Stiff-to-Soft Transition from Glass to 3D Hydrogel Substrates in Neuronal Cell Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydrogel Fabrication
2.1.1. GelMA (Gelatin Methacryloyl) Preparation
2.1.2. PEGDA (Poly(ethylene glycol) diacrylate) Preparation
2.1.3. Red-Colored Thermoset Gelatin Preparation
2.1.4. Cell Culture and Differentiation
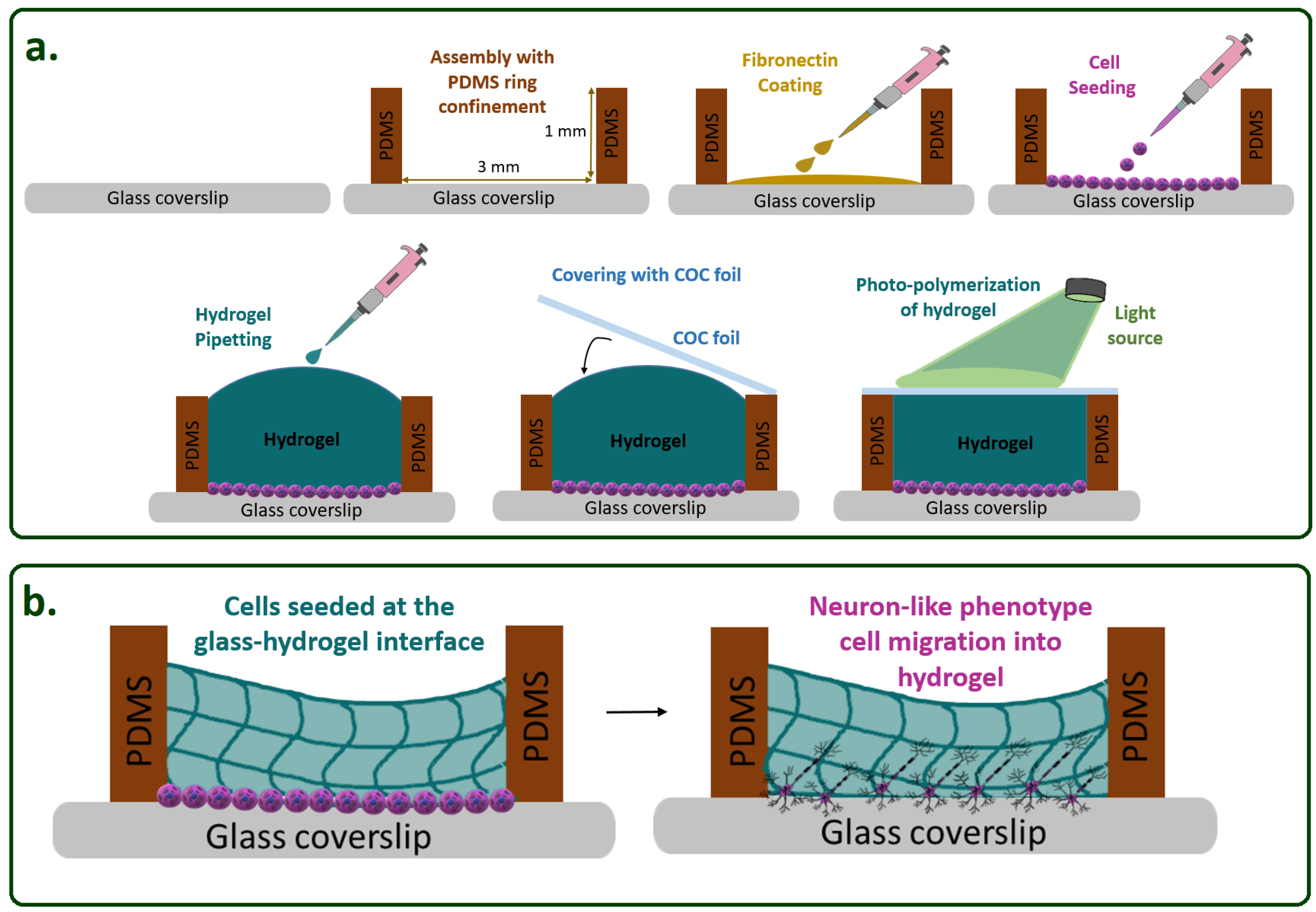
Hydrogel Construct Preparation
Cell Loading
2.1.5. Immunofluorescence Staining Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastiaens, A.J.; Frimat, J.P.; van Nunen, T.; Schurink, B.; Homburg, E.F.; Luttge, R. Advancing a MEMS-based 3D cell culture system for in vitro neuro-electrophysiological recordings. Front. Mech. Eng. 2018, 4, 21. [Google Scholar] [CrossRef]
- Xie, S.; Gardeniers, J.; Luttge, R. Nanoscale membrane actuator for in vitro mechano-stimuli responsive studies of neuronal cell networks on chip. J. Micromech. Microeng. 2018, 28, 085011. [Google Scholar] [CrossRef]
- Ye, W.; Li, H.; Yu, K.; Xie, C.; Wang, P.; Zheng, Y.; Zhang, P.; Xiu, J.; Yang, Y.; Zhang, F.; et al. 3D printing of gelatin methacrylate-based nerve guidance conduits with multiple channels. Mater. Des. 2020, 192, 108757. [Google Scholar] [CrossRef]
- Park, S.; Park, K.M. Engineered polymeric hydrogels for 3D tissue models. Polymers 2016, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Recent advances in photo-crosslinkable hydrogels for biomedical applications. BioTechniques 2019, 66, 40–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenje, M.; Cantoni, F.; Hernández, A.M.P.; Searle, S.S.; Johansson, S.; Barbe, L.; Antfolk, M.; Pohlit, H. A practical guide to microfabrication and patterning of hydrogels for biomimetic cell culture scaffolds. Organs-on-a-Chip 2020, 100003. [Google Scholar] [CrossRef]
- Kim, H.N.; Choi, N. Consideration of the Mechanical Properties of Hydrogels for Brain Tissue Engineering and Brain-on-a-chip. BioChip J. 2019, 13, 8–19. [Google Scholar] [CrossRef]
- Tsou, Y.H.; Khoneisser, J.; Huang, P.C.; Xu, X. Hydrogel as a bioactive material to regulate stem cell fate. Bioact. Mater. 2016, 1, 39–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwashita, M.; Ohta, H.; Fujisawa, T.; Cho, M.; Ikeya, M.; Kidoaki, S.; Kosodo, Y. Brain-stiffness-mimicking tilapia collagen gel promotes the induction of dorsal cortical neurons from human pluripotent stem cells. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Mazza, G.; Al-Akkad, W.; Rombouts, K.; Pinzani, M. Liver tissue engineering: From implantable tissue to whole organ engineering. Hepatol. Commun. 2018, 2, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Papavasiliou, G.; Sokic, S.; Turturro, M. Synthetic PEG hydrogels as extracellular matrix mimics for tissue engineering applications. In Biotechnology Molecular Studies and Novel Applications for Improved Quality of Human Life; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Al Rifai, N.; Hasan, A.; Kobeissy, F.; Gazalah, H.; Charara, J. Culture of PC12 neuronal cells in GelMA hydrogel for brain tissue engineering. In Proceedings of the 2015 International Conference on Advances in Biomedical Engineering (ICABME), Beirut, Lebanon, 16–18 September 2015; pp. 254–257. [Google Scholar]
- Yanagawa, F.; Sugiura, S.; Kanamori, T. Hydrogel microfabrication technology toward three dimensional tissue engineering. Regen. Therapy 2016, 3, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Bastiaens, A.; Xie, S.; Luttge, R. Nanogroove-enhanced hydrogel scaffolds for 3D neuronal cell culture: An easy access brain-on-chip model. Micromachines 2019, 10, 638. [Google Scholar] [CrossRef] [Green Version]
- Kropp, C.; Massai, D.; Zweigerdt, R. Progress and challenges in large-scale expansion of human pluripotent stem cells. Process Biochem. 2017, 59, 244–254. [Google Scholar] [CrossRef] [Green Version]
- Marchioli, G.; Zellner, L.; Oliveira, C.; Engelse, M.; de Koning, E.; Mano, J.; Karperien; van Apeldoorn, A.; Moroni, L. Layered PEGDA hydrogel for islet of Langerhans encapsulation and improvement of vascularization. J. Mater. Sci. Mater. Med. 2017, 28, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepelanova, I.; Kruppa, K.; Scheper, T.; Lavrentieva, A. Gelatin-Methacryloyl (GelMA) hydrogels with defined degree of functionalization as a versatile toolkit for 3D cell culture and extrusion bioprinting. Bioengineering 2018, 5, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.; Liu, C.; Chen, X.; Zou, Y.; Zhou, Z.; Lin, C.; Tan, G.; Zhou, L.; Ning, C.; Wang, Q. Directing induced pluripotent stem cell derived neural stem cell fate with a three-dimensional biomimetic hydrogel for spinal cord injury repair. ACS Appl. Mater. Interfaces 2018, 10, 17742–17755. [Google Scholar] [CrossRef] [PubMed]
- Dehmelt, L.; Halpain, S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2005, 6, 1–10. [Google Scholar]
- Hromadkova, L.; Bezdekova, D.; Pala, J.; Schedin-Weiss, S.; Tjernberg, L.O.; Hoschl, C.; Ovsepian, S.V. Brain-derived neurotropic factor (BDNF) promotes molecular polarization and differentiation of immature neuroblastoma cells into definitive neurons. Biochim. Biophys. Acta-(Bba)-Mol. Cell Res. 2020, 1867, 118737. [Google Scholar] [CrossRef]
- Gunhanlar, N.; Shpak, G.; Van Der Kroeg, M.; Gouty-Colomer, L.; Munshi, S.; Lendemeijer, B.; Ghazvini, M.; Dupont, C.; Hoogendijk, W.; Gribnau, J.; et al. A simplified protocol for differentiation of electrophysiologically mature neuronal networks from human induced pluripotent stem cells. Mol. Psychiatry 2018, 23, 1336–1344. [Google Scholar] [CrossRef] [Green Version]
- Satir, T.M.; Nazir, F.H.; Vizlin-Hodzic, D.; Hardselius, E.; Blennow, K.; Wray, S.; Zetterberg, H.; Agholme, L.; Bergström, P. Accelerated neuronal and synaptic maturation by BrainPhys medium increases Aβ secretion and alters Aβ peptide ratios from iPSC-derived cortical neurons. Sci. Rep. 2020, 10, 1–17. [Google Scholar]
- Nazir, F.H.; Becker, B.; Brinkmalm, A.; Höglund, K.; Sandelius, Å.; Bergström, P.; Satir, T.M.; Öhrfelt, A.; Blennow, K.; Agholme, L.; et al. Expression and secretion of synaptic proteins during stem cell differentiation to cortical neurons. Neurochem. Int. 2018, 121, 38–49. [Google Scholar] [CrossRef]
- Hansen, S.K.; Stummann, T.C.; Borland, H.; Hasholt, L.F.; Tümer, Z.; Nielsen, J.E.; Rasmussen, M.A.; Nielsen, T.T.; Daechsel, J.C.; Fog, K.; et al. Induced pluripotent stem cell-derived neurons for the study of spinocerebellar ataxia type 3. Stem Cell Res. 2016, 17, 306–317. [Google Scholar] [CrossRef] [Green Version]
- Klotz, B.J.; Oosterhoff, L.A.; Utomo, L.; Lim, K.S.; Vallmajo-Martin, Q.; Clevers, H.; Woodfield, T.B.; Rosenberg, A.J.; Malda, J.; Ehrbar, M.; et al. A versatile biosynthetic hydrogel platform for engineering of tissue analogues. Adv. Healthc. Mater. 2019, 8, 1900979. [Google Scholar] [CrossRef] [Green Version]
- Uwamori, H.; Higuchi, T.; Arai, K.; Sudo, R. Integration of neurogenesis and angiogenesis models for constructing a neurovascular tissue. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.W.; Lee, H.J.; Hwang, H.S.; Ko, K.; Han, D.W.; Ko, K. Optimization of Matrigel-based culture for expansion of neural stem cells. Anim. Cells Syst. 2015, 19, 175–180. [Google Scholar] [CrossRef]
- Kawaguchi, N.; Toriyama, K.; Nicodemou-Lena, E.; Inou, K.; Torii, S.; Kitagawa, Y. De novo adipogenesis in mice at the site of injection of basement membrane and basic fibroblast growth factor. Proc. Natl. Acad. Sci. USA 1998, 95, 1062–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakunts, K.; Gillum, N.; Karabekian, Z.; Sarvazyan, N. Formation of cardiac fibers in Matrigel matrix. Biotechniques 2008, 44, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Garnier, D.; Li, R.; Delbos, F.; Fourrier, A.; Collet, C.; Guguen-Guillouzo, C.; Chesné, C.; Nguyen, T.H. Expansion of human primary hepatocytes in vitro through their amplification as liver progenitors in a 3D organoid system. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q.; Wang, O.; Shao, K.; Lin, H.; Lei, Y. A simple and scalable hydrogel-based system for culturing protein-producing cells. PLoS ONE 2018, 13, e0190364. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Weir, M.D.; Xu, H.H. Effect of cell seeding density on proliferation and osteodifferentiation of umbilical cord stem cells on calcium phosphate cement-fiber scaffold. Tissue Eng. Part A 2011, 17, 2603–2613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talukdar, S.; Nguyen, Q.T.; Chen, A.C.; Sah, R.L.; Kundu, S.C. Effect of initial cell seeding density on 3D-engineered silk fibroin scaffolds for articular cartilage tissue engineering. Biomaterials 2011, 32, 8927–8937. [Google Scholar] [CrossRef] [Green Version]
- Tyler, W.J. The mechanobiology of brain function. Nat. Rev. Neurosci. 2012, 13, 867–878. [Google Scholar] [CrossRef]
- Lozano, R.; Stevens, L.; Thompson, B.C.; Gilmore, K.J.; Gorkin, R., III; Stewart, E.M.; in het Panhuis, M.; Romero-Ortega, M.; Wallace, G.G. 3D printing of layered brain-like structures using peptide modified gellan gum substrates. Biomaterials 2015, 67, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Koser, D.E.; Thompson, A.J.; Foster, S.K.; Dwivedy, A.; Pillai, E.K.; Sheridan, G.K.; Svoboda, H.; Viana, M.; da F Costa, L.; Guck, J.; et al. Mechanosensing is critical for axon growth in the developing brain. Nat. Neurosci. 2016, 19, 1592–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akcay, G.; Luttge, R. Stiff-to-Soft Transition from Glass to 3D Hydrogel Substrates in Neuronal Cell Culture. Micromachines 2021, 12, 165. https://doi.org/10.3390/mi12020165
Akcay G, Luttge R. Stiff-to-Soft Transition from Glass to 3D Hydrogel Substrates in Neuronal Cell Culture. Micromachines. 2021; 12(2):165. https://doi.org/10.3390/mi12020165
Chicago/Turabian StyleAkcay, Gulden, and Regina Luttge. 2021. "Stiff-to-Soft Transition from Glass to 3D Hydrogel Substrates in Neuronal Cell Culture" Micromachines 12, no. 2: 165. https://doi.org/10.3390/mi12020165
APA StyleAkcay, G., & Luttge, R. (2021). Stiff-to-Soft Transition from Glass to 3D Hydrogel Substrates in Neuronal Cell Culture. Micromachines, 12(2), 165. https://doi.org/10.3390/mi12020165





