A Small Hybrid Power System of Photovoltaic Cell and Sodium Borohydride Hydrolysis-Based Fuel Cell
Abstract
:1. Introduction
2. Design of the Hybrid Power System
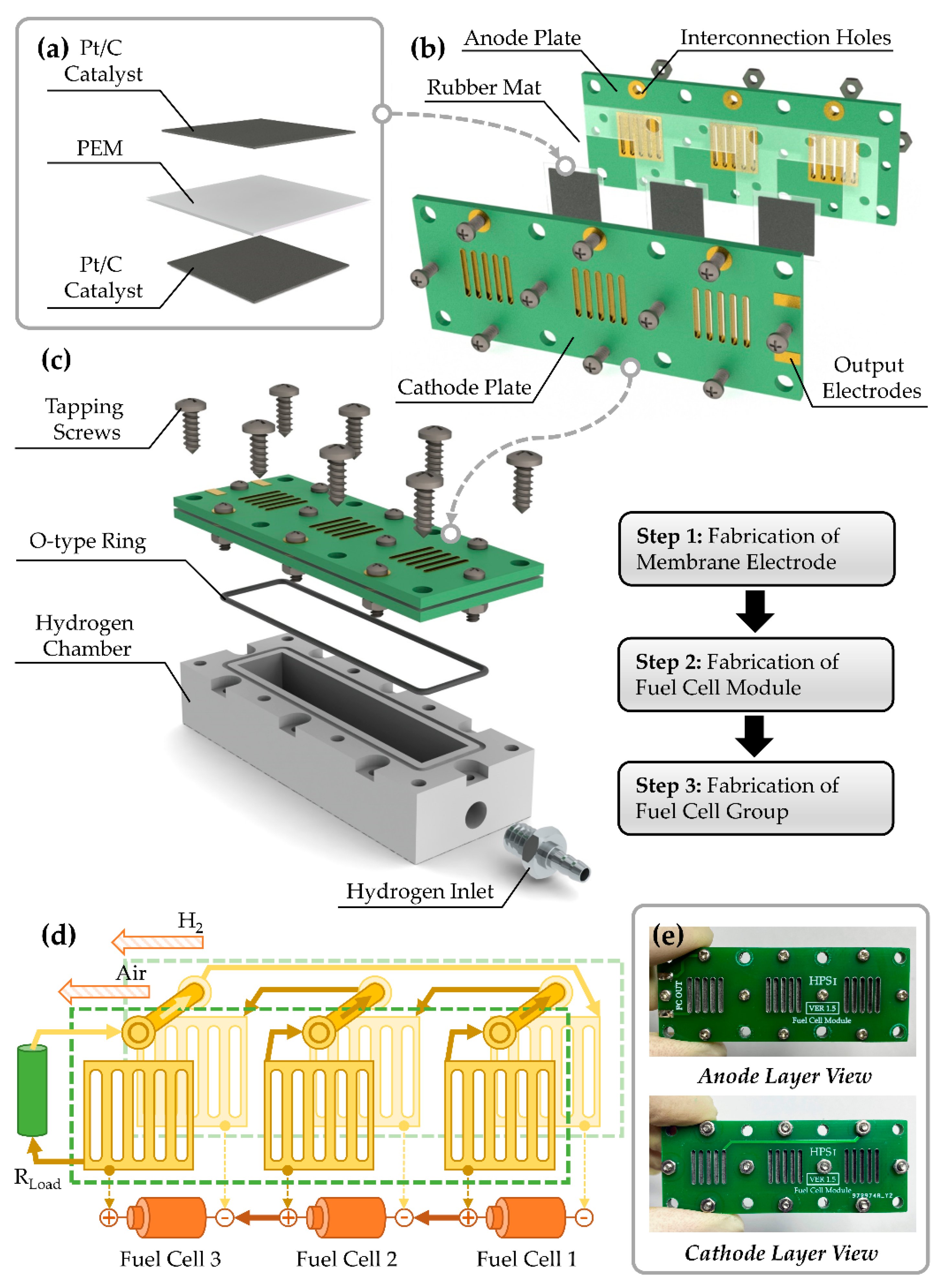
2.1. Design of Fuel Cell Group
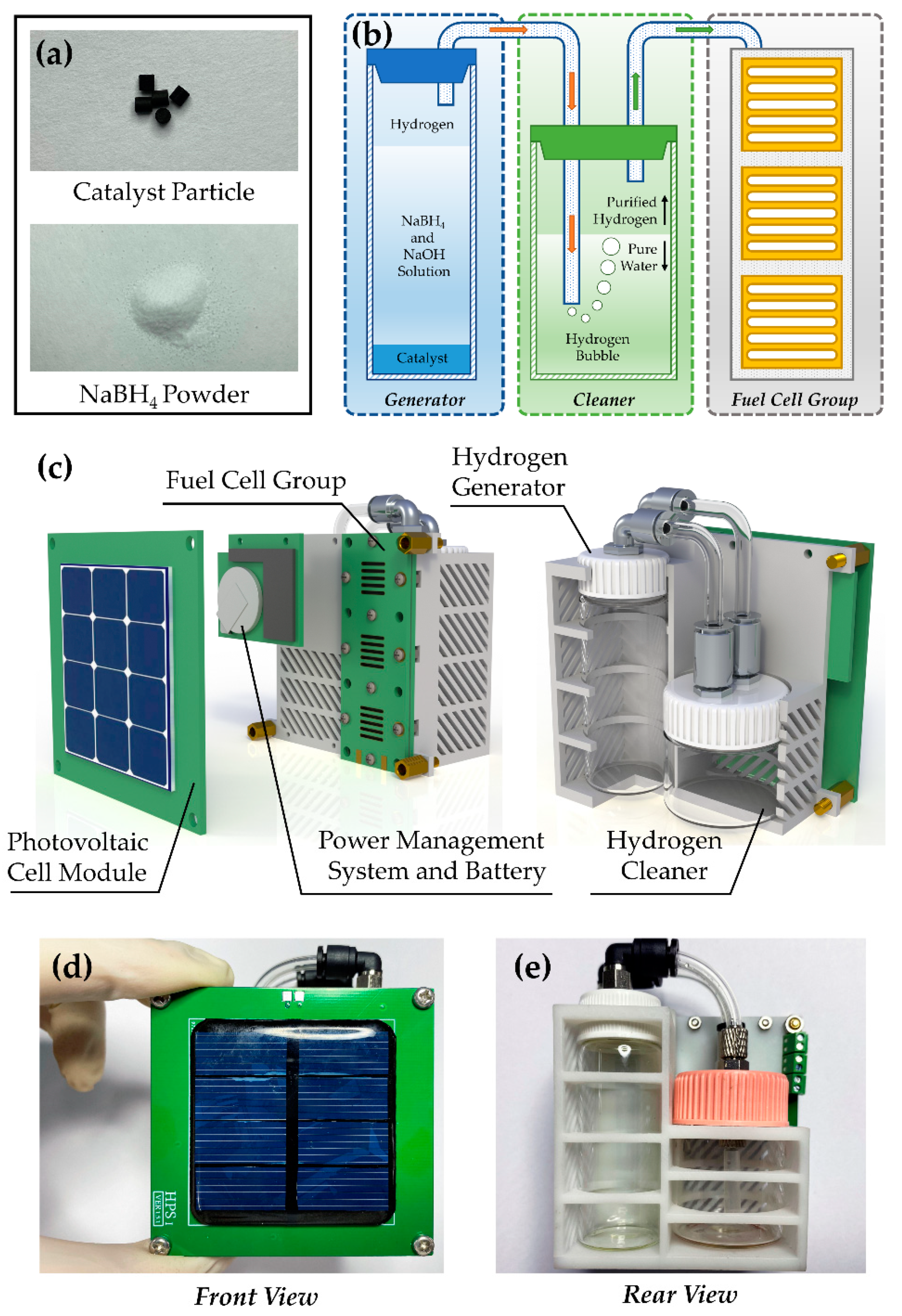
2.2. Design of Hydrogen Generator
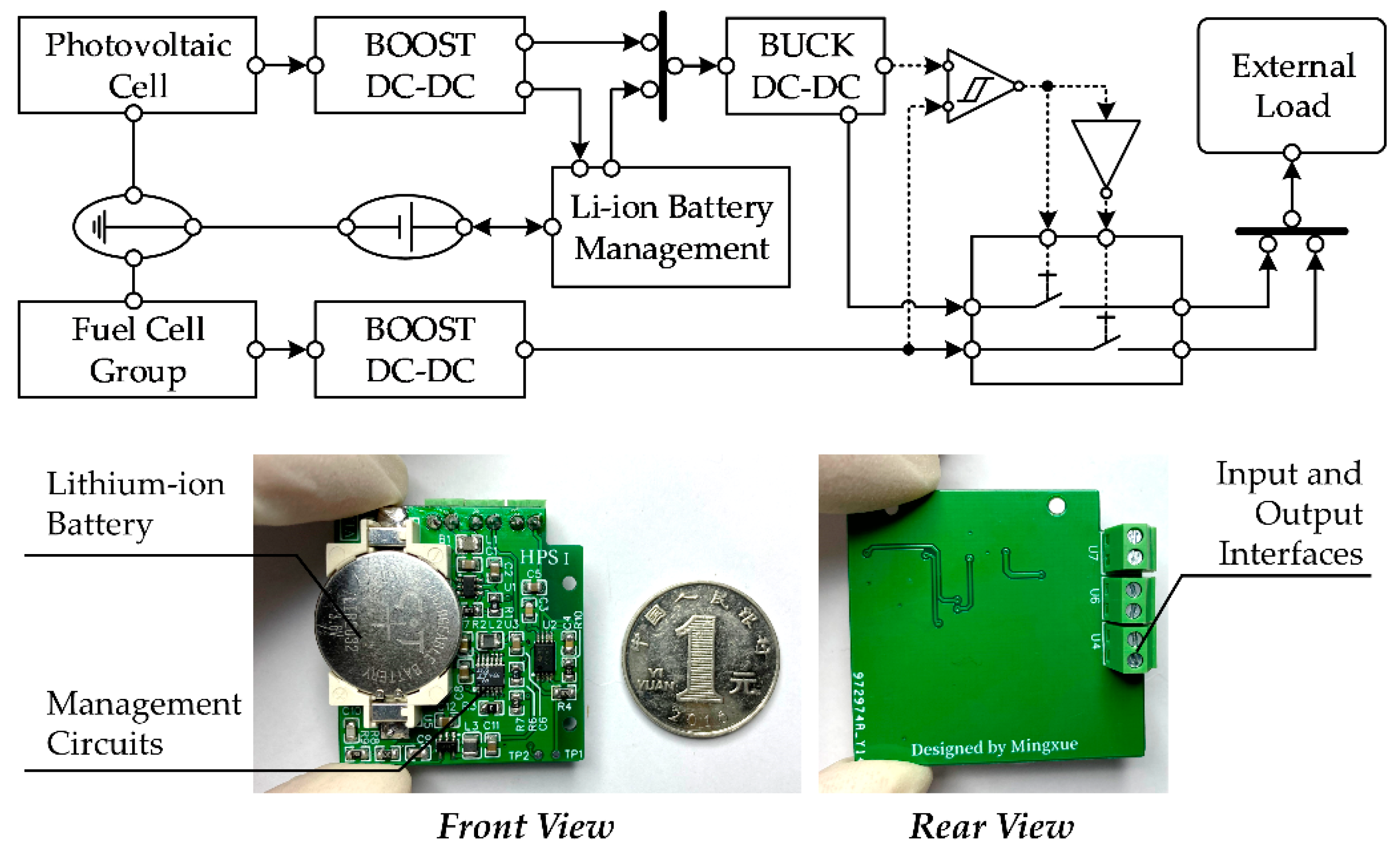
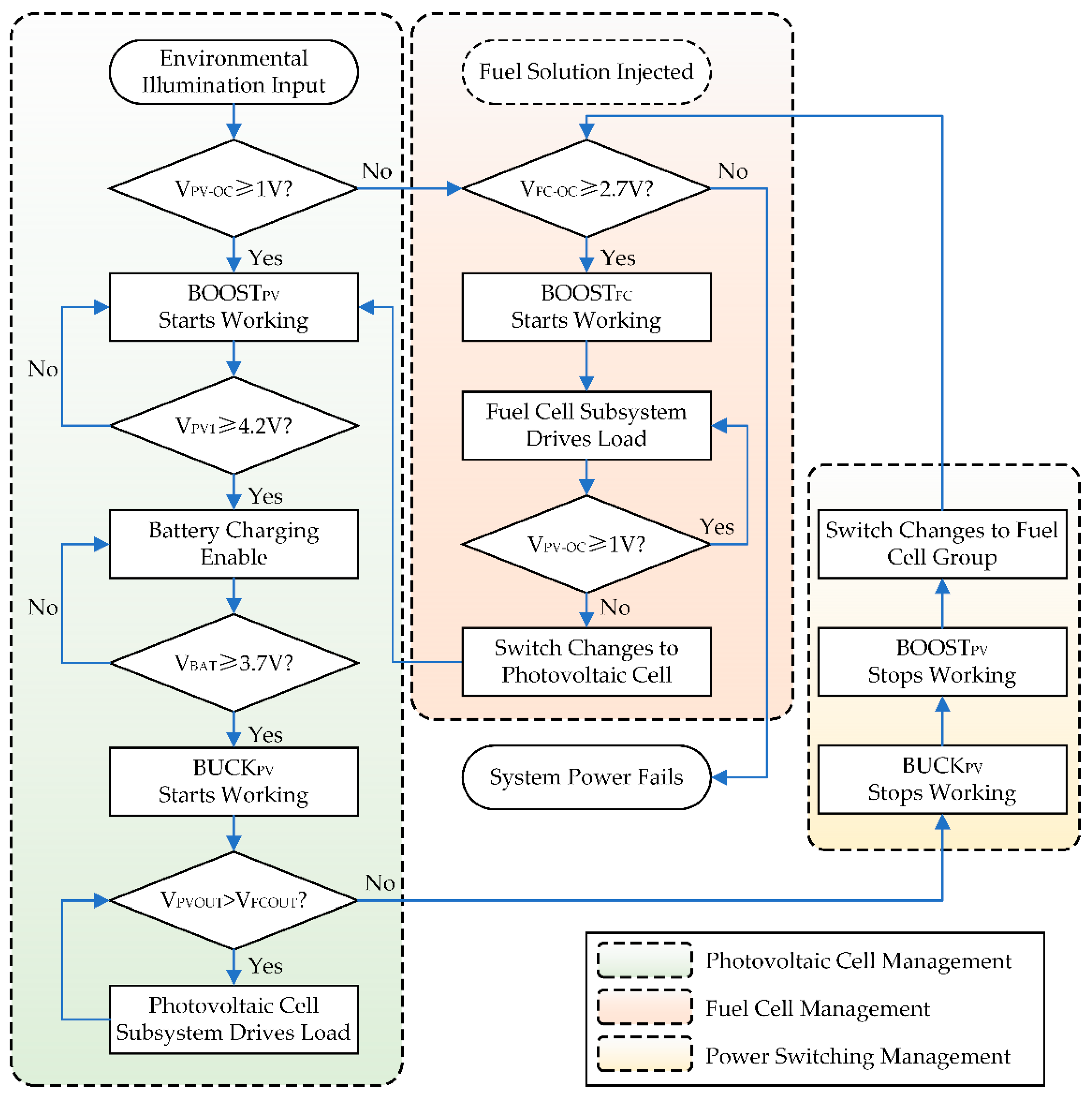
2.3. Design of the Hybrid Power Management Circuit
3. Results and Discussion
3.1. Test of the Power-Generating Devices
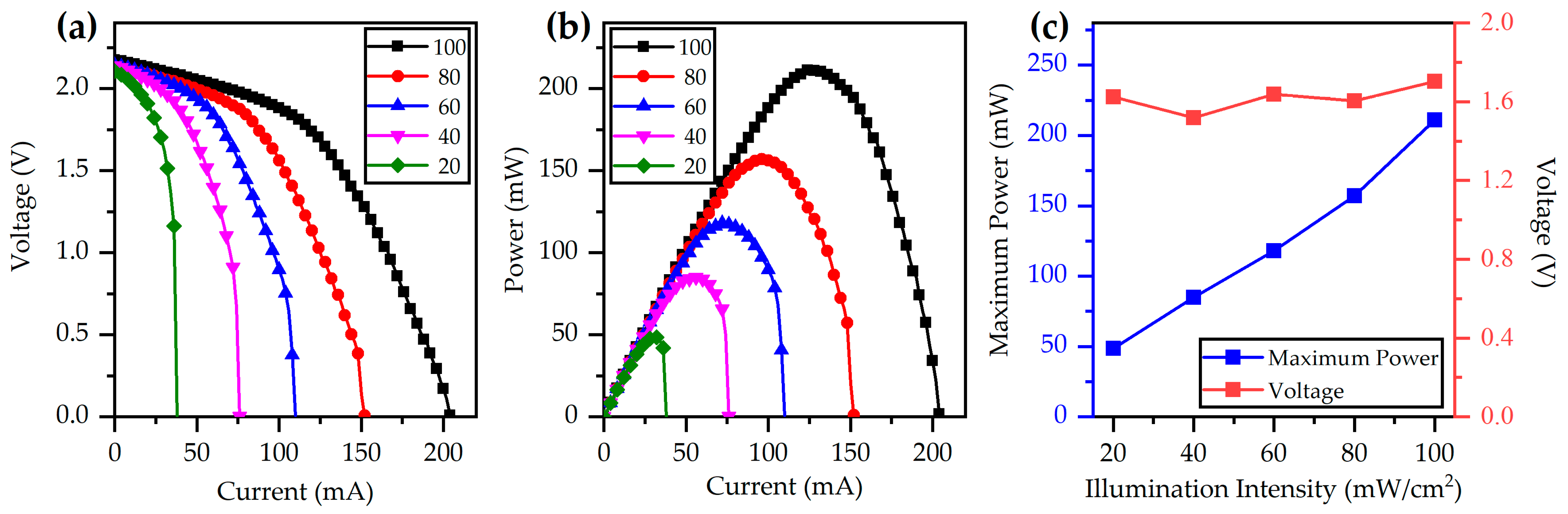
3.1.1. Photovoltaic Cell Test
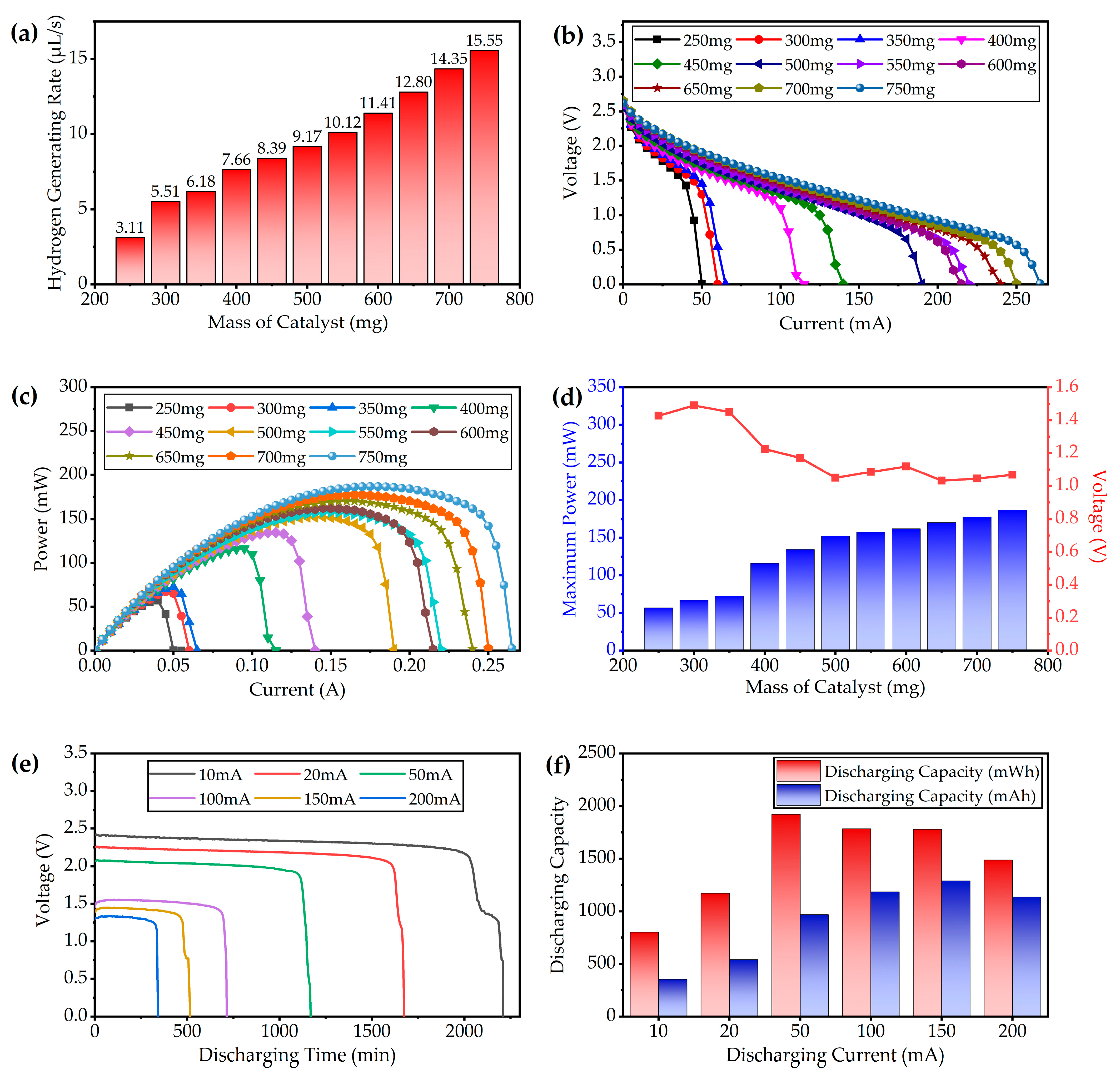
3.1.2. Fuel Cell Test
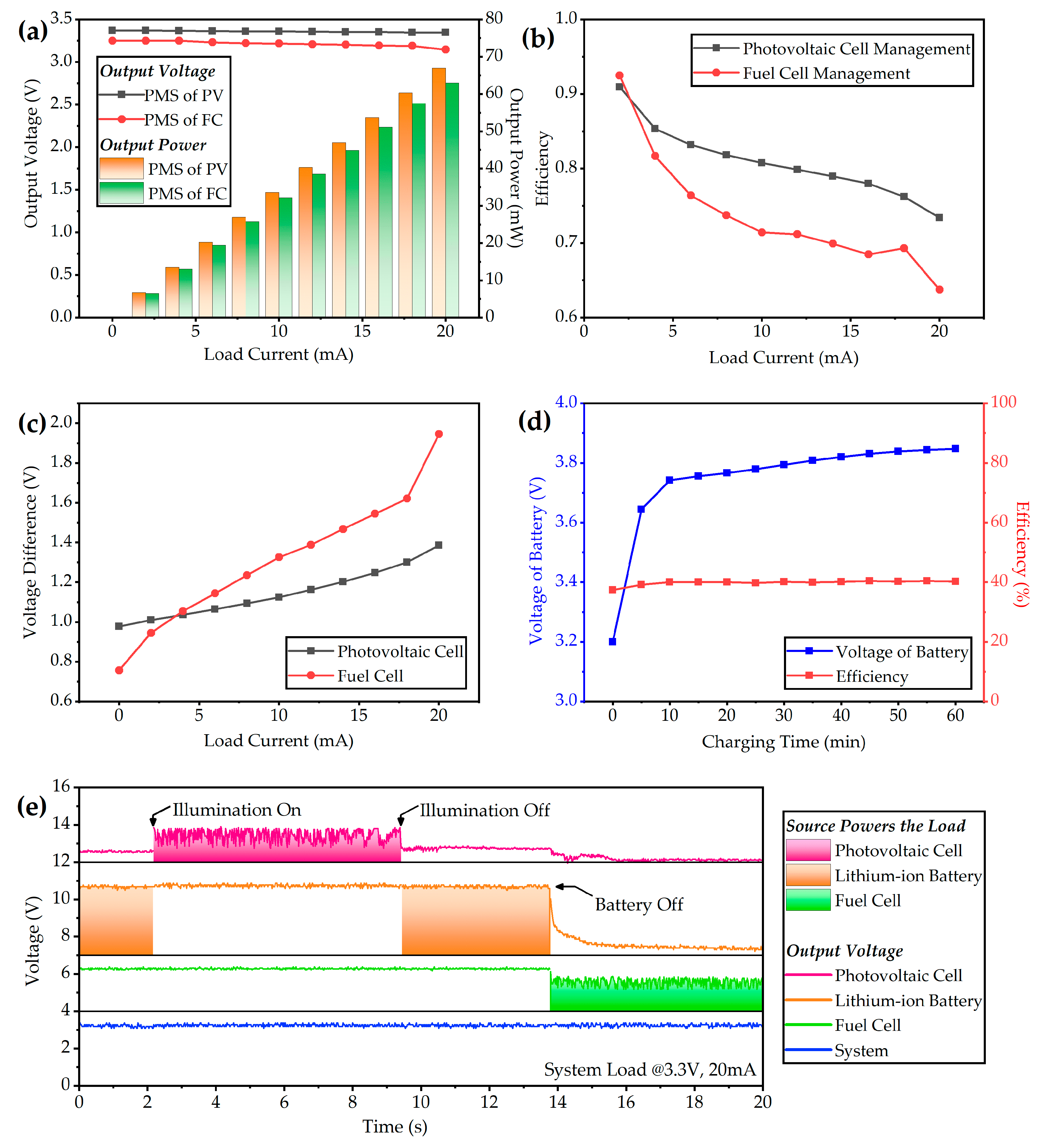
3.2. Test of the Hybrid Power System
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Duan, Q.; Zhao, A. Analysis and Prospect of Wireless Sensor Network Routing Technology. In Precision Engineering and Non-Traditional Machining; Trans Tech Publications Ltd.: Xian, China, 2012; Volume 411, p. 592. [Google Scholar]
- Diakite, H.L.; Yu, L. Energy and Bandwidth Efficient Wireless Sensor Communications for Improving the Energy Efficiency of the Air Interface for Wireless Sensor Networks. In Proceedings of the 2013 International Conference on Information Science and Technology (Icist), Yangzhou, China, 23–25 March 2013; pp. 1426–1429. [Google Scholar]
- Srivastava, S.; Singh, M.; Gupta, S. Wireless Sensor Network: A Survey. In Proceedings of the 2018 International Conference on Automation and Computational Engineering (Icace), Greater Noida, India, 3–4 October 2018; pp. 159–163. [Google Scholar]
- Marriwala, N.; Rathee, P. An Approach to Increase the Wireless Sensor Network Lifetime. In Proceedings of the 2012 World Congress on Information and Communication Technologies, Trivandrum, India, 30 Octorber–2 November 2012; pp. 495–499. [Google Scholar]
- Tripathi, A.; Gupta, H.P.; Dutta, T.; Mishra, R.; Shukla, K.K.; Jit, S. Coverage and Connectivity in WSNs: A Survey, Research Issues and Challenges. IEEE Access 2018, 6, 26971–26992. [Google Scholar] [CrossRef]
- Rasheduzzaman, M.; Pillai, P.B.; Mendoza AN, C.; De Souza, M.M. A Study of the Performance of Solar Cells for Indoor Autonomous Wireless Sensors. In Proceedings of the 2016 10th International Symposium on Communication Systems, Networks and Digital Signal Processing (Csndsp), Prague, Czech Republic, 20–22 July 2016. [Google Scholar]
- Agarwal, M.; Munjal, A.; Dusane, R. To Demonstrate the Potential Application of “Low Temperature and High Performance Silicon Heterojunction Solar Cells Fabricated Using HWCVD” in Wireless Sensor Network: An Initial Research. J. Sol. Energy Eng. Trans. ASME 2018, 140. [Google Scholar] [CrossRef]
- Ramasur, D.; Hancke, G.P. A wind energy harvester for low power Wireless Sensor Networks. In Proceedings of the 2012 IEEE International Instrumentation and Measurement Technology Conference (I2mtc), Graz, Austria, 13–16 May 2012; pp. 2623–2627. [Google Scholar]
- Farhangdoust, S.; Mehrabi, A.; Younesian, D. Bistable Wind-Induced Vibration Energy Harvester for Self-Powered Wireless Sensors in Smart Bridge Monitoring Systems. In Proceedings of the Nondestructive Characterization and Monitoring of Advanced Materials, Aerospace, Civil Infrastructure, and Transportation Xiii, SPIE-INT SOC Optical Engineering, Denver, CO, USA, 4–7 March 2019; p. 10971. [Google Scholar]
- Wang, P.D.; Yan, Z. A Survey of Vibration Energy Harvesters for Wireless Sensor Networks. In Proceedings of the 2nd International Conference on Electronic & Mechanical Engineering and Information Technology (Emeit-2012), Shenyang, China, 7 September 2012; Atlantis Press: Shenyang, China, 2012; p. 23. [Google Scholar]
- Rodriguez, C.J.; Nico, V.; Punch, J. A Vibration Energy Harvester and Power Management Solution for Battery-Free Operation of Wireless Sensor Nodes. Sensors 2019, 19, 3776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.; Singh, J.; Kuchroo, P.; Bhatia, H.; Bhagat, S.; Sharma, G.; Sidhu, E. Efficient Autonomous Solar Panel and Thermo-Electric Generator (TEG) Integrated Hybrid Energy Harvesting System. In Proceedings of the 2016 Progress in Electromagnetics Research Symposium (Piers), Shanghai, China, 8–11 August 2016; pp. 1764–1768. [Google Scholar]
- Tang, H.; He, S.S.; Peng, C.W. A Short Progress Report on High-Efficiency Perovskite Solar Cells. Nanoscale Res. Lett. 2017, 12, 410. [Google Scholar] [CrossRef] [PubMed]
- Van der Woerd, A.C.; Bais, M.A.; de Jong, L.P.; Van Roermund, A.H. A highly efficient micro-power converter between a solar cell and a rechargeable lithium-ion battery. In 5th Annual International Symposium on Smart Structures and Materials Proceedings of the Volume 3328, Smart Structures and Materials 1998: Smart Electronics and MEMS; SPIE-INT SOC Optical Engineering: San Diego, CA, USA, 1998; Volume 3328, pp. 315–325. [Google Scholar]
- Xu, T.J.; Chen, Y.H.; Dai, L.M. Efficiently photo-charging lithium-ion battery by perovskite solar cell. Nat. Commun. 2015, 6, 8103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurung, A.; Chen, K.; Khan, R.; Abdulkarim, S.S.; Varnekar, G.; Pathak, R.; Naderi, R.; Qiao, Q. Highly Efficient Perovskite Solar Cell Photocharging of Lithium Ion Battery Using DC-DC Booster. Adv. Energy Mater. 2017, 7, 1602105. [Google Scholar] [CrossRef]
- Di, Y.; Jia, S.; Yan, X.; Liang, J.; Hu, S. Available photo-charging integrated device constructed with dye-sensitized solar cells and lithium-ion battery. New J. Chem. 2020, 44, 791–796. [Google Scholar] [CrossRef]
- Ahmed, M.; Dincer, I. A review on methanol crossover in direct methanol fuel cells: Challenges and achievements. Int. J. Energy Res. 2011, 35, 1213–1228. [Google Scholar] [CrossRef]
- Ong, C.B.; Kamarudin, S.K.; Basri, S. Direct liquid fuel cells: A review. Int. J. Hydrog. Energy 2017, 42, 10142–10157. [Google Scholar] [CrossRef]
- Lai, Q.; Alligier, D.; Aguey-Zinsou, K.F.; Demirci, U.B. Hydrogen generation from a sodium borohydride-nickel core@shell structure under hydrolytic conditions. Nanoscale Adv. 2019, 1, 2707–2717. [Google Scholar] [CrossRef] [Green Version]
- Ekinci, A. Hydrogen Generation by Hydrolysis of NaBH(4)with Efficient Co-La-Mo-B Catalyst for PEM Fuel Cells. Kinet. Catal. 2020, 61, 589–594. [Google Scholar] [CrossRef]
- Netskina, O.V.; Tayban, E.S.; Rogov, V.A.; Ozerova, A.M.; Mukha, S.A.; Simagina, V.I.; Komova, O.V. Solid-state NaBH4 composites for hydrogen generation: Catalytic activity of nickel and cobalt catalysts. Int. J. Hydrog. Energy 2021, 46, 5459–5471. [Google Scholar] [CrossRef]
- Dai, P.; Zhao, X.; Xu, D.; Wang, C.; Tao, X.; Liu, X.; Gao, J. Preparation, characterization, and properties of Pt/ AL(2)O(3)/cordierite monolith catalyst for hydrogen generation from hydrolysis of sodium borohydride in a flow reactor. Int. J Hydrog. Energy 2019, 44, 28463–28470. [Google Scholar] [CrossRef]
- Hansu, T.A.; Caglar, A.; Sahin, O.; Kivrak, H. Hydrolysis and electrooxidation of sodium borohydride on novel CNT supported CoBi fuel cell catalyst. Mater. Chem. Phys. 2020, 239, 122031. [Google Scholar] [CrossRef]
- Uzundurukan, A.; Devrim, Y. Hydrogen generation from sodium borohydride hydrolysis by multi-walled carbon nanotube supported platinum catalyst: A kinetic study. Int. J. Hydrog. Energy 2019, 44, 17586–17594. [Google Scholar] [CrossRef]
- Hsueh, C.-L.; Liu, C.-H.; Chen, B.-H.; Lee, M.-S.; Chen, C.-Y.; Lu, Y.-W.; Tsau, F.; Ku, J.-R. A novel design of solid-state NaBH4 composite as a hydrogen source for 2 W PEMFC applications. J. Power Sources 2011, 196, 3530–3538. [Google Scholar] [CrossRef]
- Netskina, V.O.; Komova, O.V.; Simagina, V.I. Developing Effective Cobalt Catalysts for Hydrogen-Generating Solid-State NaBH4 Composite. Catal. Ind. 2018, 10, 166–172. [Google Scholar] [CrossRef]
- Gang, G.B.; Kwon, S. All-in-one portable electric power plant using proton exchange membrane fuel cells for mobile applications. Int. J. Hydrog. Energy 2018, 43, 6331–6339. [Google Scholar] [CrossRef]
- Jung, E.S.; Kim, H.; Kwon, S.; Oh, T.H. Fuel cell system with sodium borohydride hydrogen generator for small unmanned aerial vehicles. Int. J. Green Energy 2018, 15, 385–392. [Google Scholar] [CrossRef]
- Kwon, S.-M.; Kim, M.J.; Kang, S.; Kim, T. Development of a high-storage-density hydrogen generator using solid-state NaBH4 as a hydrogen source for unmanned aerial vehicles. Appl. Energy 2019, 251, 113331. [Google Scholar] [CrossRef]
- Kim, T.; Kwon, S. Design and development of a fuel cell-powered small unmanned aircraft. Int. J. Hydrog. Energy 2012, 37, 615–622. [Google Scholar] [CrossRef]
- Kim, K.; Kim, T.; Lee, K.; Kwon, S. Fuel cell system with sodium borohydride as hydrogen source for unmanned aerial vehicles. J. Power Sources 2011, 196, 9069–9075. [Google Scholar] [CrossRef]
- Deng, J.; Sun, B.; Xu, J.; Shi, Y.; Xie, L.; Zheng, J.; Li, X. A monolithic sponge catalyst for hydrogen generation from sodium borohydride solution for portable fuel cells. Inorg. Chem. Front. 2021, 8, 35–40. [Google Scholar] [CrossRef]
- Nunes, H.X.; Ferreira, M.J.F.; Rangel, C.M.; Pinto, A.M.F.R. Hydrogen generation and storage by aqueous sodium borohydride (NaBH4) hydrolysis for small portable fuel cells (H-2-PEMFC). Int. J. Hydrog. Energy 2016, 41, 15426–15432. [Google Scholar] [CrossRef]
- Lee, J.; Kim, T. Micro PEM fuel cell system with NaBH4 hydrogen generator. Sens. Actuators A Phys. 2012, 177, 54–59. [Google Scholar] [CrossRef]
- Gang, B.G. The Selective Zero Emission Power Systems between NaBH4-Based Fuel Cells and Solar Cells for UAVs. Int. J. Aeronaut. Space Sci. 2020, 21, 1017–1027. [Google Scholar] [CrossRef]
- Valizadeh, M.; Feyzi, M.R.; Babaei, E.; Sabahi, M. Dynamic modeling of modular fuel cell for maximum power point tracking and torque ripple reduction in direct torque control of induction motor. Turk. J. Electr. Eng. Comput. Sci. 2015, 23, 317–334. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Deng, H.; Zhang, Y.; Hou, C. A Small Hybrid Power System of Photovoltaic Cell and Sodium Borohydride Hydrolysis-Based Fuel Cell. Micromachines 2021, 12, 278. https://doi.org/10.3390/mi12030278
Li M, Deng H, Zhang Y, Hou C. A Small Hybrid Power System of Photovoltaic Cell and Sodium Borohydride Hydrolysis-Based Fuel Cell. Micromachines. 2021; 12(3):278. https://doi.org/10.3390/mi12030278
Chicago/Turabian StyleLi, Mingxue, Huichao Deng, Yufeng Zhang, and Chenjun Hou. 2021. "A Small Hybrid Power System of Photovoltaic Cell and Sodium Borohydride Hydrolysis-Based Fuel Cell" Micromachines 12, no. 3: 278. https://doi.org/10.3390/mi12030278




