Novel Platform for Regulation of Extracellular Vesicles and Metabolites Secretion from Cells Using a Multi-Linkable Horizontal Co-Culture Plate
Abstract
:1. Introduction
- The top container is invisible: Neither cell line could be simultaneously observed under a microscope. The cells in the culture vessel are usually observed from the bottom of the vessel because the culture fluid hinders observation from the top of the vessel. The upper container cannot be observed because of the short focal length of the microscope. Therefore, cells were only observed in the lower parts of the VTCP-type vessels (Figure S1a).
- Different materials could impact the results: The cells are not cultured on the same type of material surface: one group of cells is cultured on the filter material and the other on a plastic material; this difference could significantly impact the experimental results (Figure S1b).
- High cell densities are not considered: A high density of cells may prevent co-culture due to blockage of the filter. As the Boyden chamber was initially invented to evaluate cell invasion [2,8], scenarios in which cells were present at high densities were not considered (Figure S1c).
2. Materials and Methods
2.1. Device Design and Fabrication
2.2. Cell Lines
2.3. Microscopy
2.4. Cell Viability
2.5. Nanoparticle Tracking Analysis
2.6. EV Isolation
2.7. Whole-Cell Protein Extracts
2.8. Measurement of Glucose, Lactate, and Ammonium Ion Concentrations
2.9. Protein Assay and Western Blotting
2.10. Silver Staining
3. Results
3.1. Device Design and Description
3.2. Experimental Design
The Experimental Design for Evaluating HTCP
3.3. Measurement Results
3.3.1. Differences in Mass Transfer Due to Differences in Wells Containing the Materials
3.3.2. Differences in Mass Transfer Due to Differences in Wells in Which the Cells Were Placed
3.3.3. Validation of Protein Pass Rate
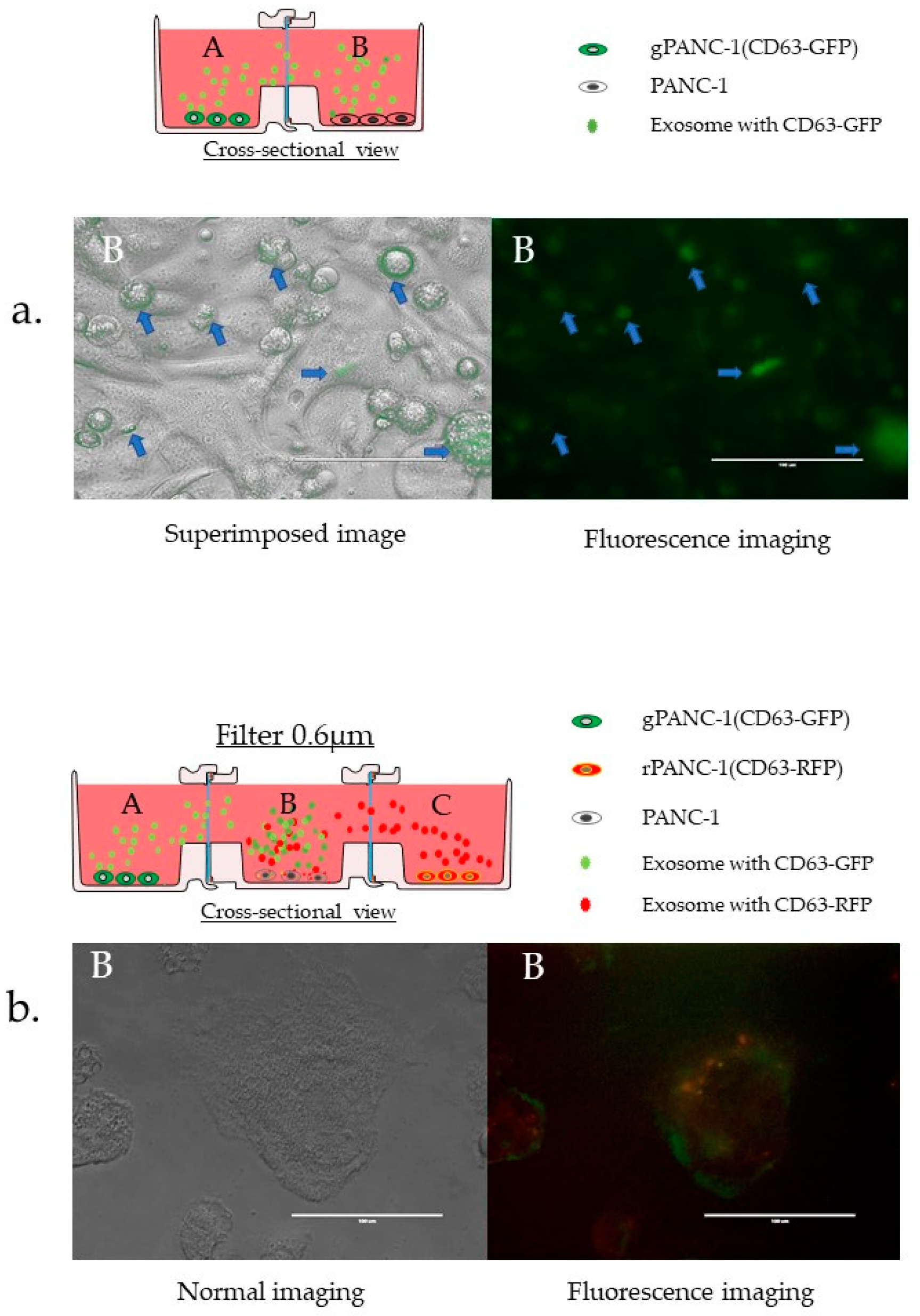
3.3.4. Difference in Exosome Transfer Due to Difference in the Pore Size of the Filter
3.3.5. Comparison of the VTCP and HTCP
3.3.6. Confirmation of EVs Migration and Cellular Uptake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DMEM | Dulbecco’s modified Eagle’s medium |
| EVs | extracellular vesicles |
| GFP | green fluorescent protein |
| gPANC1 | green fluorescence-labeled PANC-1 cell |
| HTCP | horizontal connection type co-culture plate |
| VTCP | vertical connection type co-culture plate |
| rPANC1 | red fluorescence-labeled PANC-1 cell |
References
- Shimasaki, T.; Yamamoto, S.; Arisawa, T. Exosome Research and Co-culture Study. Biol. Pharm. Bull. 2018, 41, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Boyden, S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J. Exp. Med. 1962, 115, 453–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albini, A.; Iwamoto, Y.; Kleinman, H.K.; Martin, G.R.; Aaronson, S.A.; Kozlowski, J.M.; McEwan, R.N. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987, 47, 3239–3245. [Google Scholar] [PubMed]
- Berginc, K.; Kristl, A. Transwell-grown HepG2 cell monolayers as in vitro permeability model to study drug-drug or drug-food interactions. J. Med. Food 2011, 14, 135–139. [Google Scholar] [CrossRef]
- Smith, M.P.; Young, H.; Hurlstone, A.; Wellbrock, C. Differentiation of THP1 Cells into Macrophages for Transwell Co-culture Assay with Melanoma Cells. Bio. Protoc. 2015, 5, e1638. [Google Scholar] [CrossRef] [Green Version]
- Duan, P.; Xu, H.; Zeng, Y.; Wang, Y.; Yin, Z.Q. Human bone marrow stromal cells can differentiate to a retinal pigment epithelial phenotype when co-cultured with pig retinal pigment epithelium using a transwell system. Cell. Physiol. Biochem. 2013, 31, 601–613. [Google Scholar] [CrossRef]
- Sumi, S.; Kawagoe, M.; Abe, R.; Yanai, G.; Yang, K.C.; Shirouzu, Y. A multiple-funnels cell culture insert for the scale-up production of uniform cell spheroids. Regen. Ther. 2017, 7, 52–60. [Google Scholar] [CrossRef]
- Boyden, S. Cellular Recognition of Foreign Matter. Int. Rev. Exp. Pathol. 1963, 2, 311–356. [Google Scholar] [PubMed]
- Kosaka, N.; Yoshioka, Y.; Fujita, Y.; Ochiya, T. Versatile roles of extracellular vesicles in cancer. J. Clin. Investig. 2016, 126, 1163–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobrie, A.; Colombo, M.; Raposo, G.; Thery, C. Exosome secretion: Molecular mechanisms and roles in immune responses. Traffic 2011, 12, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Chargaff, E.; West, R. The biological significance of the thromboplastic protein of blood. J. Biol. Chem. 1946, 166, 189–197. [Google Scholar] [CrossRef]
- Wolf, P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Royo, F.; Thery, C.; Falcon-Perez, J.M.; Nieuwland, R.; Witwer, K.W. Methods for Separation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey Performed by the ISEV Rigor and Standardization Subcommittee. Cells 2020, 9, 1955. [Google Scholar] [CrossRef] [PubMed]
- Iliescu, F.S.; Vrtacnik, D.; Neuzil, P.; Iliescu, C. Microfluidic Technology for Clinical Applications of Exosomes. Micromachines 2019, 10, 392. [Google Scholar] [CrossRef] [Green Version]
- Itami, Y.; Miyake, M.; Ohnishi, S.; Tatsumi, Y.; Gotoh, D.; Hori, S.; Morizawa, Y.; Iida, K.; Ohnishi, K.; Nakai, Y.; et al. Disabled Homolog 2 (DAB2) Protein in Tumor Microenvironment Correlates with Aggressive Phenotype in Human Urothelial Carcinoma of the Bladder. Diagnostics 2020, 10, 54. [Google Scholar] [CrossRef] [Green Version]
- Oishi, M.; Munesue, S.; Harashima, A.; Nakada, M.; Yamamoto, Y.; Hayashi, Y. Aquaporin 1 elicits cell motility and coordinates vascular bed formation by downregulating thrombospondin type-1 domain-containing 7A in glioblastoma. Cancer Med. 2020, 9, 3904–3917. [Google Scholar] [CrossRef]
- Garcia, N.A.; Ontoria-Oviedo, I.; Gonzalez-King, H.; Diez-Juan, A.; Sepulveda, P. Glucose Starvation in Cardiomyocytes Enhances Exosome Secretion and Promotes Angiogenesis in Endothelial Cells. PLoS ONE 2015, 10, e0138849. [Google Scholar] [CrossRef] [Green Version]
- Nakai, W.; Yoshida, T.; Diez, D.; Miyatake, Y.; Nishibu, T.; Imawaka, N.; Naruse, K.; Sadamura, Y.; Hanayama, R. A novel affinity-based method for the isolation of highly purified extracellular vesicles. Sci. Rep. 2016, 6, 33935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasquier, J.; Magal, P.; Boulange-Lecomte, C.; Webb, G.; Le Foll, F. Consequences of cell-to-cell P-glycoprotein transfer on acquired multidrug resistance in breast cancer: A cell population dynamics model. Biol. Direct 2011, 6, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wintermute, E.H.; Silver, P.A. Emergent cooperation in microbial metabolism. Mol. Syst. Biol. 2010, 6, 407. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Futaki, S. Combined treatment with a pH-sensitive fusogenic peptide and cationic lipids achieves enhanced cytosolic delivery of exosomes. Sci. Rep. 2015, 5, 10112. [Google Scholar] [CrossRef]
- Ramteke, A.; Ting, H.; Agarwal, C.; Mateen, S.; Somasagara, R.; Hussain, A.; Graner, M.; Frederick, B.; Agarwal, R.; Deep, G. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol. Carcinog. 2015, 54, 554–565. [Google Scholar] [CrossRef] [Green Version]
- Stadie, J.; Gulitz, A.; Ehrmann, M.A.; Vogel, R.F. Metabolic activity and symbiotic interactions of lactic acid bacteria and yeasts isolated from water kefir. Food Microbiol. 2013, 35, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Shi, L.; Esfandiari, L. An Electrokinetically-Driven Microchip for Rapid Entrapment and Detection of Nanovesicles. Micromachines 2020, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.L.; Khosroheidari, M.; Kanchi Ravi, R.; DiStefano, J.K. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int. 2012, 82, 1024–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rupp, A.K.; Rupp, C.; Keller, S.; Brase, J.C.; Ehehalt, R.; Fogel, M.; Moldenhauer, G.; Marme, F.; Sultmann, H.; Altevogt, P. Loss of EpCAM expression in breast cancer derived serum exosomes: Role of proteolytic cleavage. Gynecol. Oncol. 2011, 122, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Gyorgy, B.; Modos, K.; Pallinger, E.; Paloczi, K.; Pasztoi, M.; Misjak, P.; Deli, M.A.; Sipos, A.; Szalai, A.; Voszka, I.; et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood 2011, 117, e39–e48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006. Chapter 3, Unit 3 22. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Aleckovic, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; Garcia-Santos, G.; Ghajar, C.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hood, J.L.; San, R.S.; Wickline, S.A. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011, 71, 3792–3801. [Google Scholar] [CrossRef] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimasaki, T.; Yamamoto, S.; Omura, R.; Ito, K.; Nishide, Y.; Yamada, H.; Ohtomo, K.; Ishisaka, T.; Okano, K.; Ogawa, T.; et al. Novel Platform for Regulation of Extracellular Vesicles and Metabolites Secretion from Cells Using a Multi-Linkable Horizontal Co-Culture Plate. Micromachines 2021, 12, 1431. https://doi.org/10.3390/mi12111431
Shimasaki T, Yamamoto S, Omura R, Ito K, Nishide Y, Yamada H, Ohtomo K, Ishisaka T, Okano K, Ogawa T, et al. Novel Platform for Regulation of Extracellular Vesicles and Metabolites Secretion from Cells Using a Multi-Linkable Horizontal Co-Culture Plate. Micromachines. 2021; 12(11):1431. https://doi.org/10.3390/mi12111431
Chicago/Turabian StyleShimasaki, Takeo, Satoko Yamamoto, Risa Omura, Kagenori Ito, Yumiko Nishide, Hideki Yamada, Kazumi Ohtomo, Tomo Ishisaka, Keiichiro Okano, Takenori Ogawa, and et al. 2021. "Novel Platform for Regulation of Extracellular Vesicles and Metabolites Secretion from Cells Using a Multi-Linkable Horizontal Co-Culture Plate" Micromachines 12, no. 11: 1431. https://doi.org/10.3390/mi12111431
APA StyleShimasaki, T., Yamamoto, S., Omura, R., Ito, K., Nishide, Y., Yamada, H., Ohtomo, K., Ishisaka, T., Okano, K., Ogawa, T., Tsuji, H., Matsuo, Y., Minamoto, T., Tomosugi, N., Ferain, E., & Ochiya, T. (2021). Novel Platform for Regulation of Extracellular Vesicles and Metabolites Secretion from Cells Using a Multi-Linkable Horizontal Co-Culture Plate. Micromachines, 12(11), 1431. https://doi.org/10.3390/mi12111431





