Ultrafast Deep-Ultraviolet Laser-Induced Voltage Response of Pyrite
Abstract
:1. Introduction
2. Materials and Methods
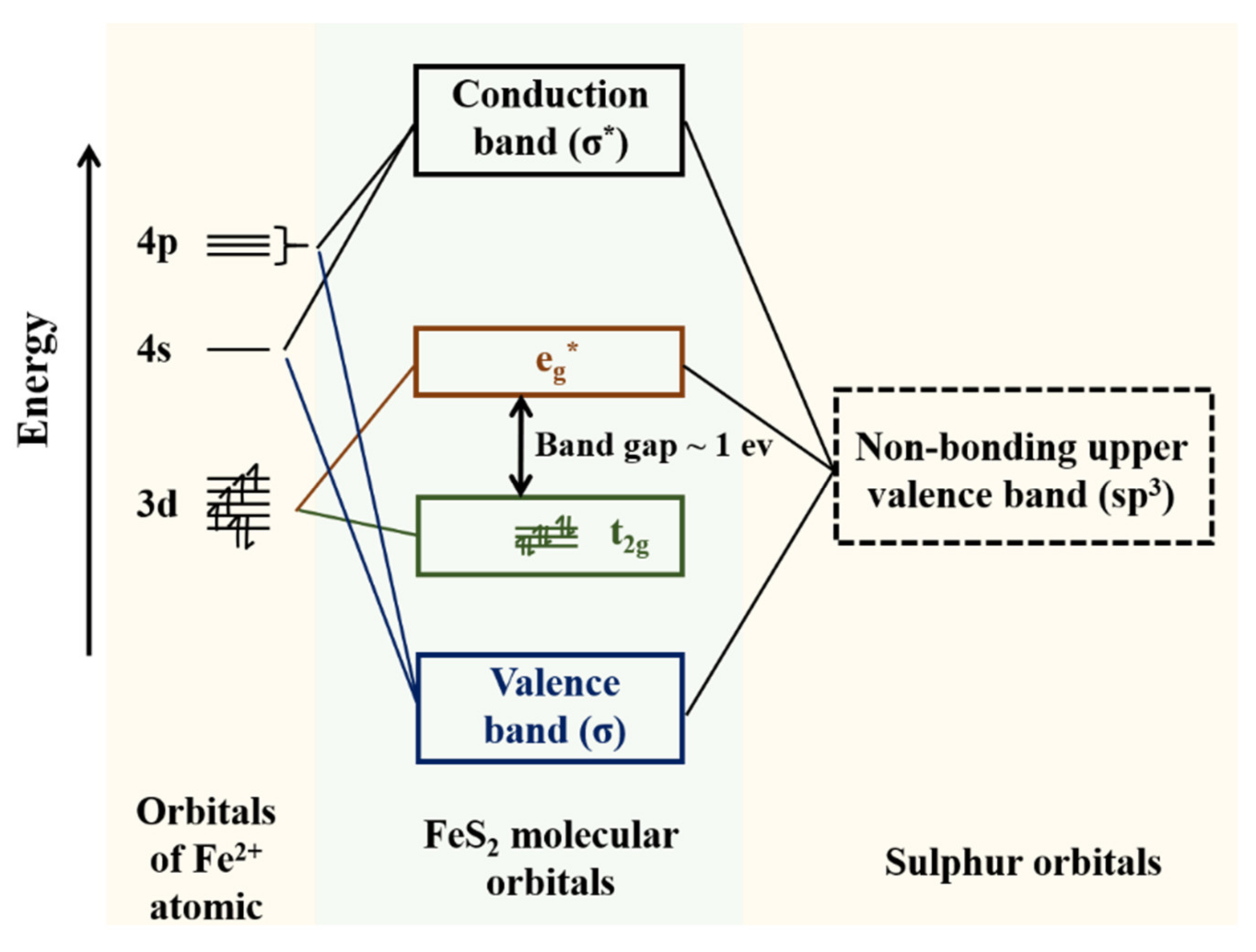
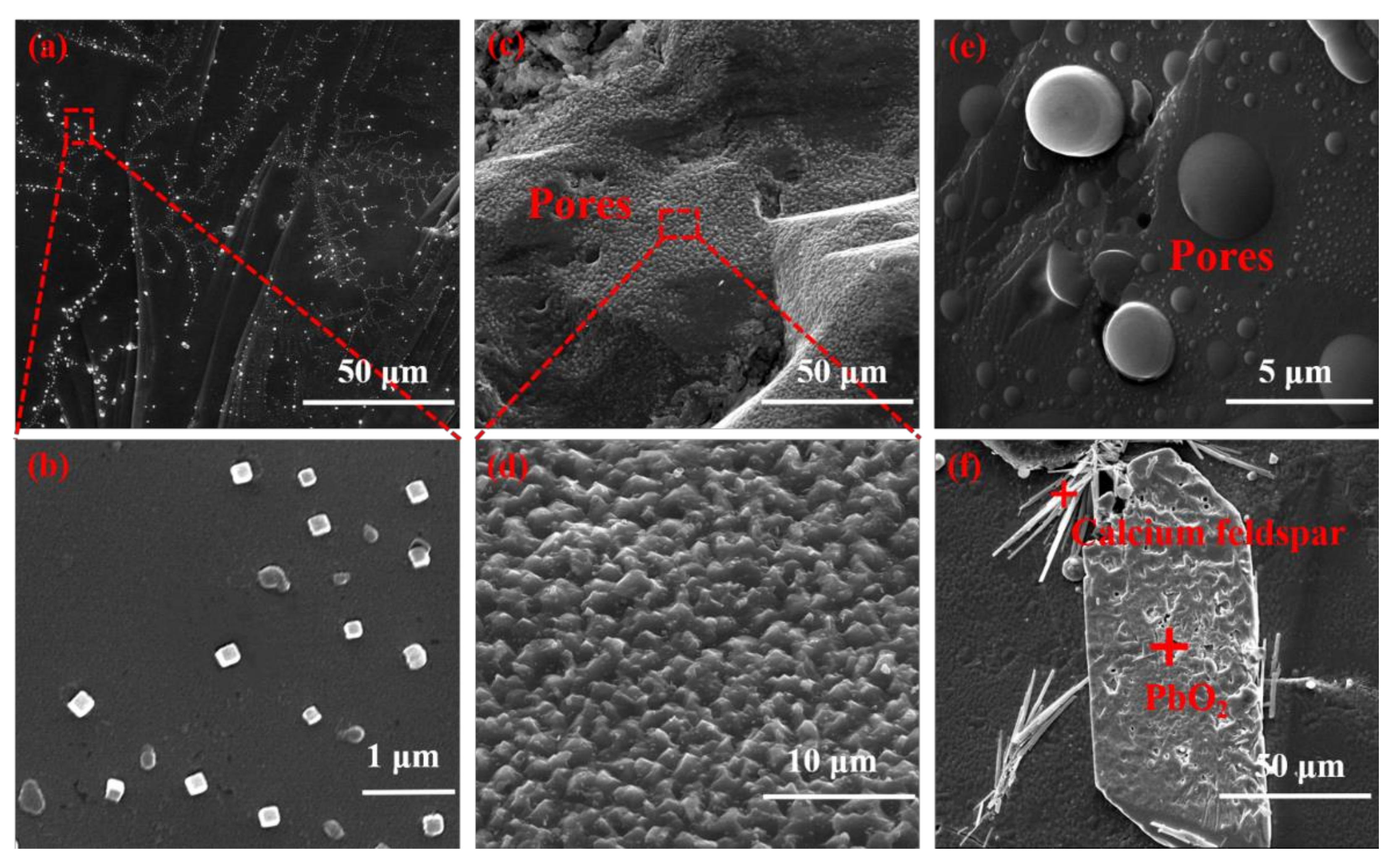
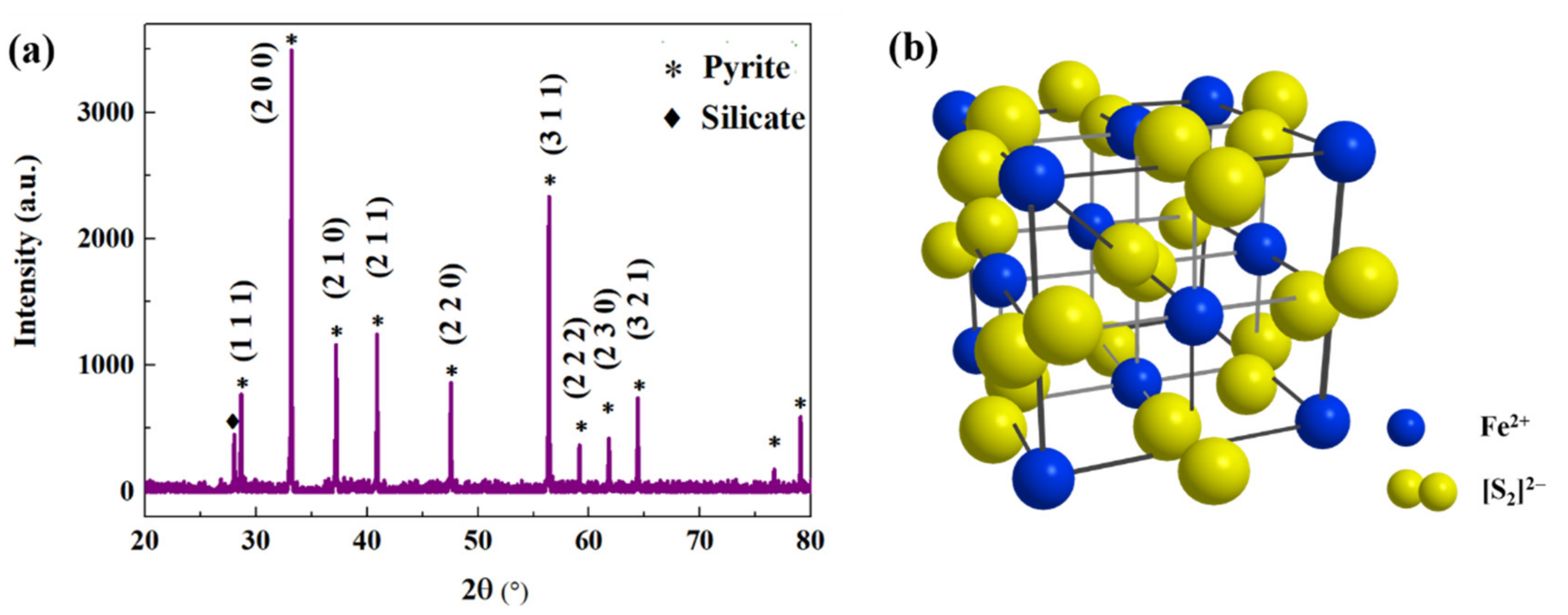
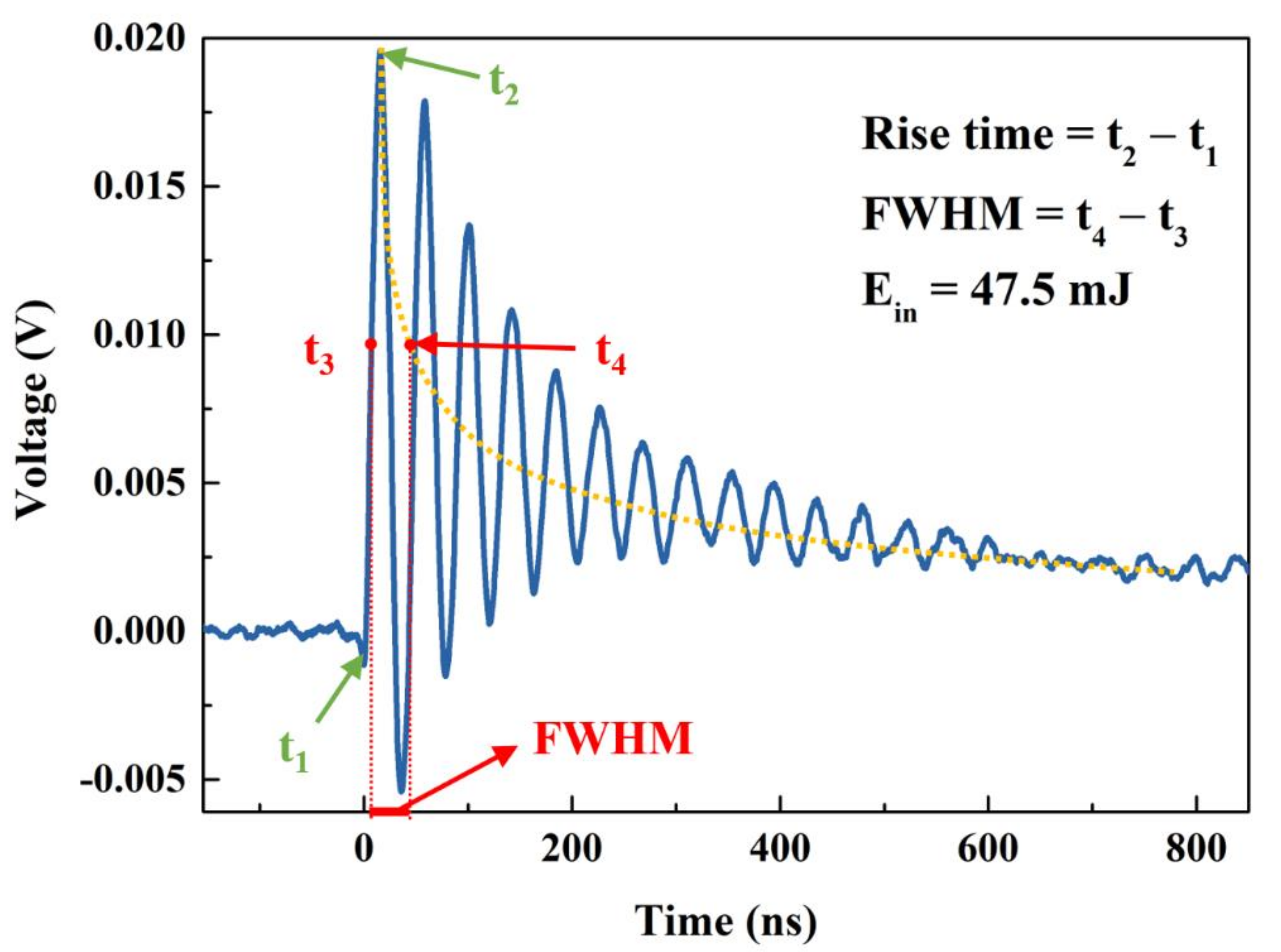
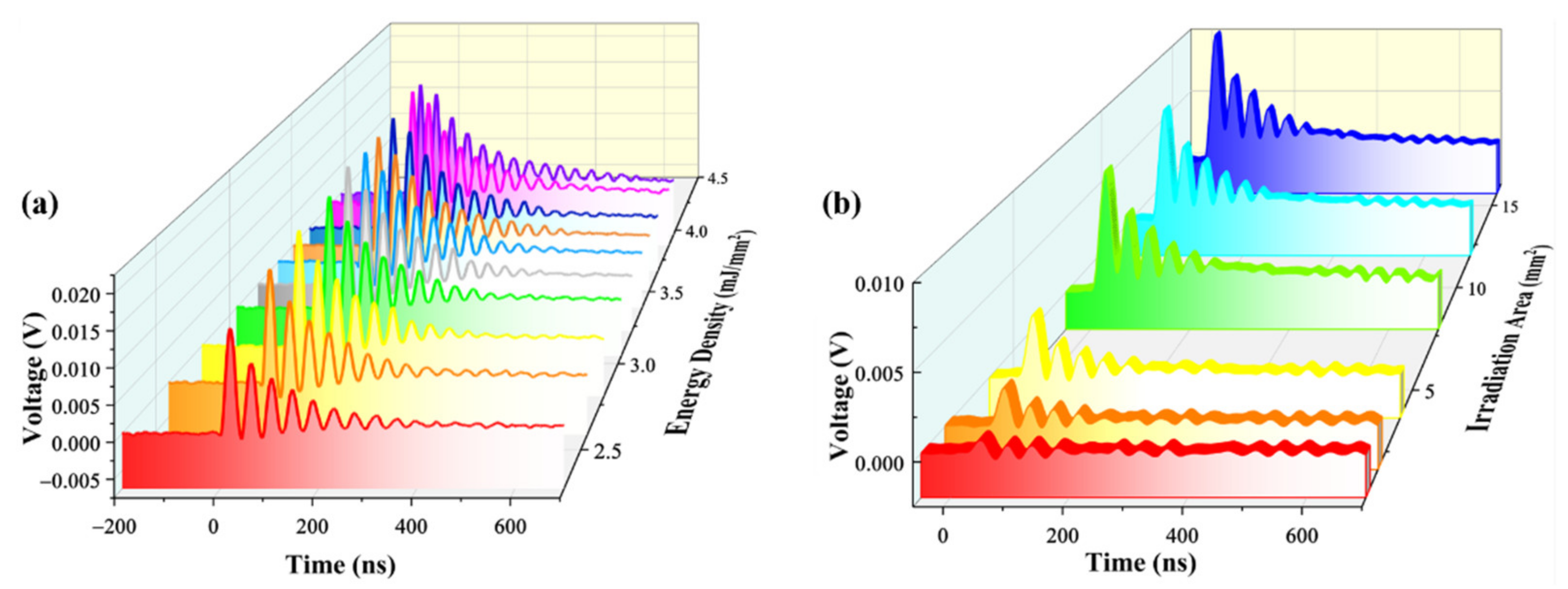
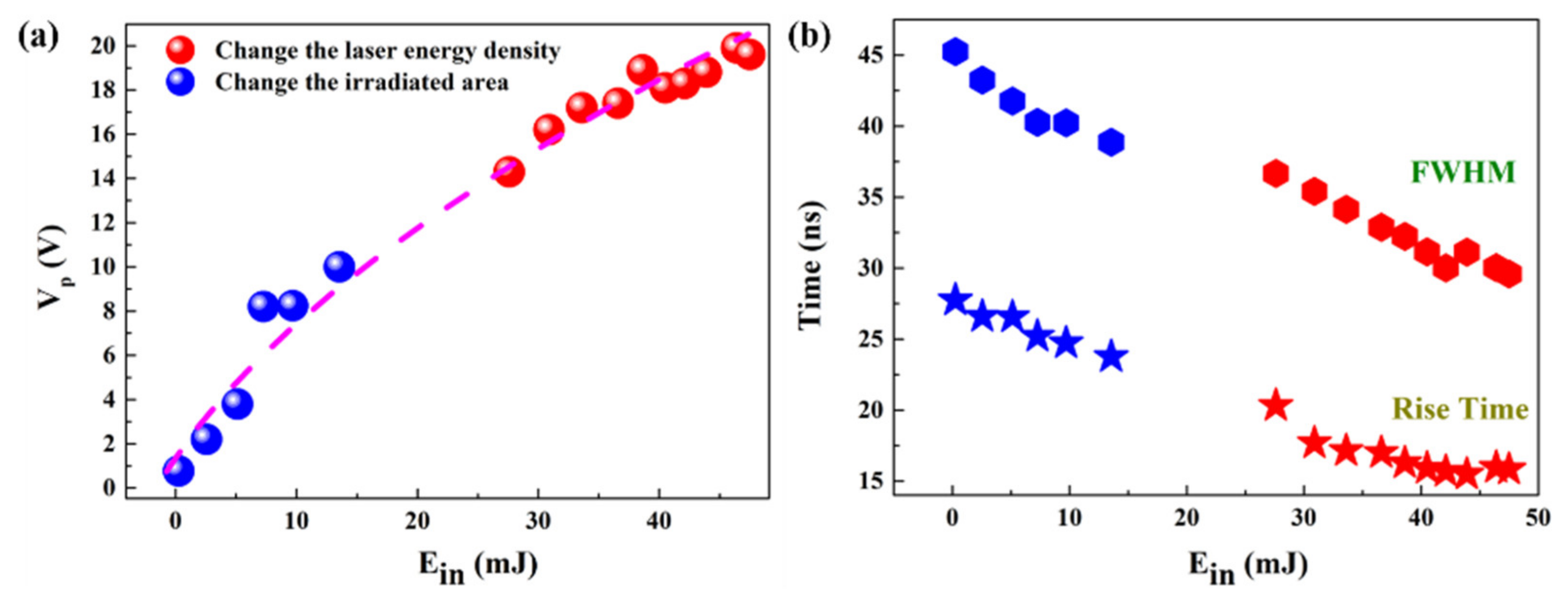
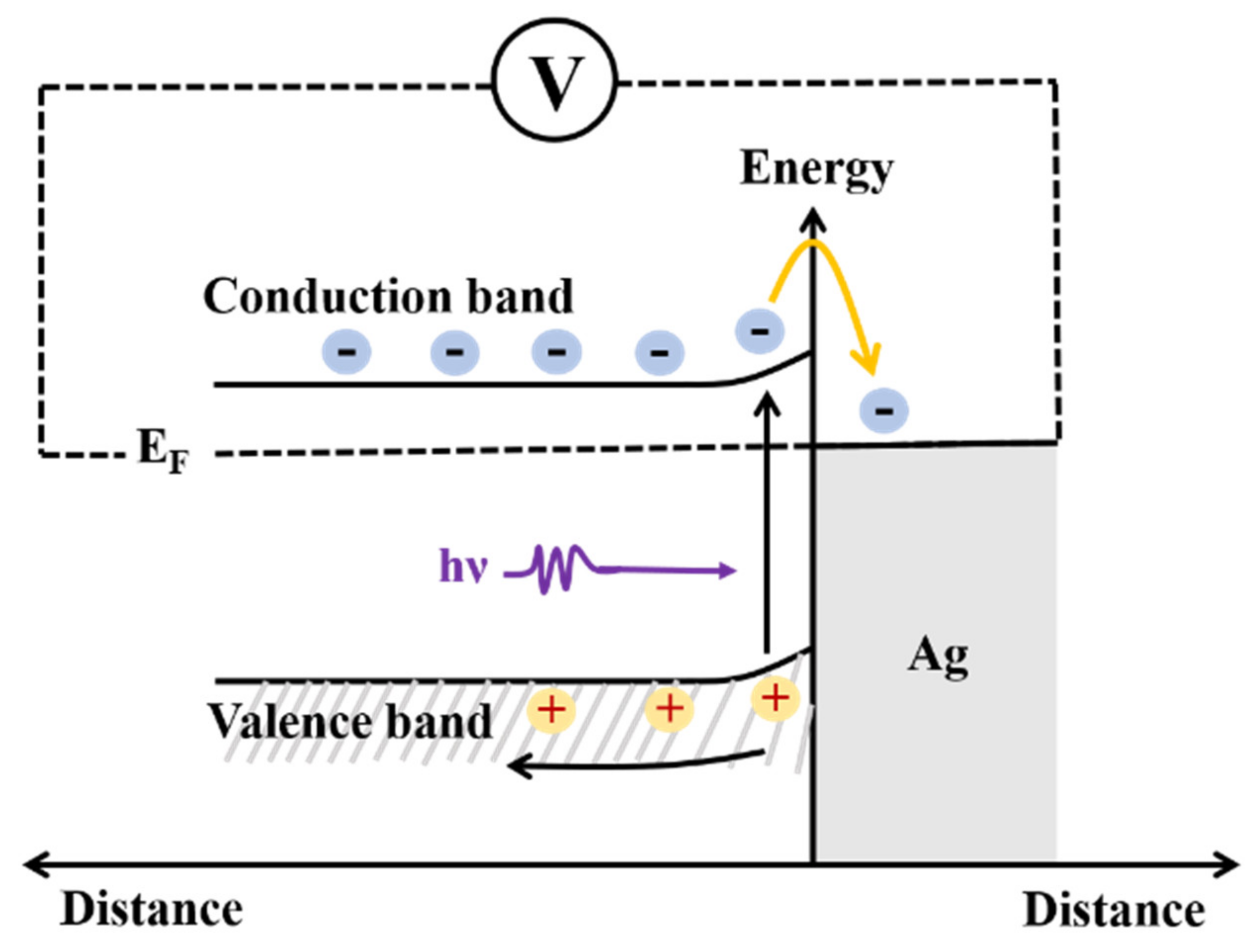
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Craig, J.R.; Vokes, F.M.; Solberg, T.N. Pyrite: Physical and chemical textures. Miner. Depos. 1998, 34, 82–101. [Google Scholar] [CrossRef]
- Ennaoui, A.; Fiechter, S.; Pettenkofer, C.; Alonso-Vante, N.; Büker, K.; Bronold, M.; Höpfner, C.; Tributsch, H. Iron disulfide for solar energy conversion. Sol. Energy Mater. Sol. Cells 1993, 29, 289–370. [Google Scholar] [CrossRef]
- Shuey, R.T. Semiconducting Ore Minerals; Elsevier: London, UK, 1975; pp. 304–318. [Google Scholar]
- Qin, H.; Jia, J.; Lin, L.; Ni, H.; Wang, M.; Meng, L. Pyrite FeS2 nanostructures: Synthesis, properties and applications. Mater. Sci. Eng. B 2018, 236-237, 104–124. [Google Scholar] [CrossRef]
- Buker, K.; Vante-Vante, N.; Tributsch, H. Photovoltaic output limitation of n-FeS2 (pyrite) Schottky barriers: A temperature-dependent characterization. J. Appl. Phys. 1992, 72, 5721–5728. [Google Scholar] [CrossRef]
- Shockley, W.; Queisser, H.J. Detailed balance limit of efficiency of p-n junction solar cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Antonucci, V.; Aricò, A.; Giordano, N.; Antonucci, P.; Russo, U.; Cocke, D.; Crea, F. Photoactive screen-printed pyrite anodes for electrochemical photovoltaic cells. Sol. Cells 1991, 31, 119–141. [Google Scholar] [CrossRef]
- Alzahrani, H.; Sulaiman, K.; Mahmoud, A.Y.; Bahabry, R.R. Ultrasensitive self-powered UV photodetector based on a novel p-n heterojunction of solution-processable organic semiconductors. Synth. Met. 2021, 278, 116830. [Google Scholar] [CrossRef]
- Li, S.; Yan, Z.; Liu, Z.; Chen, J.; Zhi, Y.; Guo, D.; Li, P.; Wu, Z.; Tang, W. A self-powered solar-blind photodetector with large: Voc enhancing performance based on the PEDOT: PSS/Ga2O3 organic-inorganic hybrid heterojunction. J. Mater. Chem. C 2020, 8, 1292–1300. [Google Scholar] [CrossRef]
- Liu, X.; Miao, X.; Zhu, M.; Peng, X.; Lu, W.; Zhang, S.; Zhan, H.; Yue, W.; Zhao, K. Mechanism of the Laser-Induced Voltage Generated in Oil Shale under the Irradiation of a 532 nm Laser. Energy Fuels 2021, 35, 1398–1403. [Google Scholar] [CrossRef]
- Chen, M.; Zhan, H.L.; Chen, R.; Meng, Z.; Zhang, S.; Zhao, K.; Zhu, J.; Yue, W. Laser-Induced Voltage of Oil Shale during Retorting. Energy Fuels 2019, 33, 10533–10536. [Google Scholar] [CrossRef]
- Miao, X.; Zhu, J.; Li, Y.; Zhan, H.; Zhao, K.; Yue, W. Transient laser-induced voltaic response in a partially illuminated dielectric core. Laser Phys. 2018, 28, 086001. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, J.; Zhan, H.L.; Meng, Z.; Zhang, S.; Chen, R.; Zhao, K.; Yue, W. Direct Detection of Oil Shale Yields: A Laser-Induced Voltage Investigation. Energy Fuels 2019, 33, 1069–1073. [Google Scholar] [CrossRef]
- Lu, Z.Q.; Sun, Q.; Zhao, K.; Liu, H.; Feng, X.; Wang, J.; Xiao, L.Z. Laser-Induced Voltage of Oil Shale for Characterizing the Oil Yield. Energy Fuels 2015, 29, 4936–4940. [Google Scholar] [CrossRef]
- Bo, R.; Zhang, F.; Bu, S.; Nasiri, N.; Di Bernardo, I.; Tran-Phu, T.; Shrestha, A.; Chen, H.; Taheri, M.; Qi, S.; et al. One-step synthesis of porous transparent conductive oxides by hierarchical self-assembly of aluminium-doped ZnO nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 9589–9599. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, N.; Bo, R.; Fu, L.; Tricoli, A. Three-dimensional nano-heterojunction networks: A highly performing structure for fast visible-blind UV photodetectors. Nanoscale 2017, 9, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Gundimeda, A.; Krishna, S.; Aggarwal, N.; Sharma, A.; Sharma, N.D.; Maurya, K.K.; Husale, S.; Gupta, G. Fabrication of non-polar GaN based highly responsive and fast UV photodetector. Appl. Phys. Lett. 2017, 110, 103507. [Google Scholar] [CrossRef]
- Aggarwal, N.; Krishna, S.; Sharma, A.; Goswami, L.; Kumar, D.; Husale, S.; Gupta, G. A Highly Responsive Self-Driven UV Photodetector Using GaN Nanoflowers. Adv. Electron. Mater. 2017, 3, 1700036. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, J.; Shi, S.; Dong, H.; Cheng, Y.; Wei, C.; Zhang, X.; Yin, S.; Li, L. Tio2 NRs based self-powered UV photodetector: Heterojunction with NiO nanoflakes and enhanced UV photoresponse. ACS Appl. Mater. Interfaces 2018, 10, 11269–11279. [Google Scholar] [CrossRef]
- Müller, A.; Konstantinidis, G.; Dragoman, M.; Neculoiu, D.; Dinescu, A.; Androulidaki, M.; Kayambaki, M.; Stavrinidis, A.; Vasilache, D.; Buiculescu, C.; et al. GaN membrane-supported UV photodetectors manufactured using nanolithographic processes. Microelectron. J. 2009, 40, 319–321. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, F.; Wu, Z. Effects of annealing on the performance of 4H-SiC metal–semiconductor–metal ultraviolet photodetectors. Mater. Sci. Semicond. Process. 2008, 11, 59–62. [Google Scholar] [CrossRef]
- Pramanik, P.; Sen, S.; Singha, C.; Roy, A.S.; Das, A.; Sen, S.; Bhattacharyya, A. Compositional inhomogeneities in AlGaN thin films grown by molecular beam epitaxy: Effect on MSM UV photodetectors. J. Appl. Phys. 2016, 120, 144502. [Google Scholar] [CrossRef]
- Wang, X.-H.; Huang, T.; Huang, F.; Zhang, S.-N.; Wang, Y.; Gao, C. Light absorption characteristic of natural pyrite. Spectrosc. Spectr. Anal. 2011, 31, 2508–2511. [Google Scholar]
- Vaughan, D.J.; Tossell, J.A. Electronic structures of sulfide minerals—Theory and experiment. Phys. Chem. Miner. 1983, 9, 253–262. [Google Scholar] [CrossRef]
- Bither, T.A.; Bouchard, R.J.; Cloud, W.H.; Donahue, P.C. Transition metal pyrite dichalcogenides. High pressure synthesis and correlation of properties. Inorg. Chem. 1968, 7, 2208–2220. [Google Scholar] [CrossRef]
- Abraitis, P.K.; Pattrick, R.A.D.; Vaughan, D.J. Variations in the compositional, textural and electrical properties of natural pyrite: A review. Int. J. Miner. Process. 2004, 74, 41–59. [Google Scholar] [CrossRef]
- Schlegel, A.; Wachter, P. Optical properties, phonons and electronic structure of iron pyrite (FeS2). J. Phys. C Solid State Phys. 1976, 9, 3363–3369. [Google Scholar] [CrossRef]
- Xian, H.; He, H.; Zhu, J.; Du, R.; Wu, X.; Tang, H.; Tan, W.; Liang, X.; Zhu, R.; Teng, H.H. Crystal habit-directed gold deposition on pyrite: Surface chemical interpretation of the pyrite morphology indicative of gold enrichment. Geochim. Cosmochim. Acta 2019, 264, 191–204. [Google Scholar] [CrossRef]
- Sai, R.; Ezzaouia, H.; Nofal, M.M. Electronic structure of iron pyrite by the LMTO_ASA method. Results Phys. 2021, 22, 103950. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, Z.; Jiang, H. Relative Stability of FeS2 polymorphs with the random phase approximation approach. J. Mater. Chem. A 2018, 15, 6606–6616. [Google Scholar] [CrossRef]
- Zhang, S.; Tran, D.T. Electrochemical verification of the redox mechanism of FeS2 in a rechargeable lithium battery. Electrochim. Acta 2015, 176, 784–789. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, K.; Zhao, S.; Zhou, N.; Zhao, H.; Lü, Z.; Wang, A. Ultrafast and spectrally broadband photovoltaic response in quartz single crystals. J. Phys. D Appl. Phys. 2009, 42, 075104. [Google Scholar] [CrossRef]
- Miao, X.; Zhu, J.; Li, Y.; Zhao, K.; Zhan, H.; Yue, W. Ultraviolet laser-induced voltage in anisotropic shale. J. Phys. D Appl. Phys. 2017, 51, 045503. [Google Scholar] [CrossRef]
- Farrell, P.; Kayser, S.; Routundo, N. Modeling and simulation of the lateral photovoltage scanning method. Comput. Math. Appl. 2021, 102, 248–260. [Google Scholar] [CrossRef]
- Bryson, L.J.; Crundwell, F.K. The anodic dissolution of pyrite (FeS2) in hydrochloric acid solutions. Hydrometallurgy 2014, 143, 42–53. [Google Scholar] [CrossRef]
- Johnson, E.O. Large-signal surface photovoltage studies with germanium. Phys. Rev. J. Arch. 1958, 111, 153. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Li, Y.; Wu, H.; Yu, Y.; Zhan, H.; Miao, X.; Zhao, K. Ultrafast Deep-Ultraviolet Laser-Induced Voltage Response of Pyrite. Micromachines 2021, 12, 1555. https://doi.org/10.3390/mi12121555
Liu X, Li Y, Wu H, Yu Y, Zhan H, Miao X, Zhao K. Ultrafast Deep-Ultraviolet Laser-Induced Voltage Response of Pyrite. Micromachines. 2021; 12(12):1555. https://doi.org/10.3390/mi12121555
Chicago/Turabian StyleLiu, Xuecong, Yudong Li, Haoqiang Wu, Yawen Yu, Honglei Zhan, Xinyang Miao, and Kun Zhao. 2021. "Ultrafast Deep-Ultraviolet Laser-Induced Voltage Response of Pyrite" Micromachines 12, no. 12: 1555. https://doi.org/10.3390/mi12121555
APA StyleLiu, X., Li, Y., Wu, H., Yu, Y., Zhan, H., Miao, X., & Zhao, K. (2021). Ultrafast Deep-Ultraviolet Laser-Induced Voltage Response of Pyrite. Micromachines, 12(12), 1555. https://doi.org/10.3390/mi12121555




