Aerosol Spray Deposition of Liquid Metal and Elastomer Coatings for Rapid Processing of Stretchable Electronics
Abstract
:1. Introduction
2. Results and Discussion
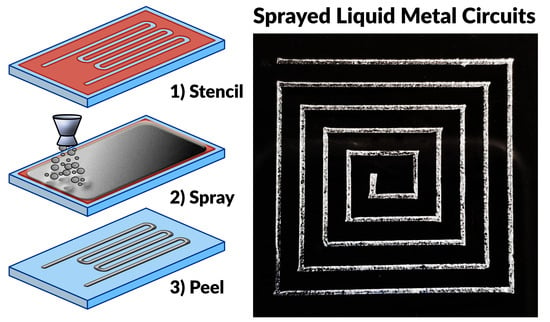
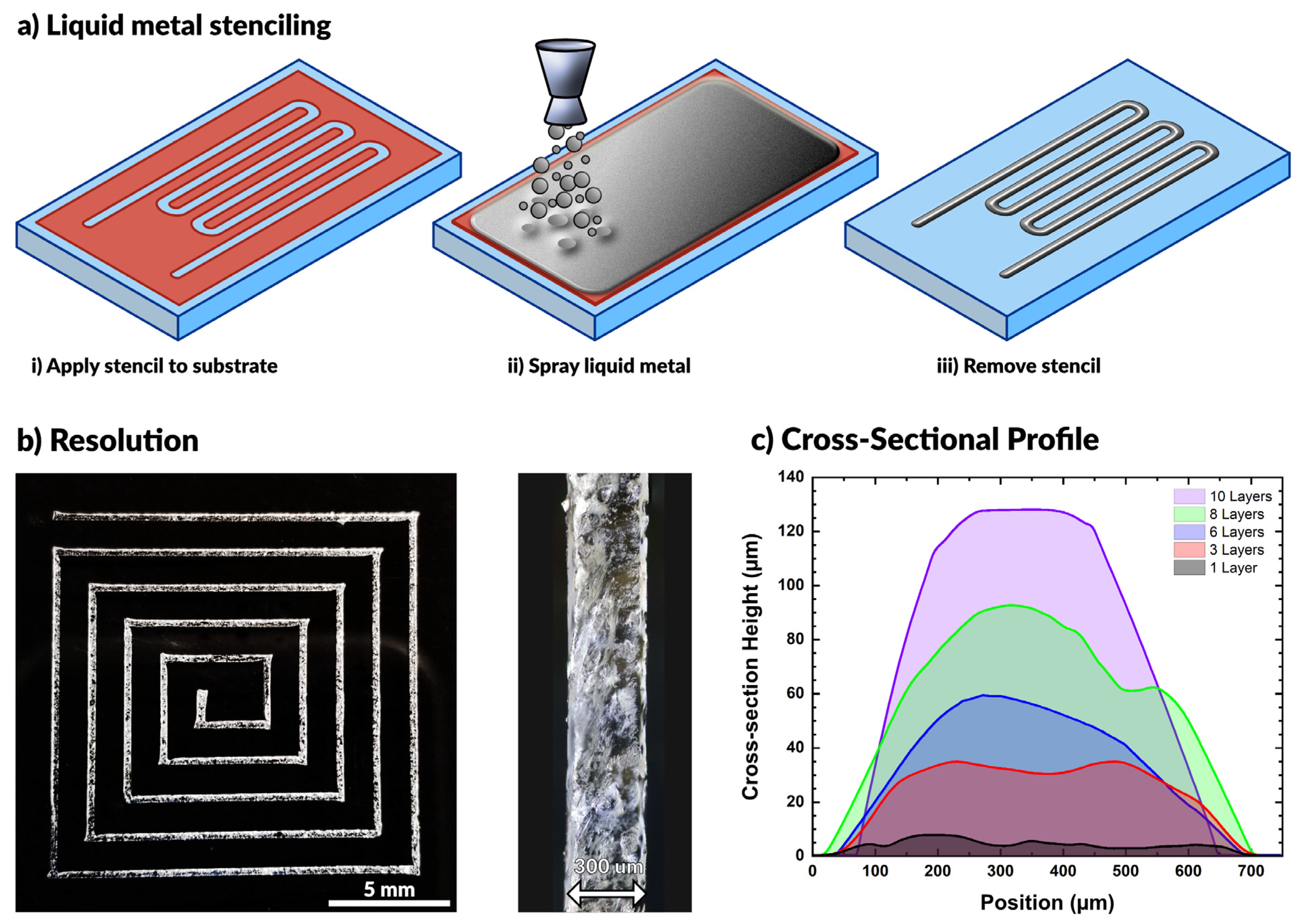
2.1. Processing Overview
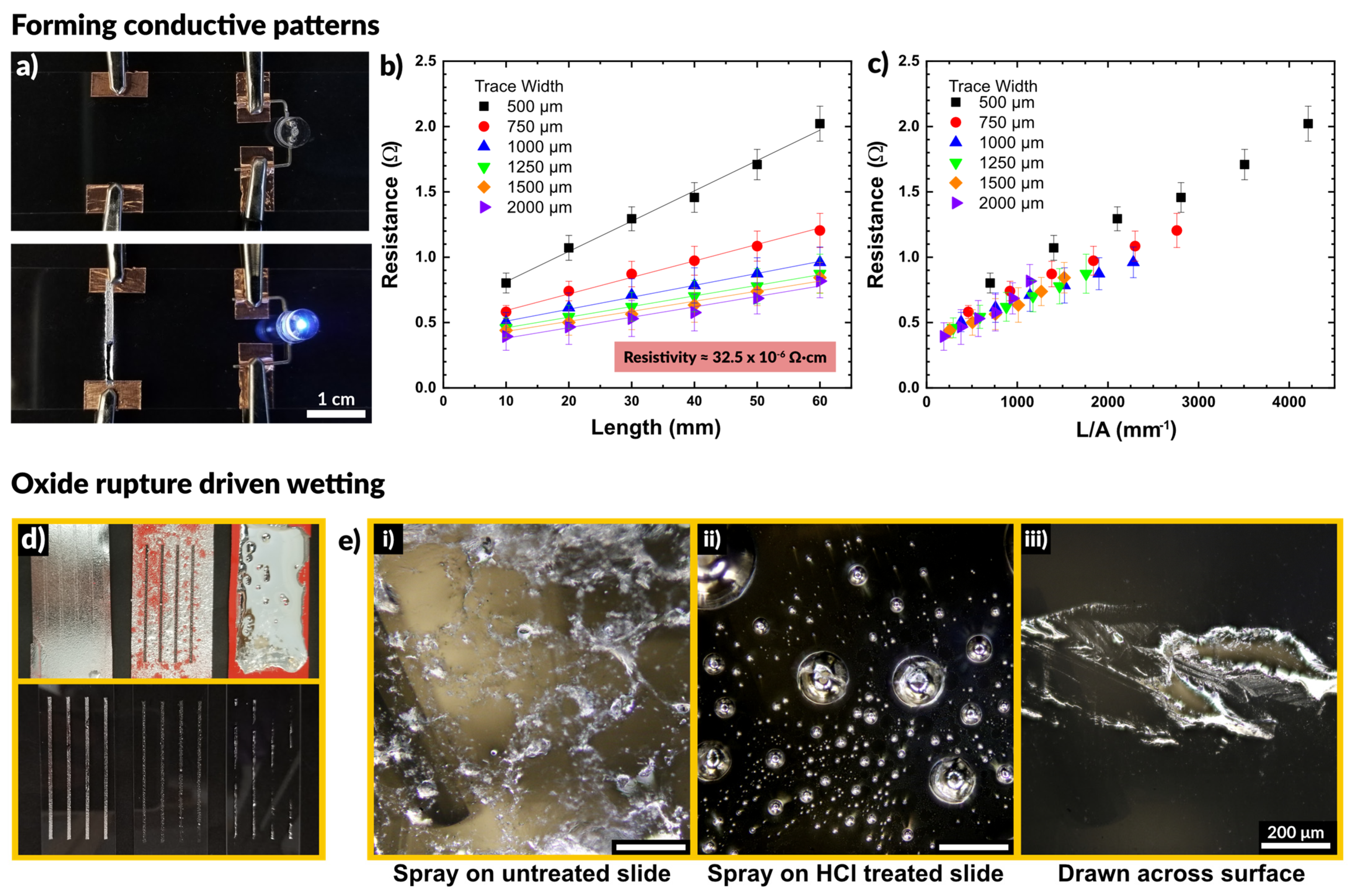
2.2. Impact of the Surface Oxide
2.3. Particle Rupture
2.3.1. Effect of Particle Size
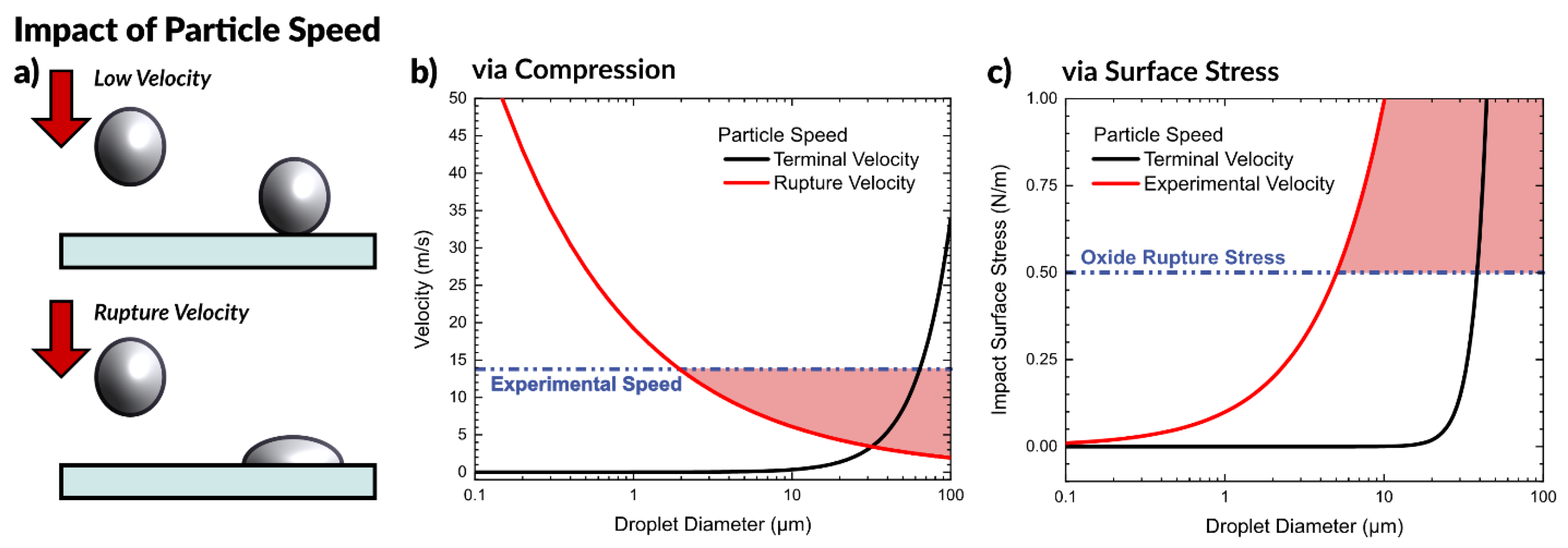
2.3.2. Effect of Particle Speed
Rupture Velocity
Surface Stress at Impact
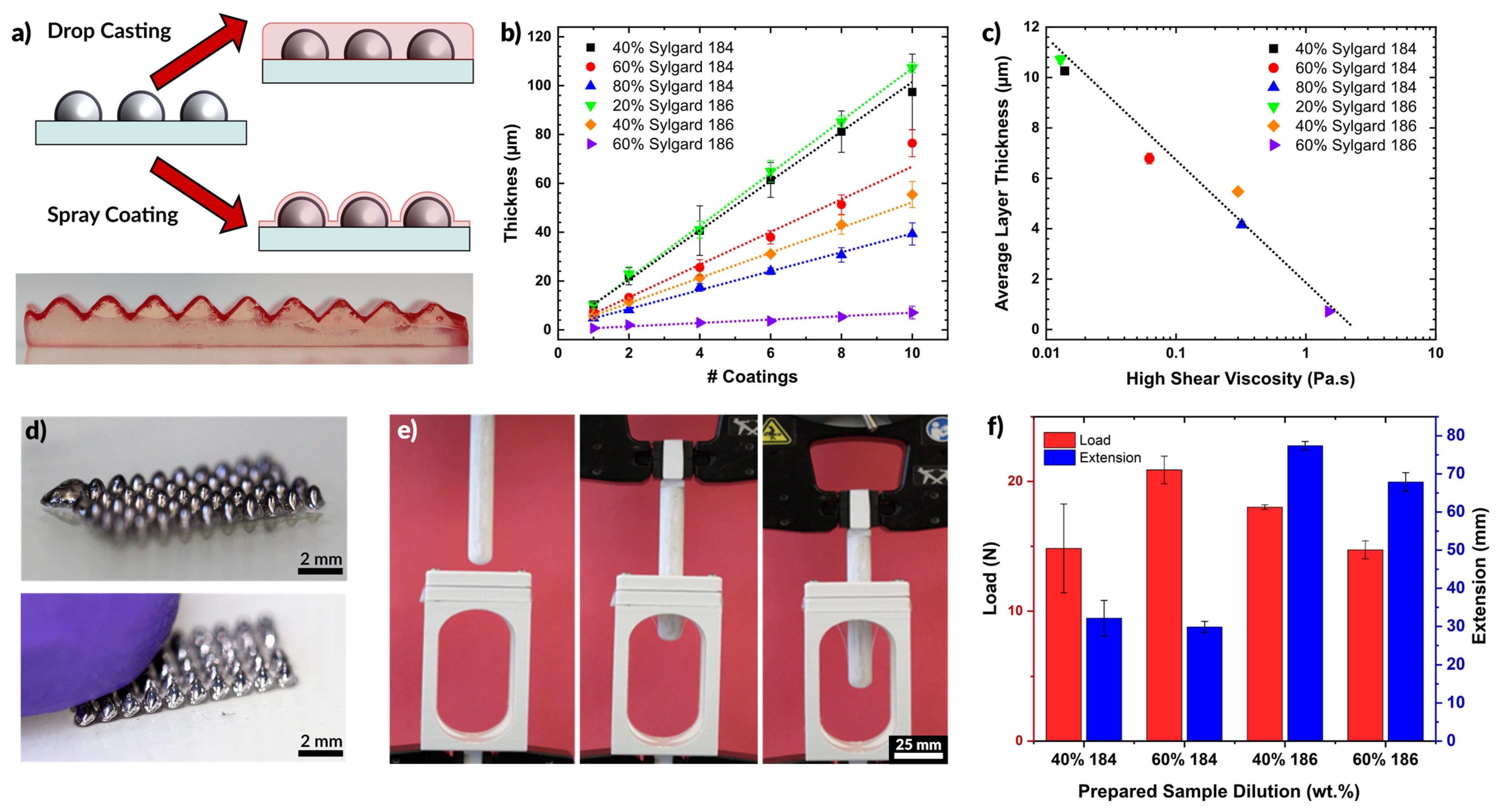
2.4. Encapsulation via Spray Coating
2.5. Function as Soft Sensors
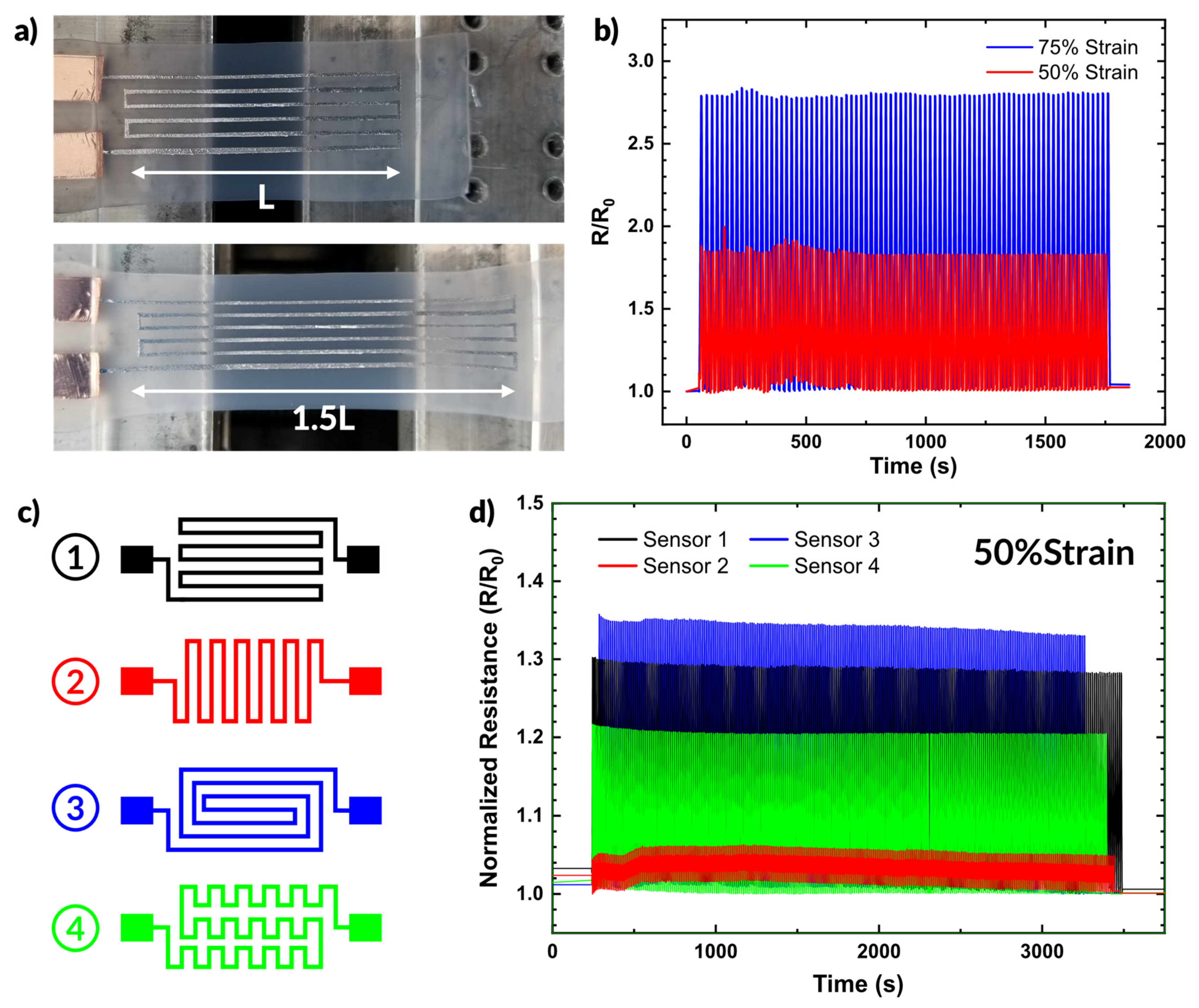
2.5.1. Strain Sensor
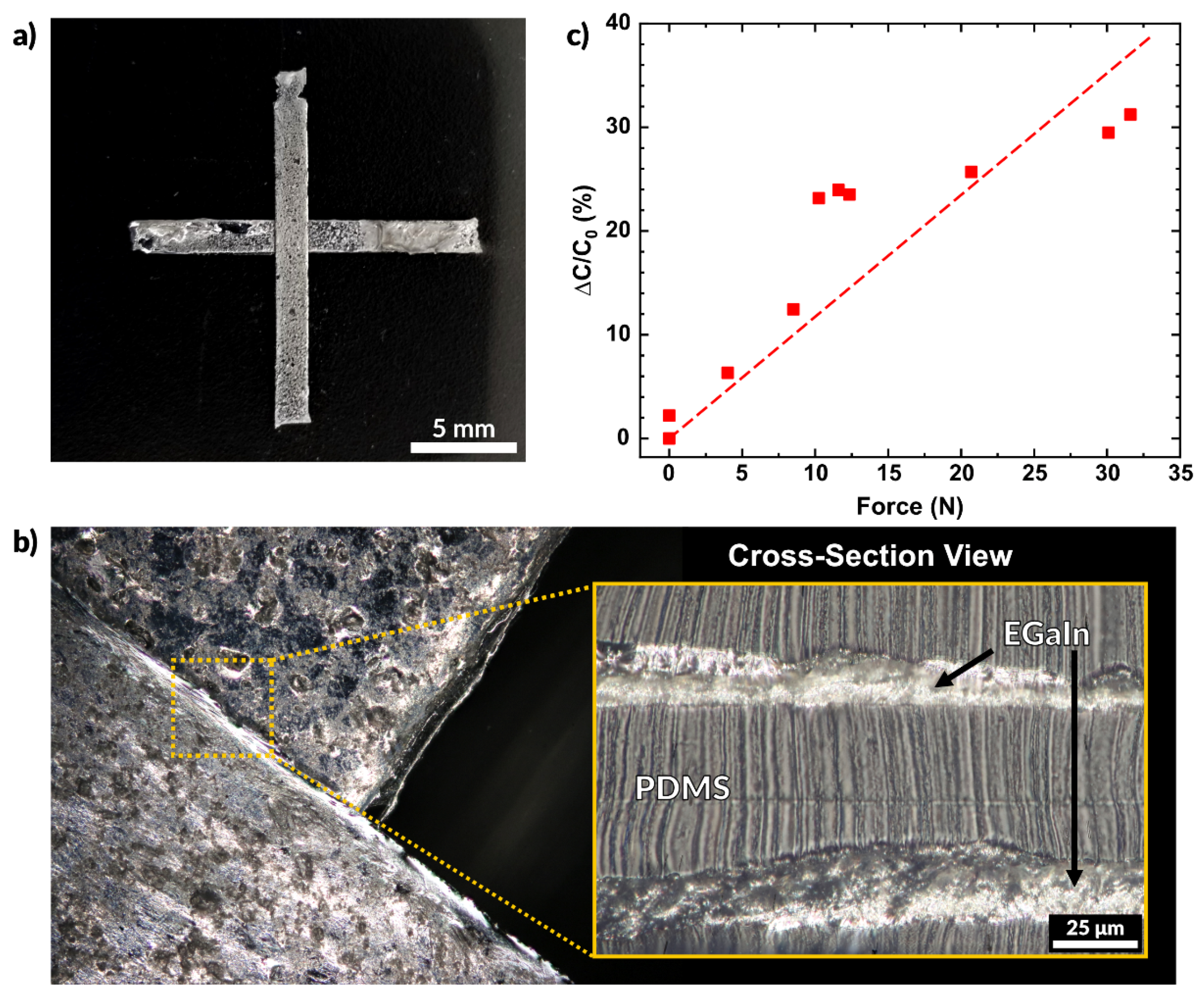
2.5.2. Multilayer Capacitive Sensor
3. Conclusions
4. Experimental
4.1. Materials
4.2. Stencil Preparation
4.3. Aerosol Deposition
4.4. Surface Profiling
4.5. Electrical Measurements
4.6. Viscosity Measurements
4.7. Force Measurements
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dickey, M.D. Stretchable and Soft Electronics Using Liquid Metals. Adv. Mater. 2017, 29, 1606425. [Google Scholar] [CrossRef] [PubMed]
- Daeneke, T.; Khoshmanesh, K.; Mahmood, N.; de Castro, I.A.; Esrafilzadeh, D.; Barrow, S.J.; Dickey, M.D.; Kalantar-Zadeh, K. Liquid Metals: Fundamentals and Applications in Chemistry. Chem. Soc. Rev. 2018, 47, 4073–4111. [Google Scholar] [CrossRef] [PubMed]
- Majidi, L.; Gritsenko, D.; Xu, J. Gallium-Based Room-Temperature Liquid Metals: Actuation and Manipulation of Droplets and Flows. Front. Mech. Eng. 2017, 3. [Google Scholar] [CrossRef] [Green Version]
- Kalantar-Zadeh, K.; Tang, J.; Daeneke, T.; O’Mullane, A.P.; Stewart, L.A.; Liu, J.; Majidi, C.; Ruoff, R.S.; Weiss, P.S.; Dickey, M.D. Emergence of Liquid Metals in Nanotechnology. ACS Nano 2019, 13, 7388–7395. [Google Scholar] [CrossRef]
- Joshipura, I.D.; Ayers, H.R.; Majidi, C.; Dickey, M.D. Methods to Pattern Liquid Metals. J. Mater. Chem. C 2015, 3, 3834–3841. [Google Scholar] [CrossRef]
- Farrell, Z.J.; Tabor, C. Control of Gallium Oxide Growth on Liquid Metal Eutectic Gallium/Indium Nanoparticles via Thiolation. Langmuir 2018, 34, 234–240. [Google Scholar] [CrossRef]
- Carey, B.J.; Ou, J.Z.; Clark, R.M.; Berean, K.J.; Zavabeti, A.; Chesman, A.S.R.; Russo, S.P.; Lau, D.W.M.; Xu, Z.-Q.; Bao, Q.; et al. Wafer-Scale Two-Dimensional Semiconductors from Printed Oxide Skin of Liquid Metals. Nat. Commun. 2017, 8, 14482. [Google Scholar] [CrossRef]
- Dickey, M.D.; Chiechi, R.C.; Larsen, R.J.; Weiss, E.A.; Weitz, D.A.; Whitesides, G.M. Eutectic Gallium-Indium (EGaIn): A Liquid Metal Alloy for the Formation of Stable Structures in Microchannels at Room Temperature. Adv. Funct. Mater. 2008, 18, 1097–1104. [Google Scholar] [CrossRef]
- Tang, S.-Y.; Lin, Y.; Joshipura, I.D.; Khoshmanesh, K.; Dickey, M.D. Steering Liquid Metal Flow in Microchannels Using Low Voltages. Lab Chip 2015, 15, 3905–3911. [Google Scholar] [CrossRef]
- Khoshmanesh, K.; Tang, S.-Y.; Zhu, J.Y.; Schaefer, S.; Mitchell, A.; Kalantar-zadeh, K.; Dickey, M.D. Liquid Metal Enabled Microfluidics. Lab Chip 2017, 17, 974–993. [Google Scholar] [CrossRef]
- Bharambe, V.; Parekh, D.P.; Ladd, C.; Moussa, K.; Dickey, M.D.; Adams, J.J. Vacuum-Filling of Liquid Metals for 3D Printed RF Antennas. Addit. Manuf. 2017, 18, 221–227. [Google Scholar] [CrossRef]
- Lin, Y.; Gordon, O.; Khan, M.R.; Vasquez, N.; Genzer, J.; Dickey, M.D. Vacuum Filling of Complex Microchannels with Liquid Metal. Lab Chip 2017, 17, 3043–3050. [Google Scholar] [CrossRef] [PubMed]
- Ozutemiz, K.B.; Wissman, J.; Ozdoganlar, O.B.; Majidi, C. EGaIn-Metal Interfacing for Liquid Metal Circuitry and Microelectronics Integration. Adv. Mater. Interfaces 2018, 5, 1701596. [Google Scholar] [CrossRef]
- Li, G.; Wu, X.; Lee, D.-W. Selectively Plated Stretchable Liquid Metal Wires for Transparent Electronics. Sens. Actuators B Chem. 2015, 221, 1114–1119. [Google Scholar] [CrossRef]
- Jiang, Y.; Su, S.; Peng, H.; Kwok, H.S.; Zhou, X.; Chen, S. Selective Wetting/Dewetting for Controllable Patterning of Liquid Metal Electrodes for All-Printed Device Application. J. Mater. Chem. C 2017, 5, 12378–12383. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Q.; Shan, C.; Zhang, J.; Yong, J.; Fang, Y.; Hou, X.; Chen, F. Tuning a Surface Super-Repellent to Liquid Metal by a Femtosecond Laser. RSC Adv. 2020, 10, 3301–3306. [Google Scholar] [CrossRef] [Green Version]
- Joshipura, I.D.; Ayers, H.R.; Castillo, G.A.; Ladd, C.; Tabor, C.E.; Adams, J.J.; Dickey, M.D. Patterning and Reversible Actuation of Liquid Gallium Alloys by Preventing Adhesion on Rough Surfaces. ACS Appl. Mater. Interfaces 2018, 10, 44686–44695. [Google Scholar] [CrossRef]
- Ma, J.; Bharambe, V.T.; Persson, K.A.; Bachmann, A.L.; Joshipura, I.D.; Kim, J.; Oh, K.H.; Patrick, J.F.; Adams, J.J.; Dickey, M.D. Metallophobic Coatings to Enable Shape Reconfigurable Liquid Metal Inside 3D Printed Plastics. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef]
- Mohammed, M.G.; Xenakis, A.; Dickey, M.D. Production of Liquid Metal Spheres by Molding. Metals 2014, 4, 465–476. [Google Scholar] [CrossRef]
- Gozen, B.A.; Tabatabai, A.; Ozdoganlar, O.B.; Majidi, C. High-Density Soft-Matter Electronics with Micron-Scale Line Width. Adv. Mater. 2014, 26, 5211–5216. [Google Scholar] [CrossRef]
- Kim, M.; Alrowais, H.; Pavlidis, S.; Brand, O. Size-Scalable and High-Density Liquid-Metal-Based Soft Electronic Passive Components and Circuits Using Soft Lithography. Adv. Funct. Mater. 2017, 27, 1604466. [Google Scholar] [CrossRef]
- Parekh, D.P.; Ladd, C.; Panich, L.; Moussa, K.; Dickey, M.D. 3D Printing of Liquid Metals as Fugitive Inks for Fabrication of 3D Microfluidic Channels. Lab Chip 2016, 16, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Oh, J.; Jeong, D.; Bae, J. Direct Wiring of Eutectic Gallium–Indium to a Metal Electrode for Soft Sensor Systems. Acs Appl. Mater. Interfaces 2019, 11, 20557–20565. [Google Scholar] [CrossRef] [PubMed]
- Gannarapu, A.; Dutta, P.; Gozen, B.A. Prediction of Steady-State Freeze Front Position during 3D Printing of Microstructures. Int. J. Heat Mass Transf. 2017, 115, 743–753. [Google Scholar] [CrossRef]
- Gannarapu, A.; Gozen, B.A. Freeze-Printing of Liquid Metal Alloys for Manufacturing of 3D, Conductive, and Flexible Networks. Adv. Mater. Technol. 2016, 1, 1600047. [Google Scholar] [CrossRef]
- Park, Y.-G.; An, H.S.; Kim, J.-Y.; Park, J.-U. High-Resolution, Reconfigurable Printing of Liquid Metals with Three-Dimensional Structures. Sci. Adv. 2019, 5, eaaw2844. [Google Scholar] [CrossRef] [Green Version]
- Boley, J.W.; White, E.L.; Chiu, G.T.-C.; Kramer, R.K. Direct Writing of Gallium-Indium Alloy for Stretchable Electronics. Adv. Funct. Mater. 2014, 24, 3501–3507. [Google Scholar] [CrossRef]
- Cook, A.; Parekh, D.P.; Ladd, C.; Kotwal, G.; Panich, L.; Durstock, M.; Dickey, M.D.; Tabor, C.E. Shear-Driven Direct-Write Printing of Room-Temperature Gallium-Based Liquid Metal Alloys. Adv. Eng. Mater. 2019, 21, 1900400. [Google Scholar] [CrossRef]
- Guo, R.; Tang, J.; Dong, S.; Lin, J.; Wang, H.; Liu, J.; Rao, W. One-Step Liquid Metal Transfer Printing: Toward Fabrication of Flexible Electronics on Wide Range of Substrates. Adv. Mater. Technol. 2018, 3, 1800265. [Google Scholar] [CrossRef]
- Daalkhaijav, U.; Yirmibesoglu, O.D.; Walker, S.; Mengüç, Y. Rheological Modification of Liquid Metal for Additive Manufacturing of Stretchable Electronics. Adv. Mater. Technol. 2018, 3, 1700351. [Google Scholar] [CrossRef]
- Song, M.; Kartawira, K.; Hillaire, K.D.; Li, C.; Eaker, C.B.; Kiani, A.; Daniels, K.E.; Dickey, M.D. Overcoming Rayleigh–Plateau Instabilities: Stabilizing and Destabilizing Liquid-Metal Streams via Electrochemical Oxidation. Proc. Natl. Acad. Sci. USA 2020, 117, 19026–19032. [Google Scholar] [CrossRef] [PubMed]
- Neumann, T.V.; Dickey, M.D. Liquid Metal Direct Write and 3D Printing: A Review. Adv. Mater. Technol. 2020, 5, 2000070. [Google Scholar] [CrossRef]
- Yoon, Y.; Kim, S.; Kim, D.; Kauh, S.K.; Lee, J. Four Degrees-of-Freedom Direct Writing of Liquid Metal Patterns on Uneven Surfaces. Adv. Mater. Technol. 2019, 4, 1800379. [Google Scholar] [CrossRef]
- Doudrick, K.; Liu, S.; Mutunga, E.M.; Klein, K.L.; Damle, V.; Varanasi, K.K.; Rykaczewski, K. Different Shades of Oxide: From Nanoscale Wetting Mechanisms to Contact Printing of Gallium-Based Liquid Metals. Langmuir 2014, 30, 6867–6877. [Google Scholar] [CrossRef]
- Watson, A.M.; Cook, A.B.; Tabor, C.E. Electrowetting-Assisted Selective Printing of Liquid Metal. Adv. Eng. Mater. 2019, 21, 1900397. [Google Scholar] [CrossRef]
- Wang, L.; Wang, M.; Lu, J.; Ardhi, R.E.A.; Liu, J.; Liu, G.; Lee, J.K. Enhanced Adhesion between Liquid Metal Ink and the Wetted Printer Paper for Direct Writing Electronic Circuits. J. Taiwan Inst. Chem. Eng. 2019, 95, 202–207. [Google Scholar] [CrossRef]
- Neumann, T.V.; Facchine, E.G.; Leonardo, B.; Khan, S.; Dickey, M.D. Direct Write Printing of a Self-Encapsulating Liquid Metal–Silicone Composite. Soft Matter 2020, 16, 6608–6618. [Google Scholar] [CrossRef]
- Zhou, L.; Fu, J.; Gao, Q.; Zhao, P.; He, Y. All-Printed Flexible and Stretchable Electronics with Pressing or Freezing Activatable Liquid-Metal–Silicone Inks. Adv. Funct. Mater. 2020, 30, 1906683. [Google Scholar] [CrossRef]
- Markvicka, E.J.; Bartlett, M.D.; Huang, X.; Majidi, C. An Autonomously Electrically Self-Healing Liquid Metal–Elastomer Composite for Robust Soft-Matter Robotics and Electronics. Nat. Mater. 2018, 17, 618–624. [Google Scholar] [CrossRef]
- Fassler, A.; Majidi, C. Liquid-Phase Metal Inclusions for a Conductive Polymer Composite. Adv. Mater. 2015, 27, 1928–1932. [Google Scholar] [CrossRef]
- Hirsch, A.; Michaud, H.O.; Gerratt, A.P.; de Mulatier, S.; Lacour, S.P. Intrinsically Stretchable Biphasic (Solid–Liquid) Thin Metal Films. Adv. Mater. 2016, 28, 4507–4512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.; Kim, T.; Kang, S.; Jin, H.; Lee, K.; Yoon, H.J. Ga-Based Liquid Metal Micro/Nanoparticles: Recent Advances and Applications. Small 2020, 16, 1903391. [Google Scholar] [CrossRef] [PubMed]
- Yarema, M.; Wörle, M.; Rossell, M.D.; Erni, R.; Caputo, R.; Protesescu, L.; Kravchyk, K.V.; Dirin, D.N.; Lienau, K.; von Rohr, F.; et al. Monodisperse Colloidal Gallium Nanoparticles: Synthesis, Low Temperature Crystallization, Surface Plasmon Resonance and Li-Ion Storage. J. Am. Chem. Soc. 2014, 136, 12422–12430. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-Y.; Qiao, R.; Yan, S.; Yuan, D.; Zhao, Q.; Yun, G.; Davis, T.P.; Li, W. Microfluidic Mass Production of Stabilized and Stealthy Liquid Metal Nanoparticles. Small 2018, 14, 1800118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boley, J.W.; White, E.L.; Kramer, R.K. Mechanically Sintered Gallium–Indium Nanoparticles. Adv. Mater. 2015, 27, 2355–2360. [Google Scholar] [CrossRef]
- Mohammed, M.G.; Kramer, R. All-Printed Flexible and Stretchable Electronics. Adv. Mater. 2017, 29, 1604965. [Google Scholar] [CrossRef]
- Ren, L.; Sun, S.; Casillas-Garcia, G.; Nancarrow, M.; Peleckis, G.; Turdy, M.; Du, K.; Xu, X.; Li, W.; Jiang, L.; et al. A Liquid-Metal-Based Magnetoactive Slurry for Stimuli-Responsive Mechanically Adaptive Electrodes. Adv. Mater. 2018, 30, 1802595. [Google Scholar] [CrossRef]
- Liu, S.; Yuen, M.C.; White, E.L.; Boley, J.W.; Deng, B.; Cheng, G.J.; Kramer-Bottiglio, R. Laser Sintering of Liquid Metal Nanoparticles for Scalable Manufacturing of Soft and Flexible Electronics. ACS Appl. Mater. Interfaces 2018, 10, 28232–28241. [Google Scholar] [CrossRef]
- Liu, S.; Reed, S.N.; Higgins, M.J.; Titus, M.S.; Kramer-Bottiglio, R. Oxide Rupture-Induced Conductivity in Liquid Metal Nanoparticles by Laser and Thermal Sintering. Nanoscale 2019, 11, 17615–17629. [Google Scholar] [CrossRef]
- Liu, S.; Yuen, M.C.; Kramer-Bottiglio, R. Reconfigurable Electronic Devices Enabled by Laser-Sintered Liquid Metal Nanoparticles. Flex. Print. Electron. 2019, 4, 015004. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J. Pressured Liquid Metal Screen Printing for Rapid Manufacture of High Resolution Electronic Patterns. RSC Adv. 2015, 5, 57686–57691. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, Y.; Liu, J. Atomized Spraying of Liquid Metal Droplets on Desired Substrate Surfaces as a Generalized Way for Ubiquitous Printed Electronics. Appl. Phys. A 2014, 116, 1091–1097. [Google Scholar] [CrossRef]
- Kent, T.A.; Ford, M.J.; Markvicka, E.J.; Majidi, C. Soft Actuators Using Liquid Crystal Elastomers with Encapsulated Liquid Metal Joule Heaters. Multifunct. Mater. 2020, 3, 025003. [Google Scholar] [CrossRef]
- Varga, M.; Ladd, C.; Ma, S.; Holbery, J.; Tröster, G. On-Skin Liquid Metal Inertial Sensor. Lab Chip 2017, 17, 3272–3278. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, J. Ink Spraying Based Liquid Metal Printed Electronics for Directly Making Smart Home Appliances. ECS J. Solid State Sci. Technol. 2015, 4, P3057. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.H.; Cruz, F.J.; Chen, S.; Gravier, L.; Liu, J.; Wu, Z.; Hjort, K.; Zhang, S.-L.; Zhang, Z.-B. Stretchable Thermoelectric Generators Metallized with Liquid Alloy. ACS Appl. Mater. Interfaces 2017, 9, 15791–15797. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Kruse, M.; Xu, B.; Tutika, R.; Zheng, W.; Bartlett, M.D.; Wu, Y.; Claussen, J.C. Flexible Thermoelectric Generators with Inkjet-Printed Bismuth Telluride Nanowires and Liquid Metal Contacts. Nanoscale 2019, 11, 5222–5230. [Google Scholar] [CrossRef] [Green Version]
- Sargolzaeiaval, Y.; Padmanabhan Ramesh, V.; Neumann, T.V.; Misra, V.; Vashaee, D.; Dickey, M.D.; Öztürk, M.C. Flexible Thermoelectric Generators for Body Heat Harvesting—Enhanced Device Performance Using High Thermal Conductivity Elastomer Encapsulation on Liquid Metal Interconnects. Appl. Energy 2020, 262, 114370. [Google Scholar] [CrossRef]
- Jeong, S.H.; Hjort, K.; Wu, Z. Tape Transfer Printing of a Liquid Metal Alloy for Stretchable RF Electronics. Sensors 2014, 14, 16311–16321. [Google Scholar] [CrossRef]
- Choonee, K.; Syms, R.R.A.; Ahmad, M.M.; Zou, H. Post Processing of Microstructures by PDMS Spray Deposition. Sens. Actuators A Phys. 2009, 155, 253–262. [Google Scholar] [CrossRef]
- Gong, X.; He, S. Highly Durable Superhydrophobic Polydimethylsiloxane/Silica Nanocomposite Surfaces with Good Self-Cleaning Ability. ACS Omega 2020, 5, 4100–4108. [Google Scholar] [CrossRef] [PubMed]
- Gui, H.; Tan, S.; Wang, Q.; Yu, Y.; Liu, F.; Lin, J.; Liu, J. Spraying Printing of Liquid Metal Electronics on Various Clothes to Compose Wearable Functional Device. Sci. China Technol. Sci. 2017, 60, 306–316. [Google Scholar] [CrossRef]
- Guo, C.; Yu, Y.; Liu, J. Rapidly Patterning Conductive Components on Skin Substrates as Physiological Testing Devices via Liquid Metal Spraying and Pre-Designed Mask. J. Mater. Chem. B 2014, 2, 5739–5745. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Hjort, K.; Wu, Z. Tape Transfer Atomization Patterning of Liquid Alloys for Microfluidic Stretchable Wireless Power Transfer. Sci. Rep. 2015, 5, 8419. [Google Scholar] [CrossRef]
- Melcher, J.L.; Elassy, K.S.; Ordonez, R.C.; Hayashi, C.; Ohta, A.T.; Garmire, D. Spray-On Liquid-Metal Electrodes for Graphene Field-Effect Transistors. Micromachines 2019, 10, 54. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Kim, S.; Kim, T.; Kim, W.; Lee, J. Spray-Coated Liquid Metal Reflectors for Transparent Hydrogel Atomic Force Microscope Cantilevers. J. Microelectromech. Syst. 2016, 25, 848–850. [Google Scholar] [CrossRef]
- Bartholomeusz, D.A.; Boutte, R.W.; Andrade, J.D. Xurography: Rapid Prototyping of Microstructures Using a Cutting Plotter. J. Microelectromech. Syst. 2005, 14, 1364–1374. [Google Scholar] [CrossRef]
- Zrnic, D.; Swatik, D.S. On the Resistivity and Surface Tension of the Eutectic Alloy of Gallium and Indium. J. Less Common Met. 1969, 18, 67–68. [Google Scholar] [CrossRef]
- Chiechi, R.C.; Weiss, E.A.; Dickey, M.D.; Whitesides, G.M. Eutectic Gallium–Indium (EGaIn): A Moldable Liquid Metal for Electrical Characterization of Self-Assembled Monolayers. Angew. Chem. Int. Ed. 2008, 47, 142–144. [Google Scholar] [CrossRef]
- Dickey, M.D. Emerging Applications of Liquid Metals Featuring Surface Oxides. ACS Appl. Mater. Interfaces 2014, 6, 18369–18379. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Wu, X.; Lee, D.-W. A Galinstan-Based Inkjet Printing System for Highly Stretchable Electronics with Self-Healing Capability. Lab Chip 2016, 16, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Yoo, J.H.; Lee, Y.; Choi, W.; Yoo, K.; Lee, J.-B. Gallium-Based Liquid Metal Inkjet Printing. In Proceedings of the 2014 IEEE 27th International Conference on Micro Electro Mechanical Systems (MEMS), San Francisco, CA, USA, 26–30 January 2014; pp. 967–970. [Google Scholar]
- Khan, M.R.; Eaker, C.B.; Bowden, E.F.; Dickey, M.D. Giant and Switchable Surface Activity of Liquid Metal via Surface Oxidation. Proc. Natl. Acad. Sci. USA 2014, 111, 14047–14051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, A.; Mashima, Y.; Iyoda, T. Reversible Size Control of Liquid-Metal Nanoparticles under Ultrasonication. Angew. Chem. Int. Ed. 2015, 54, 12809–12813. [Google Scholar] [CrossRef]
- Suarez, F.; Nozariasbmarz, A.; Vashaee, D.; Öztürk, M.C. Designing Thermoelectric Generators for Self-Powered Wearable Electronics. Energy Environ. Sci. 2016, 9, 2099–2113. [Google Scholar] [CrossRef]
- Morris, N.J.; Farrell, Z.J.; Tabor, C.E. Chemically Modifying the Mechanical Properties of Core–Shell Liquid Metal Nanoparticles. Nanoscale 2019, 11, 17308–17318. [Google Scholar] [CrossRef]
- Farrell, Z.J.; Thrasher, C.J.; Flynn, A.E.; Tabor, C.E. Silanized Liquid-Metal Nanoparticles for Responsive Electronics. ACS Appl. Nano Mater. 2020, 3, 6297–6303. [Google Scholar] [CrossRef]
- Lear, T.R.; Hyun, S.-H.; Boley, J.W.; White, E.L.; Thompson, D.H.; Kramer, R.K. Liquid Metal Particle Popping: Macroscale to Nanoscale. Extrem. Mech. Lett. 2017, 13, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Foresti, D.; Kroll, K.T.; Amissah, R.; Sillani, F.; Homan, K.A.; Poulikakos, D.; Lewis, J.A. Acoustophoretic Printing. Sci. Adv. 2018, 4, eaat1659. [Google Scholar] [CrossRef] [Green Version]
- Heintzenberg, J. Properties of the Log-Normal Particle Size Distribution. Aerosol Sci. Technol. 1994, 21, 46–48. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, Y.; Genzer, J.; Dickey, M.D. Shape-Transformable Liquid Metal Nanoparticles in Aqueous Solution. Chem. Sci. 2017, 8, 3832–3837. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Cooper, C.; Wang, M.; Adams, J.J.; Genzer, J.; Dickey, M.D. Handwritten, Soft Circuit Boards and Antennas Using Liquid Metal Nanoparticles. Small 2015, 11, 6397–6403. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.R.; Parekh, D.P.; Dickey, M.D.; Hsiao, L.C. Interfacial Rheology of Gallium-Based Liquid Metals. Langmuir 2019, 35, 11774–11783. [Google Scholar] [CrossRef] [PubMed]
- Elton, E.S.; Reeve, T.C.; Thornley, L.E.; Joshipura, I.D.; Paul, P.H.; Pascall, A.J.; Jeffries, J.R. Dramatic Effect of Oxide on Measured Liquid Metal Rheology. J. Rheol. 2019, 64, 119–128. [Google Scholar] [CrossRef]
- Fassler, A.; Majidi, C. 3D Structures of Liquid-Phase GaIn Alloy Embedded in PDMS with Freeze Casting. Lab Chip 2013, 13, 4442–4450. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Mondal, K.; Treadway, R.M.; Kumar, V.; Ma, S.; Holbery, J.D.; Dickey, M.D. Silicones for Stretchable and Durable Soft Devices: Beyond Sylgard-184. ACS Appl. Mater. Interfaces 2018, 10, 11261–11268. [Google Scholar] [CrossRef]
- Bartlett, M.D.; Fassler, A.; Kazem, N.; Markvicka, E.J.; Mandal, P.; Majidi, C. Stretchable, High-k Dielectric Elastomers through Liquid-Metal Inclusions. Adv. Mater. 2016, 28, 3726–3731. [Google Scholar] [CrossRef]
- Fassler, A.; Majidi, C. Soft-Matter Capacitors and Inductors for Hyperelastic Strain Sensing and Stretchable Electronics. Smart Mater. Struct. 2013, 22, 055023. [Google Scholar] [CrossRef]
- Yang, J.; Tang, D.; Ao, J.; Ghosh, T.; Neumann, T.V.; Zhang, D.; Piskarev, E.; Yu, T.; Truong, V.K.; Xie, K.; et al. Ultrasoft Liquid Metal Elastomer Foams with Positive and Negative Piezopermittivity for Tactile Sensing. Adv. Funct. Mater. 2020, 30, 2002611. [Google Scholar] [CrossRef]
- Inglesfield, J.E. The Structure and Phase Changes of Gallium. J. Phys. C Solid State Phys. 1968, 1, 1337–1346. [Google Scholar] [CrossRef]
- Koster, J.N. Directional Solidification and Melting of Eutectic GaIn. Cryst. Res. Technol. 1999, 34, 1129–1140. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neumann, T.V.; Kara, B.; Sargolzaeiaval, Y.; Im, S.; Ma, J.; Yang, J.; Ozturk, M.C.; Dickey, M.D. Aerosol Spray Deposition of Liquid Metal and Elastomer Coatings for Rapid Processing of Stretchable Electronics. Micromachines 2021, 12, 146. https://doi.org/10.3390/mi12020146
Neumann TV, Kara B, Sargolzaeiaval Y, Im S, Ma J, Yang J, Ozturk MC, Dickey MD. Aerosol Spray Deposition of Liquid Metal and Elastomer Coatings for Rapid Processing of Stretchable Electronics. Micromachines. 2021; 12(2):146. https://doi.org/10.3390/mi12020146
Chicago/Turabian StyleNeumann, Taylor V., Berra Kara, Yasaman Sargolzaeiaval, Sooik Im, Jinwoo Ma, Jiayi Yang, Mehmet C. Ozturk, and Michael D. Dickey. 2021. "Aerosol Spray Deposition of Liquid Metal and Elastomer Coatings for Rapid Processing of Stretchable Electronics" Micromachines 12, no. 2: 146. https://doi.org/10.3390/mi12020146
APA StyleNeumann, T. V., Kara, B., Sargolzaeiaval, Y., Im, S., Ma, J., Yang, J., Ozturk, M. C., & Dickey, M. D. (2021). Aerosol Spray Deposition of Liquid Metal and Elastomer Coatings for Rapid Processing of Stretchable Electronics. Micromachines, 12(2), 146. https://doi.org/10.3390/mi12020146