The measurement of the absorbance of a colorimetric reaction is tied to the sample geometry. The absorbance is exponentially coupled to the optically penetrated distance. For this purpose, a laser is set up and its emission is attenuated when it passes through a phosphate-selective color-forming reagent.
2.1. Design and Fabrication
In our work, the emission source is an organic second-order DFB laser in a sandwich construction. Optical emission is achieved through a thin layer of an organic semiconductor polymer. Organic semiconductors are conjugated molecules, with the semiconducting properties arising from the overlap of molecular orbitals [
14]. Organic semiconductors, which intrinsically offer a four-level laser architecture, achieve their gain by the recombination of excited singlet exciton states. While excited singlet states contribute to optical gain, other excited states, such as triplet excitons and polarons, are considered detrimental to lasing [
15]. Both triplet excitons and polarons are the major product of charge recombination [
15,
16]; thus, efficient organic lasers mostly employ pulsed optical pumping [
17,
18] (see section Optical Setup below). In addition to the pump configuration used in this work, it is also possible to pump the organic laser with a pulsed inorganic light emitting diode or laser diode [
19,
20,
21].
The organic semiconductor materials used have high absorption for the pump radiation [
16], which enables a thin nanometer-scale organic layer to act as a planar waveguide. The superposition of frequency-selective diffraction at a spatial corrugation of the boundary surface and the guiding planar layer leads to a DFB resonator structure.
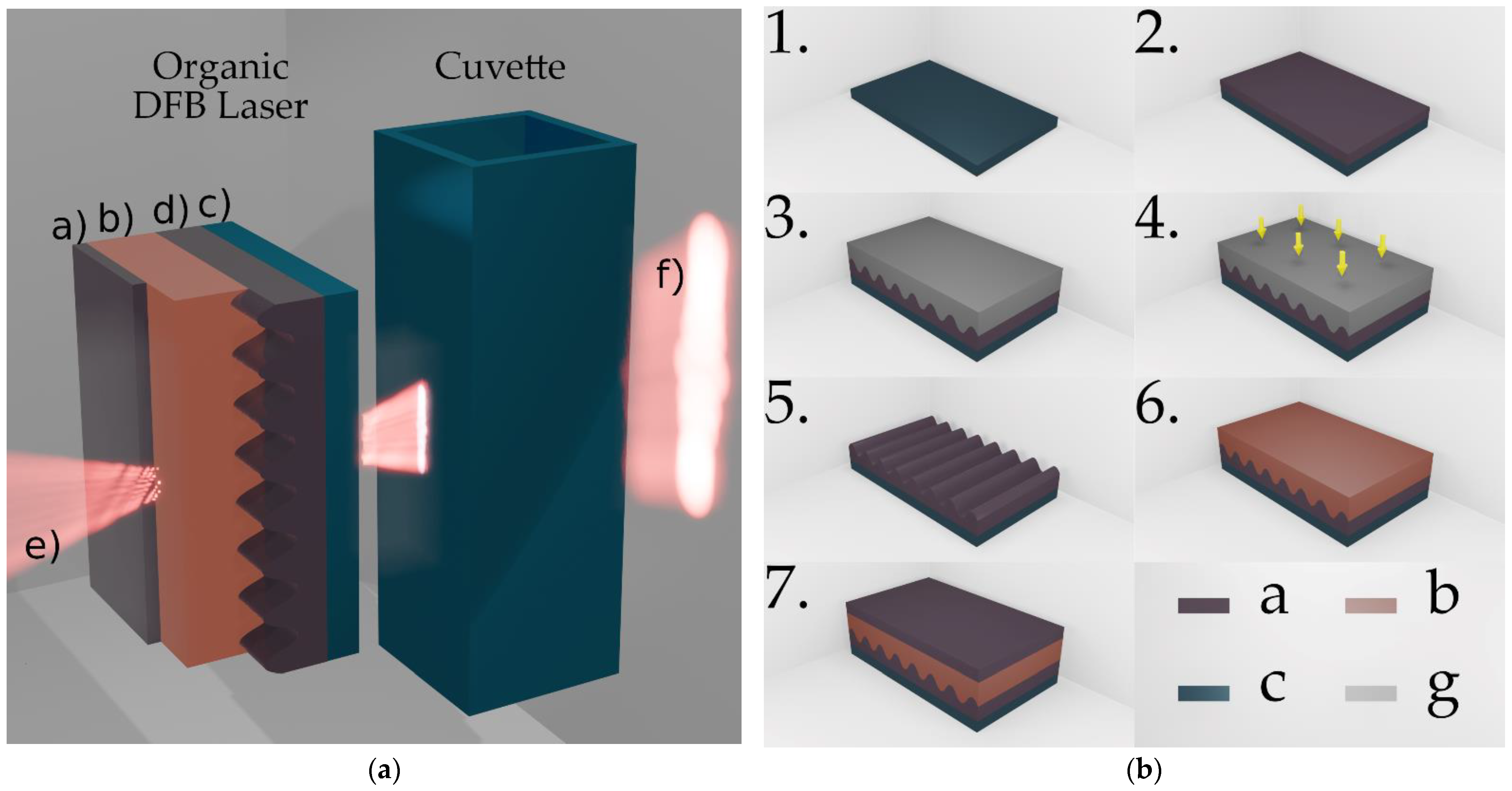
The process steps for manufacturing the organic DFB laser are illustrated in
Figure 1b. A schematic cross section of the device is shown in
Figure 1a. The three functional laser layers DFB substrate, organic emission layer and encapsulation layer are stacked on a commercially available microscope glass slide, which from now on is denoted as an auxiliary substrate (see
Figure 1b Step 1).
The DFB substrate carries a sinusoidal one-dimensional grating with a period of
. In order to create the DFB substrate, a replica of a master grating was molded into a Fluorolink
® MD700 (PFPE-urethane methacrylate) layer on top of an auxiliary substrate. Here, the master grating used is a segment of a patterned fused silica wafer produced by laser interference lithography. MD700, diluted with 2% by weight of the photoinitiator Darocur
® 1173, was deposited on the auxiliary substrate (
Figure 1b Step 2). The master was lightly pressed into the MD700 with the patterned surface pointing downwards (
Figure 1b Step 3). The ultraviolet (UV)-curable MD700 was exposed for 60 s through the master using a mercury vapor lamp with a power density of
(
Figure 1b Step 4). Subsequently, the master can be removed and later be reused, the crosslinked MD700 now carries a negative replication of the master grating (
Figure 1b Step 5). The depth of the sinusoidal structure in the MD700 with
agrees well with the structure depth of the glass master with
. The active organic emission layer consists of a guest–host system of MEH–PPV (ADS100RE) and F8BT (ADS233YE). The guest, poly(2-methoxy,5-(2′-(ethyl)hexyloxy)-p-phenylene vinylene), and the host, poly(9,9-dioctylfluorene-alt-benzothiadiazole), were dissolved in toluene at an experimentally optimized weight ratio of 6%:94%. In a spin coating process, the active organic material was applied to the DFB substrate at a spin speed of 750 rpm in a toluene-saturated atmosphere (
Figure 1b Step 6). This results in an approximately
thick layer that forms the optical waveguide of the laser cavity. To reduce photooxidation and increase the life expectancy of the laser, the organic layer is encapsulated with an additional MD700 layer (
Figure 1b Step 7). As illustrated by the beam lobes in
Figure 1a, all layers have a high transmittance in the visible spectrum and laser emission can be radiated in bottom and top directions.
2.2. Methods and Materials
The basic detection principle follows the Beer–Lambert law, a traditional theory of attenuation of radiation intensity when passing an absorbing sample. In our case, attenuation of transmitted light is used to determine an analyte concentration. This can be expressed as follows:
where
is the absorbance of the sample at a specified wavelength
,
is the initial light intensity before passing the sample,
is the transmitted light intensity behind the sample,
is the optical path length,
is the specific extinction coefficient of the analyte, and
is the concentration of the analyte. For the determination of the absorbance according to Beer–Lambert, the initial light intensity and the transmitted light intensity behind the sample, independent of other losses, must be known.
A beam splitter is conventionally used to determine the initial intensity
. Using a beam splitter increases the complexity of miniaturized setups, which is why the excitation energy of the emission source is often used as a measure of the emission intensity [
8,
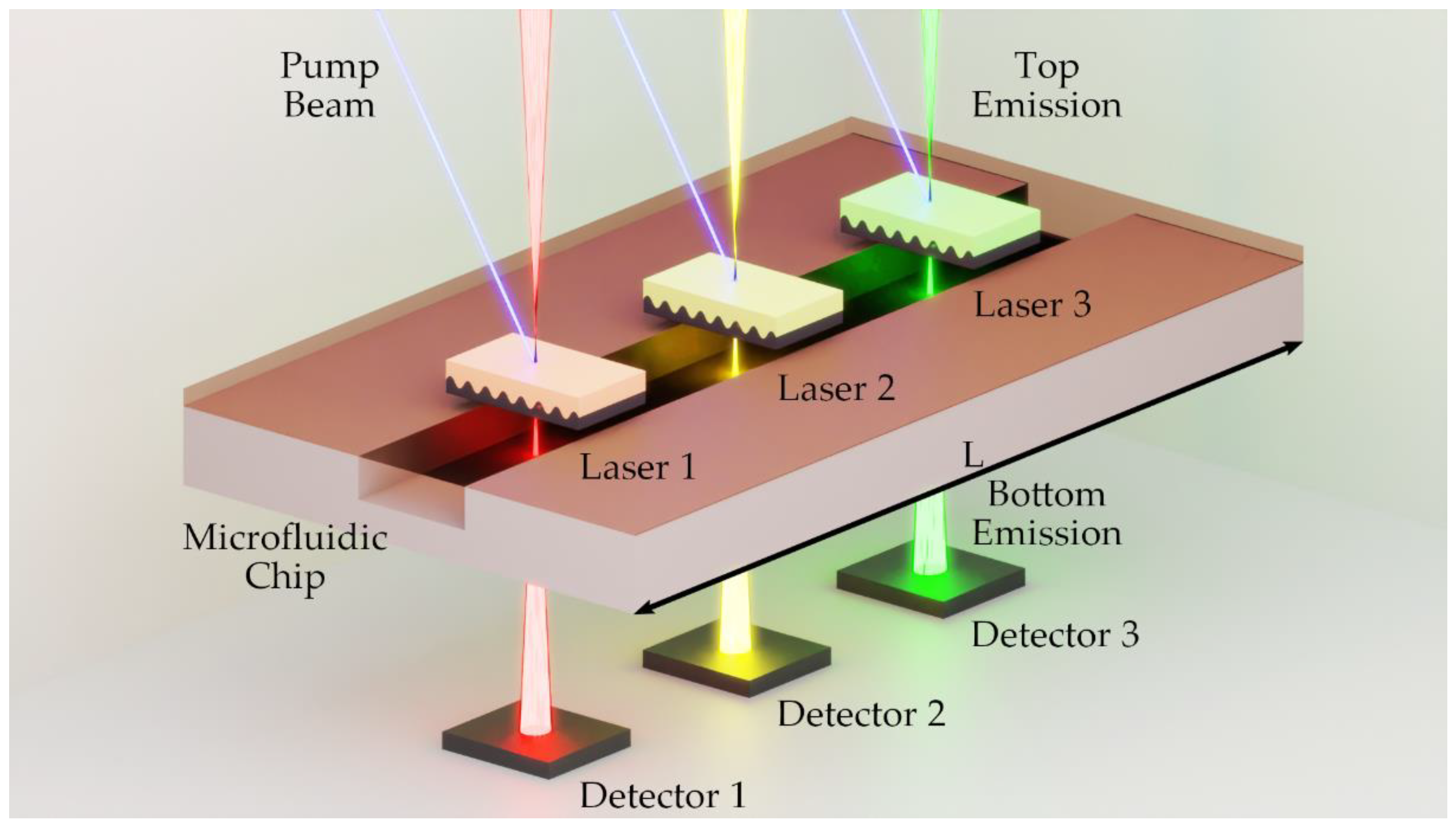
22]. This approach simplifies the measurement setup but runs the risk that changes in the emission source intensity will lead to measurement errors. To avoid both problems, we used the surface emission in two different directions of an organic second-order DFB laser [
23]. A DFB resonator grating diffracts an incident wave into transmitted and reflected orders, comparable to a beam splitter. As shown in
Figure 2, the emission intensity of the organic DFB laser is split into top
and bottom
directions. The two surface emissions are based on the same diffraction condition and are identical in emission wavelength and emission intensity due to the symmetry of the layers [
24].
To use the two-sided emission for absorbance measurements, the laser is placed between two detectors. One detector is arranged for each surface emission in top and bottom emission directions. This provides the possibility to measure intensity in the top and bottom directions for each laser pulse. The fluid to be measured is placed between the laser and a detector. The cuvette containing a sample is therefore in the beam path of the bottom emission of the laser (see
Figure 2). As mentioned above, the absorbance can be determined with the help of the Beer-Lambert law. The optical attenuation considered here is only due to the absorption of an optical wave by a medium. To determine the absorbance, at least two measurements must be made.
A reference measurement is performed, where the laser emission transmits a cuvette filled with deionized (DI) water, and a solution measurement is performed, where the emission transmits a cuvette filled with a measurement solution. Each of the two measurements generates two values: the intensity in the top direction
and the intensity in the bottom direction
. Both intensities are then used to calculate a reference ratio
and a solution ratio
(Equations (2) and (3)).
The two emission ratios differ in scattering and absorption losses during optical penetration of the cuvettes. The reference ratio includes losses due to scattering as well as absorption caused by the DI water and the cuvette. The solution ratio includes approximately the same scattering losses as the reference ratio. In addition, the solution ratio also depends on the absorption losses of the analyte. When additional scattering in the measurement solution is negligible and the emission ratio of the laser is constant in the top and bottom directions, the ratio of and is equal to the ratio of the intensity before and after passing the absorbing solution (Equation (4)). Hence, Equation (5) shows the Beer–Lambert law (Equation (1)) with substituted intensity ratios.
Here, the analyte to be detected is phosphate. Since phosphate itself has no absorbance line in the visible spectrum, a color forming reaction is used for detection [
5,
10,
25,
26]. To determine the phosphate concentration, a colorimetric reaction known as the MG method is used. It has a fast color forming reaction without heating, and a larger sensitivity than the mostly used molybdenum blue reaction [
26].
2.3. Chemicals and Reagents
For phosphate detection, a working solution of the analyte (phosphate) and a MG reagent are mixed. For all stock and calibration solutions, DI water produced by an ultrapure Direct-Q Water treatment system was used, which has a conductivity of .
The MG reagent is based on three stock solutions. A sulfuric acid was prepared from a commercially purchased solution of sulfuric acid (; Merck Chemicals Ltd., Darmstadt, Germany). Then a sodium molybdate solution was prepared by mixing 500 mL DI water and 10 g sodium molybdate dihydrate (; Merck Chemicals Ltd., Darmstadt, Germany).
To obtain a homogeneous fluid phase [
5,
25], a protective colloid based on
poly(vinyl alcohol) (PVA) (
; Merck Chemicals Ltd., Darmstadt, Germany) was dissolved in
DI water heated to near boiling point. This solution was filtered with a folded filter paper disc (Grade 589/3 blue) and poured into a graduated flask. Then,
of MG G (
; Thermo Fisher GmbH, Kandel, Germany) was added to
of the PVA solution. This solution was homogenized and mixed with DI water to provide a total volume of
of the MG reagent.
On the basis of a potassium phosphate stock solution (; Merck Chemicals Ltd., Darmstadt, Germany) with a phosphate concentration of , different phosphate working solutions were prepared. The used phosphate working solutions correspond to a phosphate concentration range from to . To achieve a high accuracy of the phosphate working solutions, only the highest phosphate working solution () was prepared from the phosphate solution. The highest working solution () was then diluted to the smaller phosphate working solutions of , , , , and by mixing with DI water. In addition, a phosphate working solution was prepared from the 0.5 mg/L phosphate working solution.
2.4. Measurement Procedure
Phosphate detection based on the MG method enables the detection of low concentrations and provides low cross sensitivity [
25,
27]. For our measurements, the above-mentioned stock solutions were mixed, which resulted in a MG reagent. This MG reagent together with the phosphate sample led to the phosphate detection. The MG G solution (
was added to
sulfuric acid (0.25 mL) and filled with
sodium molybdate solution (
. After 10 min color development of the MG reagent (
, a phosphate sample (
was pipetted in. The MG reagent together with a phosphate working solution was filled into a cuvette, denoted from now on as “MG+P”. The cuvette was left to rest for 60 min for colorimetric development before measuring the absorbance.
The absorbance of every sample was measured in the organic DFB laser absorption spectrometer (see
Figure 1a and
Figure 2) and, for comparison, it was also measured using a commercial UV-VIS spectrometer (UV-1800; Shimadzu, Kyōto, Japan). The detection range of the MG method lies between
and
phosphate [
25]. At higher phosphate concentrations of more than
, turbidity occurs in the sample, which makes photometric absorption measurements impossible [
10].
2.5. Optical Setup
Figure 3 and
Figure 4 outline the optical pump setup and emission measurement setup of the organic DFB laser in our laboratory. With a cuvette being placed between the organic laser and the bottom emission detection (
Figure 4d)), we also used this setup as our organic laser absorption spectrometer. Sketched in blue is the UV pump radiation required to optically pump the organic DFB laser. The pump pulse of a frequency tripled passively Q-switched ND:YAG laser (FTSS355-Q2; CryLas, Berlin, Germany) with a wavelength of
exhibits a pulse duration of
. All measurements are carried out at a pump pulse repetition rate of
. The use of neutral density (ND) filters as a variable ND filter mounted on a stage combined with a revolver equipped with discrete ND filters allows variation of the pump energy. A beam splitter (92:8) was positioned in the pump beam behind the ND filters for pump pulse energy monitoring. Pump energy calibration of the monitor photodiode was performed using an energy sensor (Pe10b; Gentec, Quebec, QC, Canada). A focusing unit consisting of a collimator and two plano-convex lenses was used to create an elliptical pump spot with variable beam diameter in length (
to
) and fixed diameter in width (
). The organic DFB laser was arranged at an angle (
) in the pump beam. The oblique pump configuration at a steep angle allows the measurement of organic laser emission at small distances without affecting the pump radiation. In addition, the steep angle prevents the sample fluid from being exposed to the UV pump source. The actual pump spot on the surface of the organic laser thus also exhibited an elliptical shape but with transformed dimensions (
,
), with the longer beam waist oriented orthogonally to the grating lines of the DFB resonator. The organic DFB laser emission in both directions is outlined in red in
Figure 3. The detection of the emission of the organic DFB laser as well as the monitoring of the pump emission was done with amplified Si Photodetectors (PDA10A; fixed gain; Thorlabs, Newton, MA, USA). In order to calibrate the pump energy monitor diode, the organic DFB laser was removed from the setup and an energy sensor was used instead. The photodetectors were read out with an oscilloscope (MSO9254A; Agilent, Santa Clara, CA, USA). To avoid discrepancies in bottom and top emission detection, nominally identical photodiodes and connectors were used.
As mentioned above, we used an organic DFB laser as emission source for absorption spectroscopy. A DFB resonator generates feedback based on a periodically distributed change of the refractive index and the optical gain. Resonance for the guided mode is enabled in close vicinity of the Bragg wavelength (
) given by the Bragg condition [
14,
28].
where
is the period of the spatial modulation,
is the effective refractive index of the guided mode, and
is the order of diffraction. Thus, the Bragg condition describes the wavelength-selective resonance based on the diffraction of a guided mode at a periodically changing interface [
29]. A change in lasing wavelength can be achieved by changes in periodicity [
21,
30,
31] or guidance of the mode. One common way to change the guiding of the mode is to vary the thickness of the core layer of the planar waveguide [
14,
32,
33]. The laser emission wavelength can thus be varied through changing the thickness of the organic layer that forms the core layer of the waveguide.
Variation of the emission wavelength offers the possibility to optimize the laser to the color forming reaction for measuring a specific analyte.
Figure 5a plots the absorbance of MG+P in the visible spectrum for three different phosphate concentrations. In the visible spectrum, MG+P shows two dominant absorption peaks. The two peaks
and
, mark possible measurement wavelengths. Both absorption peaks could potentially be used to determine the phosphate concentration. Our absorbance measurements were performed at
because this peak offers the highest measurement sensitivity. Our organic laser was designed for an emission wavelength close to the absorption maximum
. The resulting emission spectrum of the organic laser with a peak wavelength of
is shown in
Figure 5a.
Optical alignment is performed with two five-axis translation stages (i5000; Luminos, Ottawa, Canada). The organic DFB laser is mounted on one stage (
Figure 4g) and the detection instruments for recording the top and bottom organic laser emission on another (
Figure 4h). In order to record both the optical spectrum and the intensity, the detection unit is mounted in a cage system (SR 30mm; Thorlabs, Newton, USA) on the translation stage. The fibers of the fiber-coupled spectrometer can easily be replaced by photodiodes without change in alignment. Furthermore, the detection units for top and bottom emission are mechanically connected to each other and can be moved symmetrically around the organic DFB laser. The emission spectrum was used as orientation for the alignment of the optical measuring equipment. The shift of spectral emission as a function of divergence angle of the organic DFB laser must be taken into account when aligning the measuring equipment [
34]. Using two multimode fibers (
) and a spectrometer, the fibers fixed to each other are aligned to the pump spot. Using rotational alignment of the fibers around the pump spot, fine tuning in the adjustment of the spectral emission is achieved. The inset in
Figure 5b shows emission spectra in top and bottom direction, measured with a spectrometer (USB 2000; Ocean Optics, Orlando, FL, USA). The emission spectra in the different emission directions show hardly any spectral differences. The peak wavelengths are
and
, and the spectral bandwidths are
and
. The emission in the top direction is approximately
times smaller than the bottom emission. To avoid spectral differences due to measurement set up, both spectra were measured with the same spectrometer. Therefore, the spectrum of the pulsed laser was measured in each emission direction one after the other. The emissions in top and bottom direction are, thus, not associated with the same pump pulse. Any deviation in the pump pulse causes deviations in emission intensity. Both measurement fibers showed no discernible deviation in alignment to the surface normal of the organic laser. Fibers were positioned at a distance of
to the laser.
Examination of the laser characteristics is a common option to prove laser activity [
35]. Thus, the optical output energy is plotted in
Figure 5b for different pump energies. Laser action occurs above the laser threshold, where the slope efficiency is dramatically increased by the transition from spontaneous emission to stimulated emission. The threshold is reached at the same pump energy density for top (
) and bottom (
) emissions. The emission ratio of the organic DFB laser in top and bottom emissions appears to differ during measurement of the laser characteristics (laser output energy as a function of pump energy). The ratio of the slope efficiencies of top and bottom emissions is 0.88 ± 0.035, which deviates slightly from the theoretical prediction of 1 [
24]. In order to achieve different pump energy densities, the optical power of the pump pulse must be varied. This energy variation is done by mechanical ND filters. It is assumed that small mechanical interventions in the pump beam guidance, which occur by mechanically shifting the ND filters, lead to a local change of the pump spot. The local change in pump position on the organic laser could lead to a discrepancy in measurement acquisition. The photodiodes aligned in advance with the pump position then no longer measure in the center of the organic DFB laser beam. Measurements carried out after realigning the diodes gave an emission ratio of 1, as predicted by Streifer et al. [
24].
2.6. Data Acquisition and Processing
As mentioned previously, the emission intensity is measured with photodiodes. The acquisition of the pulsed DFB laser signals with free beam photodiodes was performed at a distance of 40 mm, which compensates for the difference between the active area of the photodiode and the area of the fiber core. It is known from literature that the pulse duration of organic laser emission correlates closely with the pump pulse duration, which here is 1.9 ns [
36].
Figure 6a,b show the photovoltage
of the photodiodes over time for an organic laser pulse. The observed pulse duration is three to five times longer than the pump pulse and varies in duration.
To determine the absorbance of a sample according to the Beer–Lambert law, the optical intensity has to be measured (see Equation (1)). According to Equation (7), the intensity should be directly proportional to . Our pulsed measurements suffer from low bandwidth () of the employed photodiodes and transimpedance amplifiers. Transimpedance circuits can be described by a second-order transfer function, which leads to overshoots with a damped decaying behavior and increased rise times. Since we acquired our absorbance data by calculating signal ratios, differences in the transfer function of the measuring channels have an effect on the measured ratio. However, the use of nominally identical photodiodes, amplifiers, connectors as well as an identical data acquisition scheme keep these differences negligible. Because both laser pulses (emitted from top and bottom) originate from the same emission source, their actual pulse duration must be identical. The deformation of the signal due to the electronic circuit and its energy buffering properties can be neglected since the physical pulses differ only in their pulse height and not in their duration.
Based on this, it is possible to use the emitted pulse energy
instead of intensity for further calculations. According to Equation (8), the energy
of a pulse is proportional to the intensity
integrated over the pulse duration. The solid angle
is defined by the distances and the active area of the photodiode.
The emitted pulse energy was thus obtained by integration of the main peak of the photovoltage .
For the determination of the absorbance, the ratio of top and bottom emission intensities is needed (see Equation (5)). As outlined in Equation (9) and explained above,
and
can be replaced by
and
.