Development of Rapid and High-Precision Colorimetric Device for Organophosphorus Pesticide Detection Based on Microfluidic Mixer Chip
Abstract
1. Introduction
2. Experimental
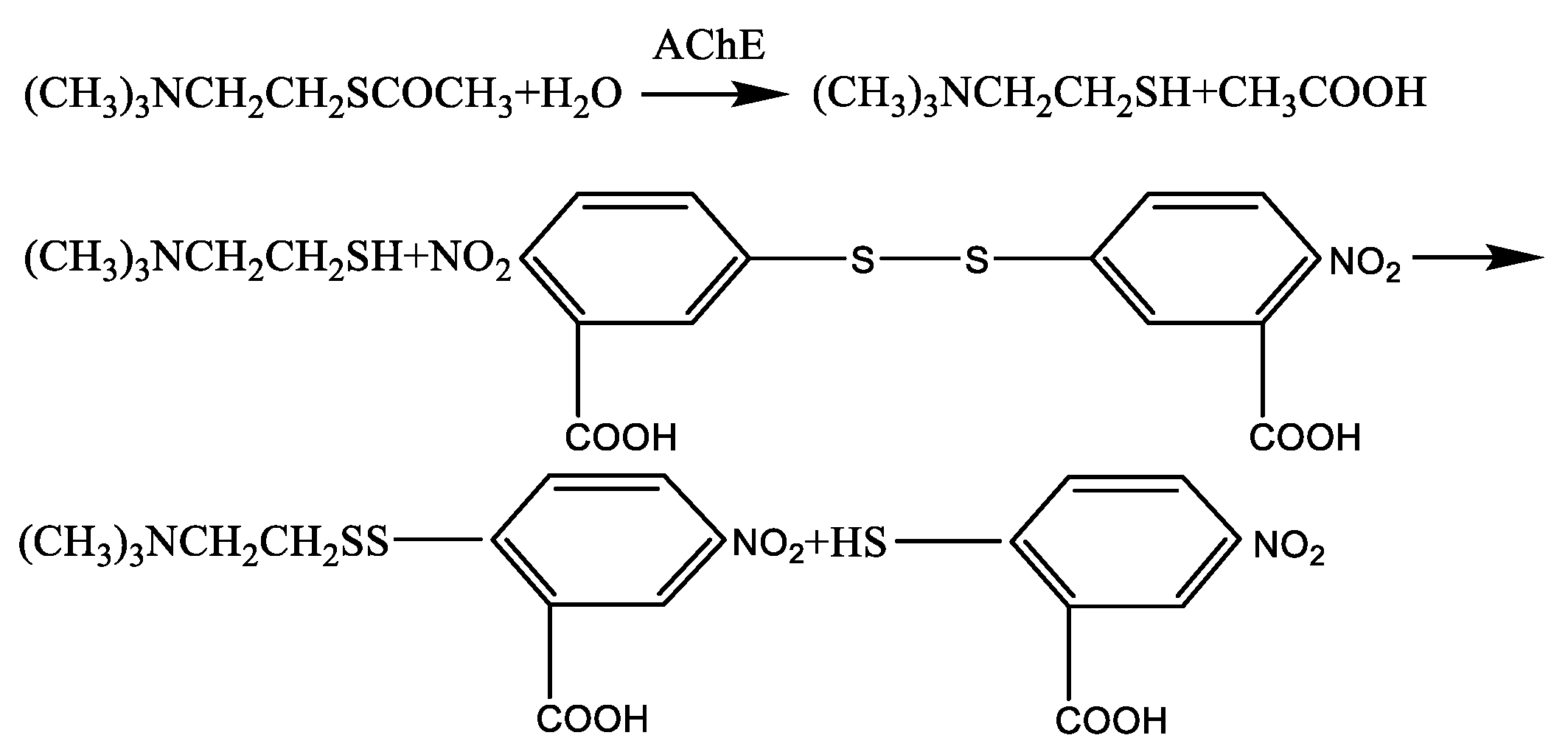
2.1. Basic Theory
2.2. Materials and Methods
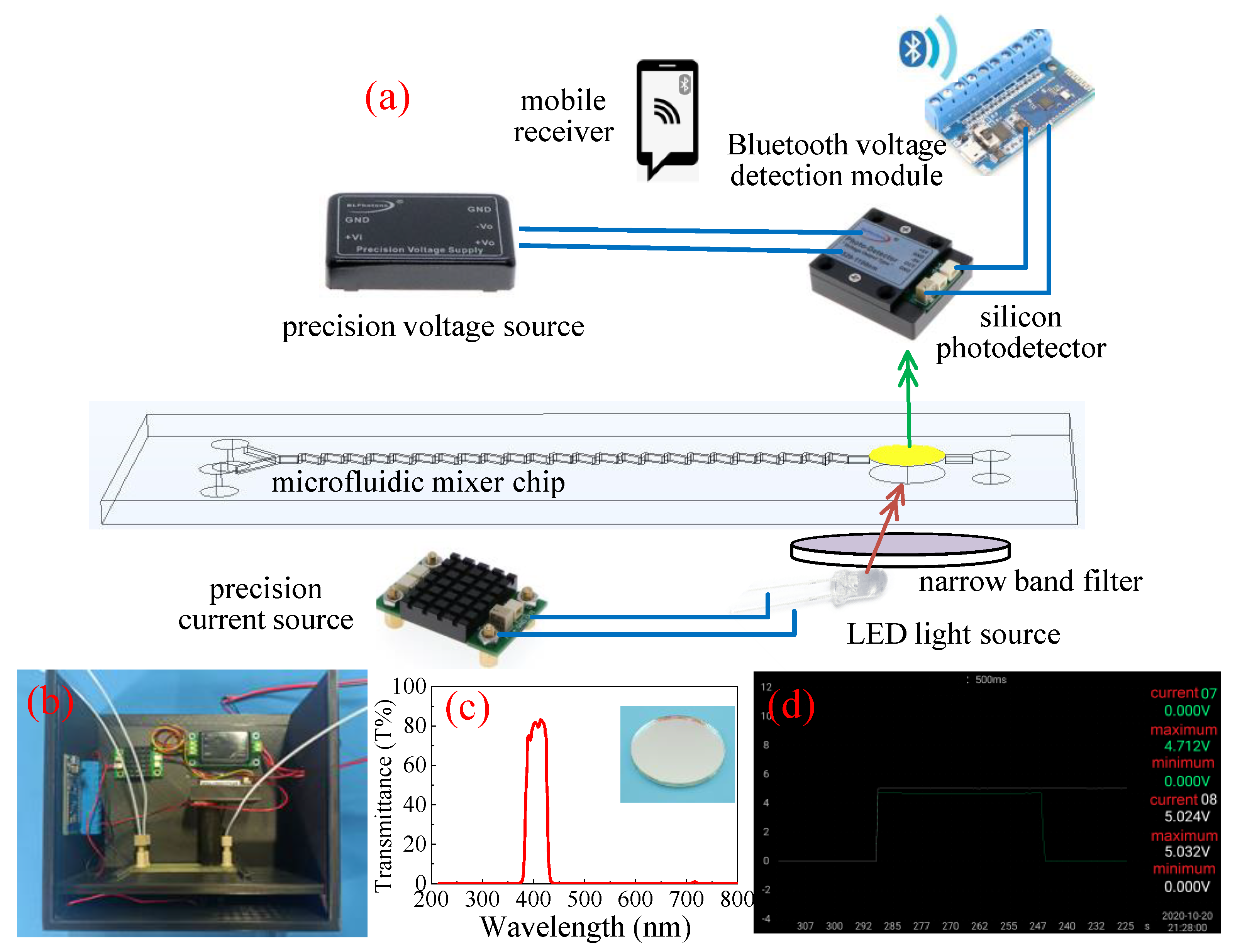
2.3. Characterization and Instruments
3. Results and Discussion
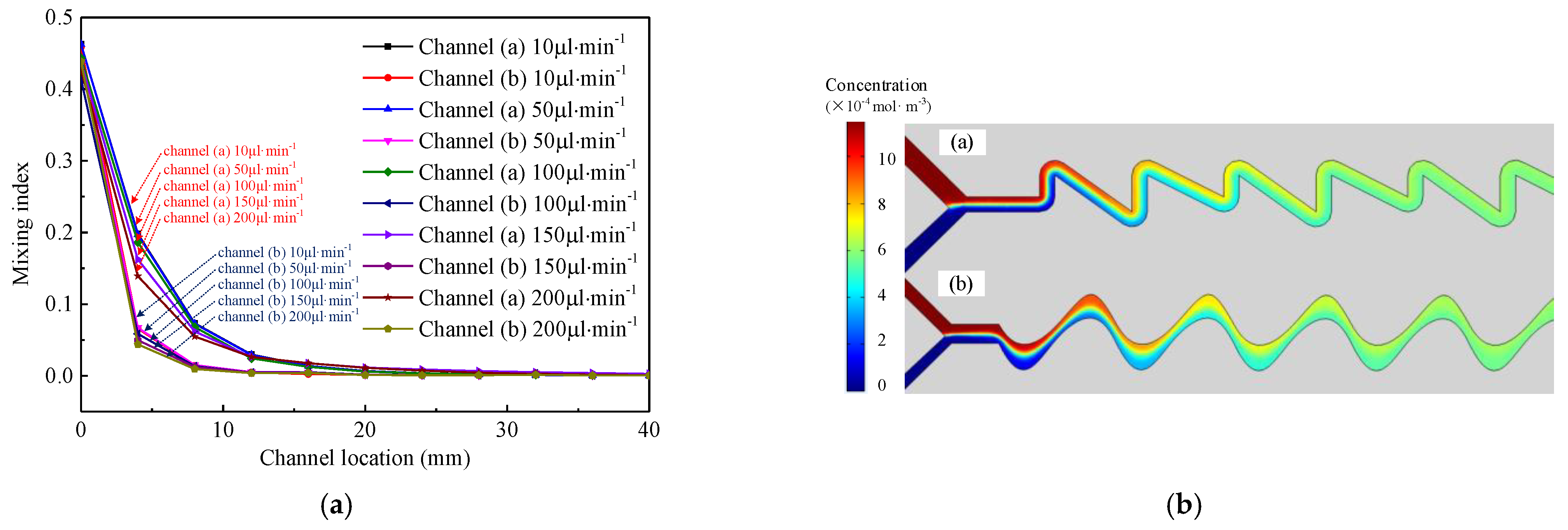
3.1. Influence of Microchannel Shape on Mixing Efficiency
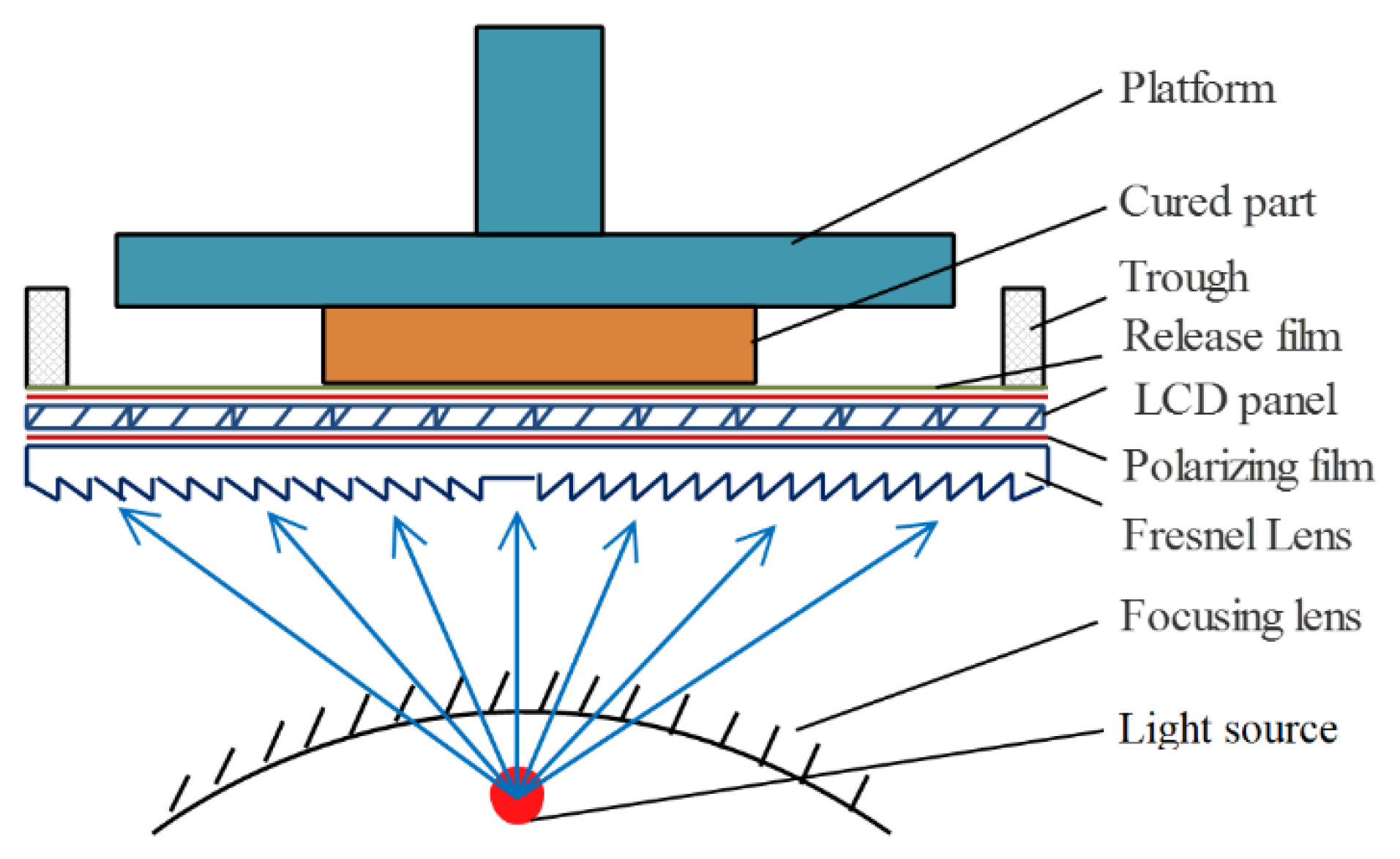
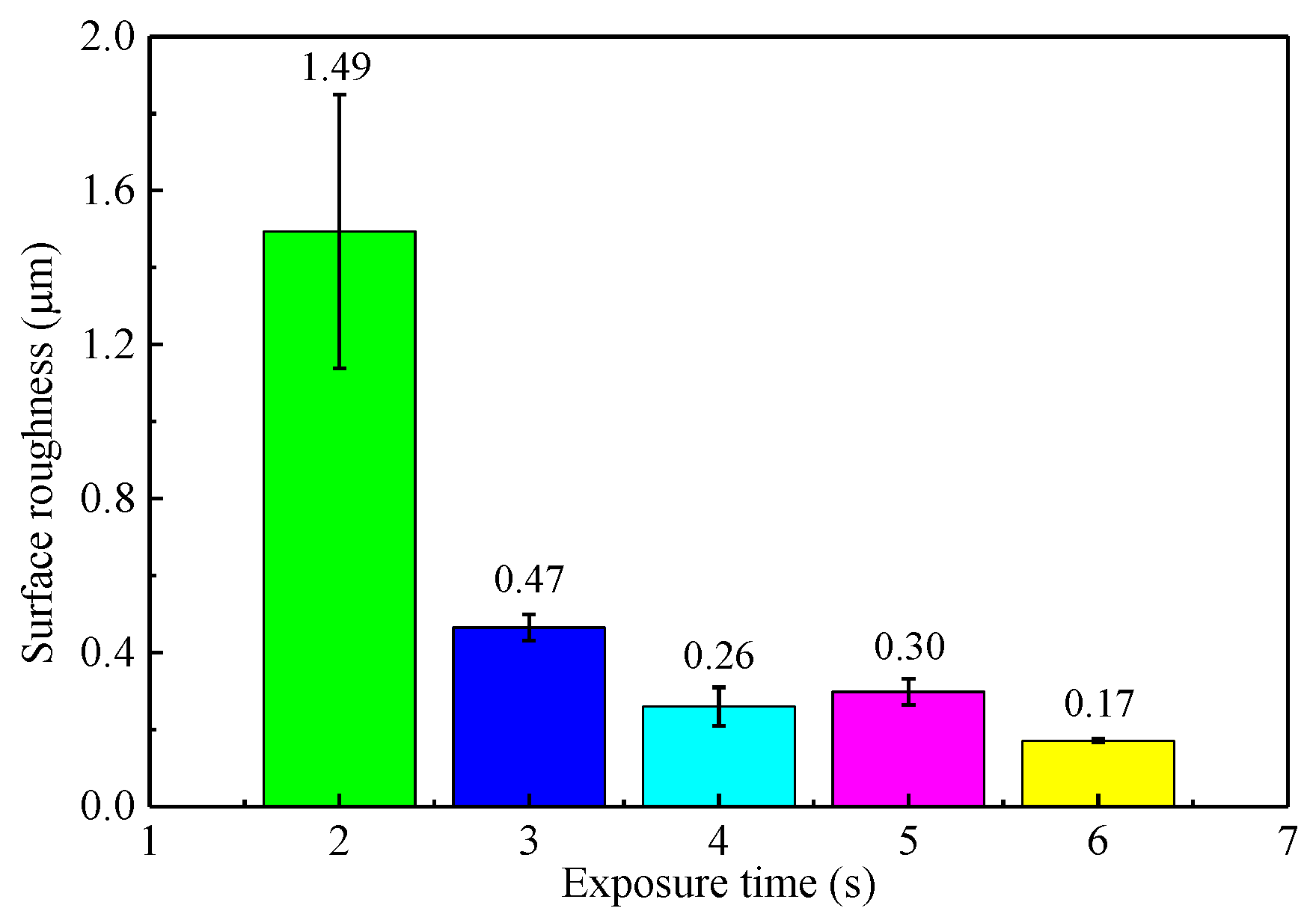
3.2. Influence of Printing Parameters on Chip Fabrication Accuracy
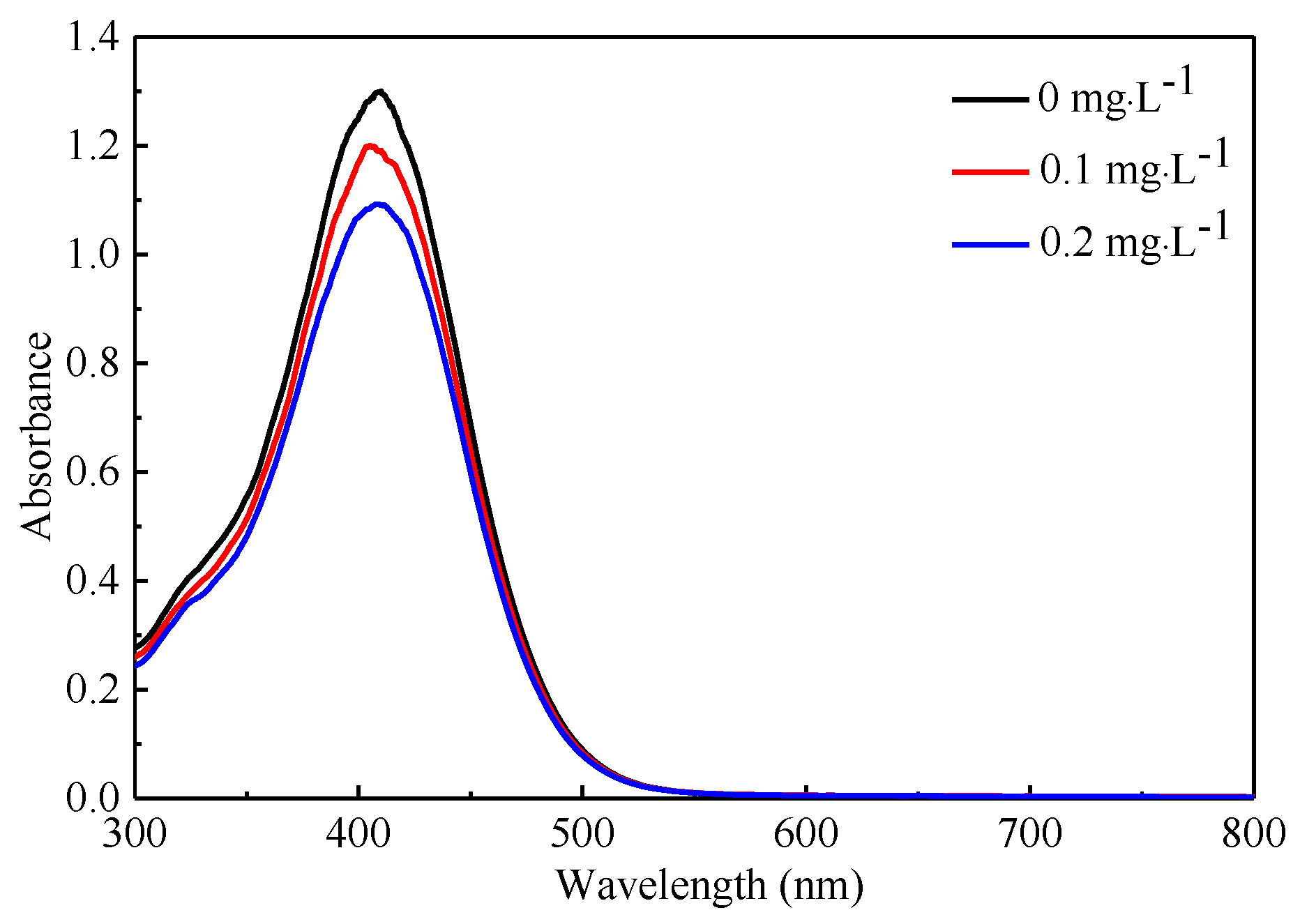
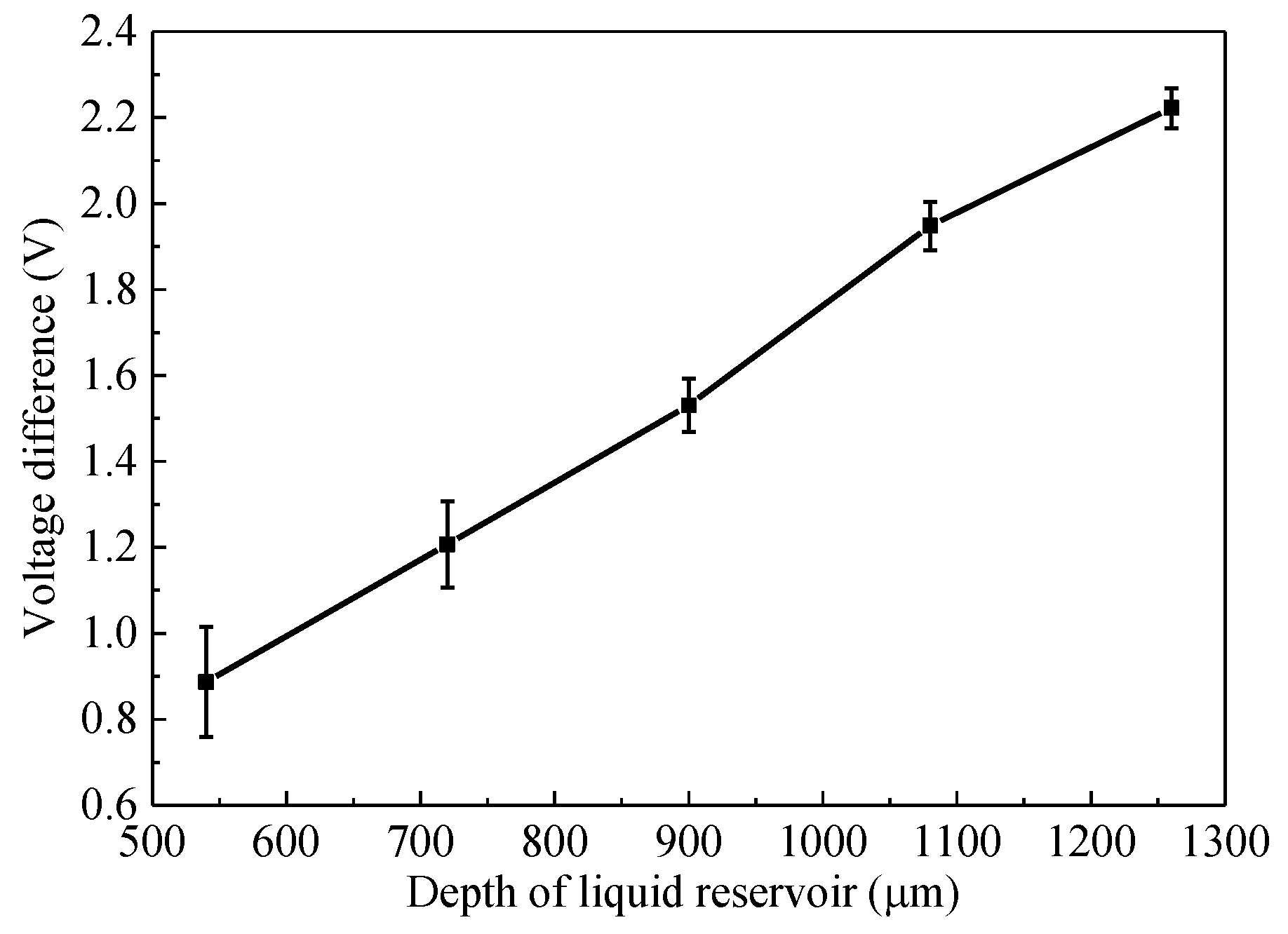
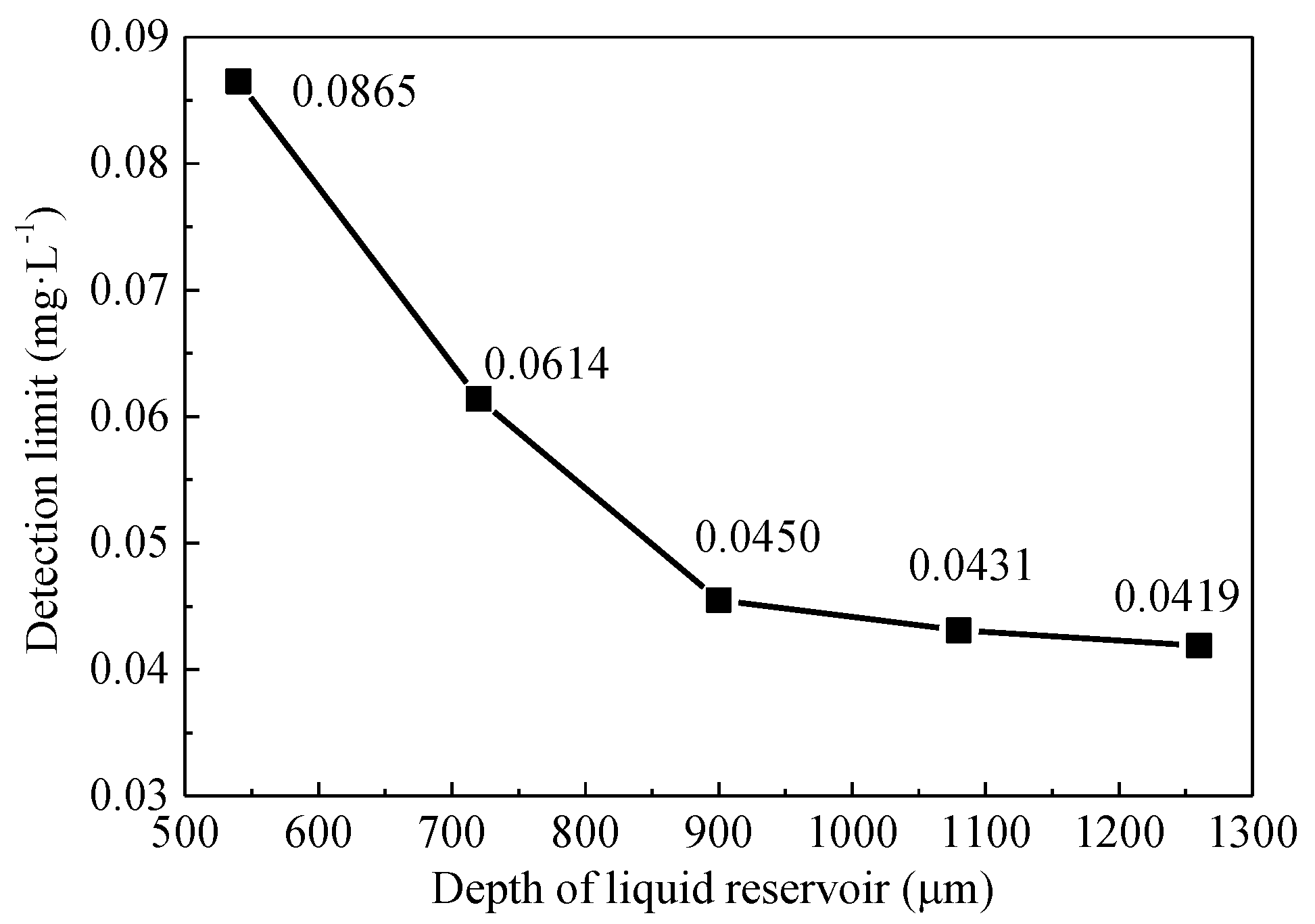
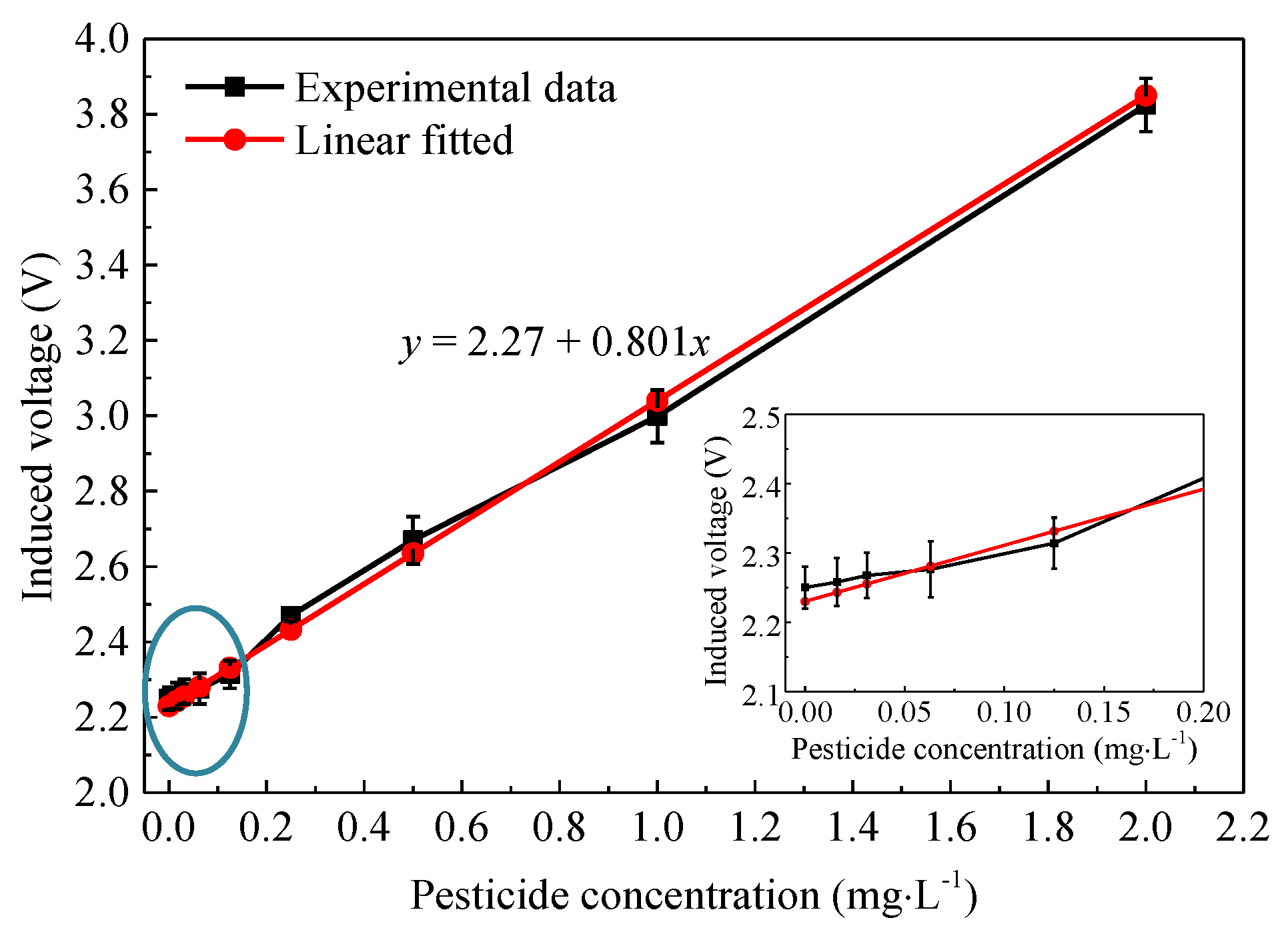
3.3. Relationshiop between Pesticide Concentration and Detection Voltage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Curl, C.L.; Fenske, R.A.; Elgethun, K. Organophosphorus pesticide exposure of urban and suburban preschool children with organic and conventional diets. Environ. Health Perspect. 2003, 111, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Jariyal, M.; Jindal, V.; Mandal, K.; Gupta, K.V.; Singh, B. Bioremediation of organophosphorus pesticide phorate in soil by microbial consortia. Ecotoxicol. Environ. Saf. 2018, 159, 310–316. [Google Scholar] [CrossRef]
- Yassine, A.; Omar, B.; Karine, G.S. Study of the degradation of an organophosphorus pesticide using electrogenerated hydroxyl radicals or heat-activated persulfate. Sep. Purif. Technol. 2018, 208, 27–33. [Google Scholar]
- Zhe, Z.; Godefroy, S.B.; Hanyang, L.; Baoguo, S.; Yongxiang, F. Transformation of china’s food safety standard setting system—Review of 50 years of change, opportunities and challenges ahead. Food Control 2018, 93, 106–111. [Google Scholar]
- Chen, X.; Liu, X.; Dong, B.; Hu, J. Simultaneous determination of pyridaben, dinotefuran, dn and uf in eggplant ecosystem under open-field conditions: Dissipation behaviour and residue distribution. Chemosphere 2018, 195, 245–251. [Google Scholar] [CrossRef]
- Lara-Ortega, F.J.; Robles-Molina, J.; Brandt, S.; Alexander, S.; Franzke, J. Use of dielectric barrier discharge ionization to minimize matrix effects and expand coverage in pesticide residue analysis by liquid chromatography-mass spectrometry. Anal. Chim. Acta 2018, 1020, 76–85. [Google Scholar] [CrossRef]
- Siyuan, B.I.; Zhu, Z.; Chi, W.; Chen, M.; Wang, X.; Zhou, S. Determination of eight organophosphorus pesticide residues in pepper by quechers-gas chromatography. Agric. Biotechnol. 2018, 7, 126–128. [Google Scholar]
- Li, X.Z.; Yu, Z.; Yang, T.Y.; Ding, J.H. Detection of organophosphorus pesticide residue on the surface of apples using sers. Spectrosc. Spectr. Anal. 2013, 33, 2711–2720. [Google Scholar]
- Andreas, H.A.; Tunemalm, K.; Fredrik, E. Crystal structures of acetylcholinesterase in complex with organophosphorus compounds suggest that the acyl pocket modulates the aging reaction by precluding the formation of the trigonal bipyramidal transition state. Biochemistry 2015, 46, 4815–4825. [Google Scholar]
- Wirdatmadja, S.A.; Moltchanov, D.; Balasubramaniam, S.; Koucheryavy, Y. Microuidic system protocols for integrated on-chip communications and cooling. IEEE Access 2017, 5, 2417–2429. [Google Scholar] [CrossRef]
- Agostini, M.; Greco, G.; Cecchini, M. Full-saw microfluidics-based lab-on-a-chip for biosensing. IEEE Access 2019, 7, 70901–70909. [Google Scholar] [CrossRef]
- Jia, H.; Guo, Y.; Sun, X.; Wang, X. An Electrochemical Immunosensor Based on Microfluidic Chip for Detection of Chlorpyrifos. Int. J. Electrochem. Sci. 2015, 10, 8750–8758. [Google Scholar]
- Deng, S.; Yang, T.; Zhang, W.; Ren, C.; Zhang, J.; Zhang, Y. Rapid detection of trichlorfon residues by a microfluidic paper-based phosphorus-detection chip (μppc). New J. Chem. 2019, 43, 7194–7197. [Google Scholar] [CrossRef]
- Asghar, W.; Sher, M.; Khan, N.S.; Vyas, J.M.; Demirci, U. Microfluidic Chip for Detection of Fungal Infections. ACS Omega 2019, 4, 7474–7481. [Google Scholar] [CrossRef]
- Suh, Y.K.; Kang, S. A review on mixing in microfluidics. Micromachines 2010, 1, 82–111. [Google Scholar] [CrossRef]
- Fan, L.L.; Zhu, X.L.; Zhao, H.; Zhe, J.; Zhao, L. Rapid microfluidic mixer utilizing sharp corner structures. Microfluid. Nanofluid. 2017, 21, 36–47. [Google Scholar] [CrossRef]
- Plevniak, K.; Campbell, M.; Myers, T.; Hodges, A.; He, M. 3d Printed Auto-Mixing Chip Enables Rapid Smartphone Diagnosis of Anemia. Biomicrofluidics 2016, 10, 54113–54123. [Google Scholar] [CrossRef]
- Xu, B.; Guo, J.; Fu, Y.; Chen, X.; Guo, J. A Review on Microfluidics in the Detection of Food Pesticide Residues. Electrophoresis 2020, 41, 821–832. [Google Scholar] [CrossRef]
- Sinko, G.; Calic, M.; Bosak, A.; Kovarik, Z. Limitation of the Ellman Method: Cholinesterase Activity Measurement in the Presence of Oximes. Anal. Biochem. 2007, 370, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Neves, D.A.; Paulo, A.A.; Eliane, N.S.; Paulo, S.L. Microcalorimetric Study of Acetylcholine and Acetylthiocholine Hydrolysis by Acetylcholinesterase. Adv. Enzyme Res. 2017, 5, 1–12. [Google Scholar] [CrossRef]
- Roeser, H.P.; Bohr, A.; Haslam, D.T.; López, J.S.; Stepper, M.; Nikoghosyan, A.S. Size Quantization in High-Temperature Superconducting Cuprates and a Link to Einstein’s Diffusion Law. Acta Astronaut. 2012, 76, 37–41. [Google Scholar] [CrossRef]
- Ernesto, B. Limit of detection and limit of quantification determination in gas chromatography. In Advances in Gas Chromatography; INTECH: London, UK, 2014; Available online: https://www.intechopen.com/books/advances-in-gas-chromatography/limit-of-detection-and-limit-of-quantification-determination-in-gas-chromatography (accessed on 10 January 2021).
- Loock, H.-P.; Wentzell, P.D. Detection limits of chemical sensors: Applications and misapplications. Sens. Actuators B Chem. 2012, 173, 157–163. [Google Scholar] [CrossRef]
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008, 29, S49–S54. [Google Scholar] [PubMed]
- Taylor, C.; Hood, P. A numerical solution of the navier-stokes equations using the finite element technique. Comput. Fluids 2017, 1, 73–100. [Google Scholar] [CrossRef]
- Liu, R.H.; Stremler, M.A.; Sharp, K.V.; Olsen, M.G.; Santiago, J.G.; Adrian, R.J. Passive mixing in a three-dimensional serpentine microchannel. J. Microelectromech. Syst. 2000, 9, 190–197. [Google Scholar] [CrossRef]
- Xie, J.; He, Y.; Ma, W.; Liu, T.; Chen, J. Study on the Liquid Crystal Display Mask Photo-Curing of Photosensitive Resin Reinforced with Graphene Oxide. J. Appl. Polym. Sci. 2020, 137, 49538–49546. [Google Scholar] [CrossRef]
- Zhu, B.; Cai, Y.; Wu, Z.; Niu, F.; Yang, H. Dielectrophoretic microfluidic chip integrated with liquid metal electrode for red blood cell stretching manipulation. IEEE Access 2019, 7, 152224–152232. [Google Scholar] [CrossRef]
- Dvir, H.; Silman, I.; Harel, M.; Rosenberry, T.L.; Sussman, J.L. Acetylcholinesterase: From 3d Structure to Function. Chem. Biol. Interact. 2010, 187, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Li, W.; Chu, W.; Yu, J.; Wu, M.; Liang, Y.; Yin, D.; Wang, P.; Wang, Z.; Wang, M.; et al. A Microfluidic Mixer of High Throughput Fabricated in Glass Using Femtosecond Laser Micromachining Combined with Glass Bonding. Micromachines 2020, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Park, J.E.; Kang, T.G.; Ahn, K.H. Design Optimization for a Microfluidic Crossflow Filtration System Incorporating a Micromixer. Micromachines 2019, 10, 836. [Google Scholar] [CrossRef]
- Yong, H.; Yan, W.; Fu, J.Z.; Wu, W.B. Fabrication of paper-based microfluidic analysis devices: A review. RSC Adv. 2015, 5, 78109–78127. [Google Scholar]
- Bhakta, S.A.; Borba, R.; Toba, M., Jr.; Garcia, C.D.; Carrilho, E. Determination of nitrite in saliva using microfluidic paper-based analytical devices. Anal. Chim. Acta 2014, 809, 117–122. [Google Scholar] [CrossRef] [PubMed]





| Parameter | Value |
|---|---|
| Density (g·cm−3) | 1.05–1.25 |
| Tensile modulus (GPa) | 1.8–2.8 |
| Tensile strength (MPa) | 64–72 |
| Bending modulus (GPa) | 1.8–2.3 |
| Heat distortion temperature (℃) | 44–47 |
| Elongation at break (%) | 8–13 |
| Parameter | Value |
|---|---|
| Boltzmann constant (J·K−1) | 1.38 × 10−23 |
| Absolute temperature (K) | 293.15 |
| Dynamic viscosity (Pa·s) | 10 × 10−3 |
| Water density (Kg·m−3) | 1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Pang, H.; Sun, R.; Wang, T.; Meng, X.; Zhou, Z. Development of Rapid and High-Precision Colorimetric Device for Organophosphorus Pesticide Detection Based on Microfluidic Mixer Chip. Micromachines 2021, 12, 290. https://doi.org/10.3390/mi12030290
Xie J, Pang H, Sun R, Wang T, Meng X, Zhou Z. Development of Rapid and High-Precision Colorimetric Device for Organophosphorus Pesticide Detection Based on Microfluidic Mixer Chip. Micromachines. 2021; 12(3):290. https://doi.org/10.3390/mi12030290
Chicago/Turabian StyleXie, Jiaqing, Haoran Pang, Ruqian Sun, Tao Wang, Xiaoyu Meng, and Zhikang Zhou. 2021. "Development of Rapid and High-Precision Colorimetric Device for Organophosphorus Pesticide Detection Based on Microfluidic Mixer Chip" Micromachines 12, no. 3: 290. https://doi.org/10.3390/mi12030290
APA StyleXie, J., Pang, H., Sun, R., Wang, T., Meng, X., & Zhou, Z. (2021). Development of Rapid and High-Precision Colorimetric Device for Organophosphorus Pesticide Detection Based on Microfluidic Mixer Chip. Micromachines, 12(3), 290. https://doi.org/10.3390/mi12030290







