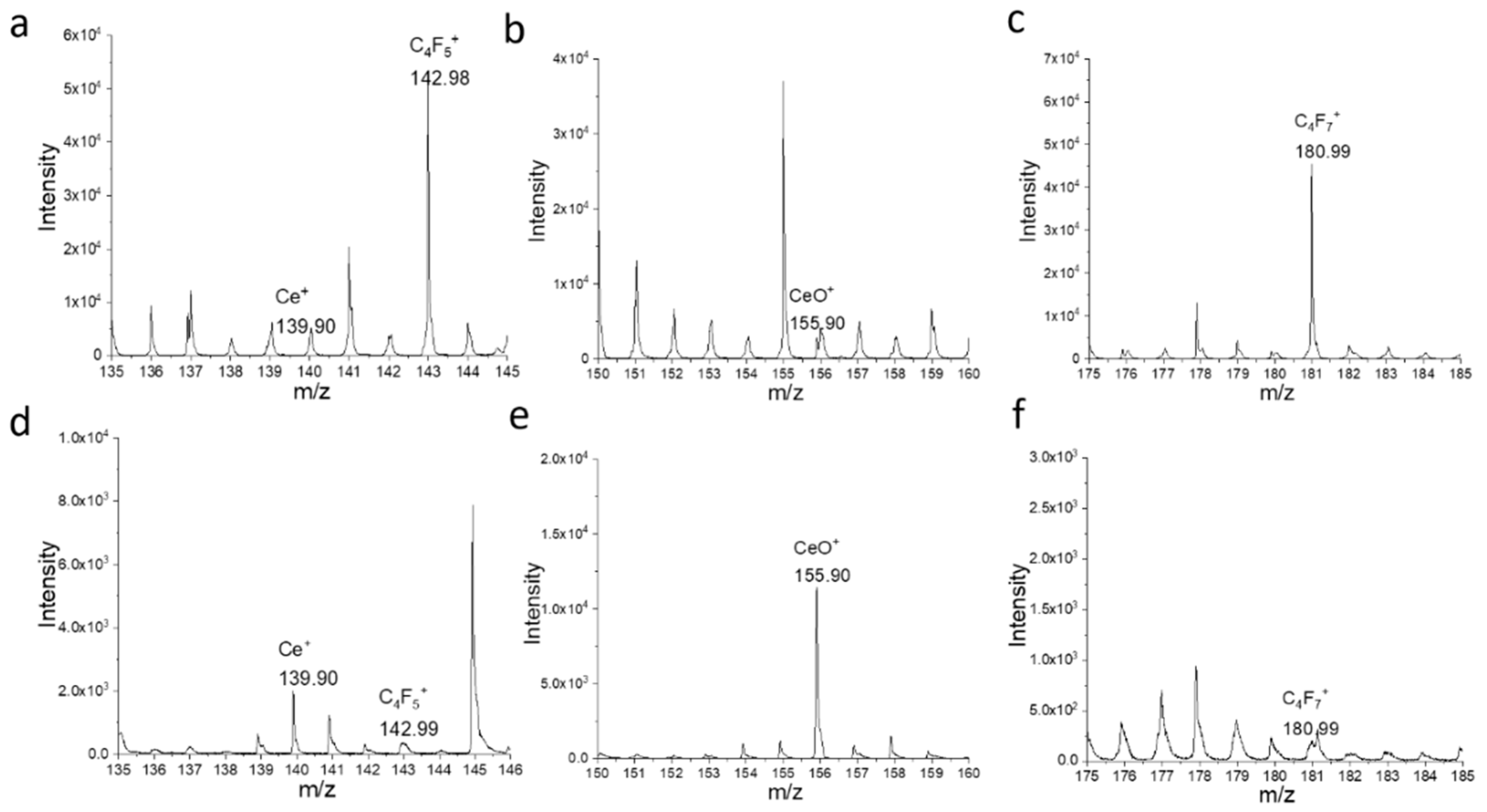
3.3. ToF-SIMS Spectral Analysis and 2D Mapping
3.4. XPS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cureton, W.F.; Tracy, C.L.; Lang, M. Review of Swift Heavy Ion Irradiation Effects in CeO2. Quantum Beam Sci. 2021, 5, 19. [Google Scholar] [CrossRef]
- García-Ostos, C.; Rodríguez-Ortiz, J.A.; Arévalo, C.; Cobos, J.; Gotor, F.J.; Torres, Y. Fabrication and characterization of CeO2 pellets for simulation of nuclear fuel. Nucl. Eng. Des. 2016, 298, 160–167. [Google Scholar] [CrossRef]
- Torres, Y.; García-Ostos, C.; Arévalo, C.; Gotor, F.J.; Pavón, J.J.; Trueba, P.; Rodríguez-Ortiz, J.A. Processing and characterization of surrogate nuclear materials with controlled radial porosity. J. Nucl. Sci. Technol. 2017, 54, 167–173. [Google Scholar] [CrossRef]
- Fernandez, S.; Nieto, M.I.; Cobos, J.; Moreno, R. CeO2 pellet fabrication as spent fuel matrix analogue. J. Eur. Ceram. Soc. 2016, 36, 3505–3512. [Google Scholar] [CrossRef]
- Nelson, A.T.; Rittman, D.R.; White, J.T.; Dunwoody, J.T.; Kato, M.; McClellan, K.J. An Evaluation of the Thermophysical Properties of Stoichiometric CeO2 in Comparison to UO2 and PuO2. J. Am. Ceram. Soc. 2014, 97, 3652–3659. [Google Scholar] [CrossRef]
- Einstein, A.J.; Pascual, T.N.; Mercuri, M.; Karthikeyan, G.; Vitola, J.V.; Mahmarian, J.J.; Better, N.; Bouyoucef, S.E.; Hee-Seung Bom, H.; Lele, V.; et al. Current worldwide nuclear cardiology practices and radiation exposure: Results from the 65 country IAEA Nuclear Cardiology Protocols Cross-Sectional Study (INCAPS). Eur. Heart J. 2015, 36, 1689–1696. [Google Scholar] [CrossRef] [Green Version]
- Sunder, S.; Miller, N.H.; Shoesmith, D.W. Corrosion of uranium dioxide in hydrogen peroxide solutions. Corros. Sci. 2004, 46, 1095–1111. [Google Scholar] [CrossRef]
- Shoesmith, D.W.; Sunder, S.; Bailey, M.G.; Wallace, G.J. The Corrosion of Nuclear Fuel (UO2) In Oxygenated Solutions. Corros. Sci. 1989, 29, 1115–1128. [Google Scholar] [CrossRef]
- Christensen, H.; Sunder, S.; Shoesmith, D.W. Oxidation of unclear fuel (UO2) by the products of water radiolysis: Development of a kinetic model. J. Alloy. Compd. 1994, 213, 93–99. [Google Scholar] [CrossRef]
- Sunder, S.; Strandlund, L.K.; Shoesmith, D.W. Anodic oxidation and dissolution of CANDU fuel (UO2) in slightly alkaline sodium perchlorate solutions. Electrochim. Acta 1998, 43, 2359–2372. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Liu, B.; Wu, K.; Thevuthasan, S.; Baer, D.R.; Zhu, Z.; Yu, X.Y.; Wang, F. In Situ Mass Spectrometric Monitoring of the Dynamic Electrochemical Process at the Electrode-Electrolyte Interface: A SIMS Approach. Anal. Chem. 2017, 89, 960–965. [Google Scholar] [CrossRef]
- Yang, L.; Yu, X.Y.; Zhu, Z.; Iedema, M.J.; Cowin, J.P. Probing liquid surfaces under vacuum using SEM and ToF-SIMS. Lab Chip 2011, 11, 2481–2484. [Google Scholar] [CrossRef]
- Yang, L.; Yu, X.-Y.; Zhu, Z.; Thevuthasan, T.; Cowin, J.P. Making a hybrid microfluidic platform compatible forin situimaging by vacuum-based techniques. J. Vac. Sci. Technol. A Vac. Surf. Films 2011, 29. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Industerial Safety Safety Guidelines for Nuclear Facilities; IAEA Nuclear Energy Series NP-T-3.3; International Atomic Energy Agency: Vienna, Austria, 2018; p. 264. [Google Scholar]
- Canadian Nuclear Safety Commission. Radiobioassy Protocols for Responding to Abnormal Intakes of Radionuclides; G-147; Canadian NuclearSafety Commission: Ottawa, ON, Canada, 2003; p. 27. [Google Scholar]
- Son, J.; Buck, E.C.; Riechers, S.L.; Yu, X.Y. Stamping Nanoparticles onto the Electrode for Rapid Electrochemical Analysis in Microfluidics. Micromachines 2021, 12, 60. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef] [PubMed]
- Nagao, Y. Highly Oriented Sulfonic Acid Groups in a Nafion Thin Film on Si Substrate. J. Phys. Chem. C 2013, 117, 3294–3297. [Google Scholar] [CrossRef]
- Paul, D.K.; McCreery, R.; Karan, K. Proton Transport Property in Supported Nafion Nanothin Films by Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2014, 161, F1395–F1402. [Google Scholar] [CrossRef] [Green Version]
- Kaw, K.A.Y.; Palisoc, S.T.; Natividad, M.T. A morphological and cyclic voltammetric investigation of spin-coated hexaammineruthenium (III)-incorporated Nafion® thin films. Philipp. Sci. Lett. 2014, 7, 171–178. [Google Scholar]
- Palisoc, S.; Natividad, M.; Tadios, S. Fabrication and morphological characterization of Nafion thin films spin coated on silica. J. Optoelectron. Adv. Mater. 2014, 16, 759. [Google Scholar]
- Parnian, M.J.; Rowshanzamir, S.; Alipour Moghaddam, J. Investigation of physicochemical and electrochemical properties of recast Nafion nanocomposite membranes using different loading of zirconia nanoparticles for proton exchange membrane fuel cell applications. Mater. Sci. Energy Technol. 2018, 1, 146–154. [Google Scholar] [CrossRef]
- Liu, B.; Yu, X.Y.; Zhu, Z.; Hua, X.; Yang, L.; Wang, Z. In situ chemical probing of the electrode-electrolyte interface by ToF-SIMS. Lab Chip 2014, 14, 855–859. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, Y.; Hua, X.; Liu, S.; Zhu, Z.; Yu, X.Y. Capturing the transient species at the electrode-electrolyte interface by in situ dynamic molecular imaging. Chem. Commun. 2016, 52, 10952–10955. [Google Scholar] [CrossRef] [PubMed]
- Paparazzo, E. Use and mis-use of X-ray photoemission spectroscopy Ce3d spectra of Ce2O3 and CeO2. J. Phys. Condens. Matter 2018, 30, 343003. [Google Scholar] [CrossRef]
- Hiesgen, R.; Aleksandrova, E.; Meichsner, G.; Wehl, I.; Roduner, E.; Friedrich, K.A. High-resolution imaging of ion conductivity of Nafion® membranes with electrochemical atomic force microscopy. Electrochim. Acta 2009, 55, 423–429. [Google Scholar] [CrossRef]
- Guo, R.; Talma, A.G.; Datta, R.N.; Dierkes, W.K.; Noordermeer, J.W.M. Novel Surface Modification of Sulfur by Plasma Polymerization and its Application in Dissimilar Rubber-Rubber Blends. Plasma Chem. Plasma Process. 2010, 30, 679–695. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, S.; Ratner, B.D. ToF-SIMS quantification of albumin adsorbed on plasma-deposited fluoropolymers by partial least-squares regression. Surf. Interface Anal. 2000, 29, 837–844. [Google Scholar] [CrossRef]
- Wheeler, D.W. Kinetics and mechanism of the oxidation of cerium in air at ambient temperature. Corros. Sci. 2016, 111, 52–60. [Google Scholar] [CrossRef]
- Zhu, Y.; Jain, N.; Hudait, M.K.; Maurya, D.; Varghese, R.; Priya, S. X-ray photoelectron spectroscopy analysis and band offset determination of CeO2 deposited on epitaxial (100), (110), and (111)Ge. J. Vac. Sci. Technol. B 2014, 32, 011217. [Google Scholar] [CrossRef]
- Grygar, T.; Marken, F.; Schröder, U.; Scholz, F. Electrochemical Analysis of Solids. A Review. Collect. Czechoslov. Chem. Commun. 2002, 67, 163–208. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, P.K.; Bard, A.J. Clay-Modified Electrodes. J. Am. Chem. Soc. 1983, 105, 5691–5693. [Google Scholar] [CrossRef]
- Murugan, R.; Ravi, G.; Yuvakkumar, R.; Rajendran, S.; Maheswari, N.; Muralidharan, G.; Hayakawa, Y. Pure and Co doped CeO2 nanostructure electrodes with enhanced electrochemical performance for energy storage applications. Ceram. Int. 2017, 43, 10494–10501. [Google Scholar] [CrossRef]
- Yao, J.; Wu, F.; Qiu, X.; Li, N.; Su, Y. Effect of CeO2-coating on the electrochemical performances of LiFePO4/C cathode material. Electrochim. Acta 2011, 56, 5587–5592. [Google Scholar] [CrossRef]
- Rosalbino, F.; Angelini, E.; De Negri, S.; Saccone, A.; Delfino, S. Influence of the rare earth content on the electrochemical behaviour of Al–Mg–Er alloys. Intermetallics 2003, 11, 435–441. [Google Scholar] [CrossRef]



| No. | Nafion (%) | Spin Rate (rpm) | Curing Time (min) | Footprint (mm × mm2) | Avg. Thickness (µm) | Note |
|---|---|---|---|---|---|---|
| 1 | 5 | 500 | 30 | 2.1 × 2.2 | 0.13 | Nafion only |
| 2 | 5 | 1000 | 30 | 2.1 × 2.1 | 0.12 | Nafion only |
| 3 | 20 | 500 | 30 | 2.4 × 1.7 | 1.18 | Nafion only |
| 4 | 20 | 1000 | 30 | 2.0 × 2.1 | 0.83 | Nafion only |
| 5 | 20 | 500 | 30 | 2.5 × 2.4 | 14.21 | Nafion over CeO2 deposit |
| 6 | 20 | 1000 | 30 | 1.8 × 1.8 | 6.45 | Nafion over CeO2 deposit |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, J.; Buck, E.C.; Riechers, S.L.; Tripathi, S.; Strange, L.E.; Engelhard, M.H.; Yu, X.-Y. Studying Corrosion Using Miniaturized Particle Attached Working Electrodes and the Nafion Membrane. Micromachines 2021, 12, 1414. https://doi.org/10.3390/mi12111414
Son J, Buck EC, Riechers SL, Tripathi S, Strange LE, Engelhard MH, Yu X-Y. Studying Corrosion Using Miniaturized Particle Attached Working Electrodes and the Nafion Membrane. Micromachines. 2021; 12(11):1414. https://doi.org/10.3390/mi12111414
Chicago/Turabian StyleSon, Jiyoung, Edgar C. Buck, Shawn L. Riechers, Shalini Tripathi, Lyndi E. Strange, Mark H. Engelhard, and Xiao-Ying Yu. 2021. "Studying Corrosion Using Miniaturized Particle Attached Working Electrodes and the Nafion Membrane" Micromachines 12, no. 11: 1414. https://doi.org/10.3390/mi12111414