Highly Sensitive Hybrid Nanostructures for Dimethyl Methyl Phosphonate Detection
Abstract
:1. Introduction
2. Materials and Methods
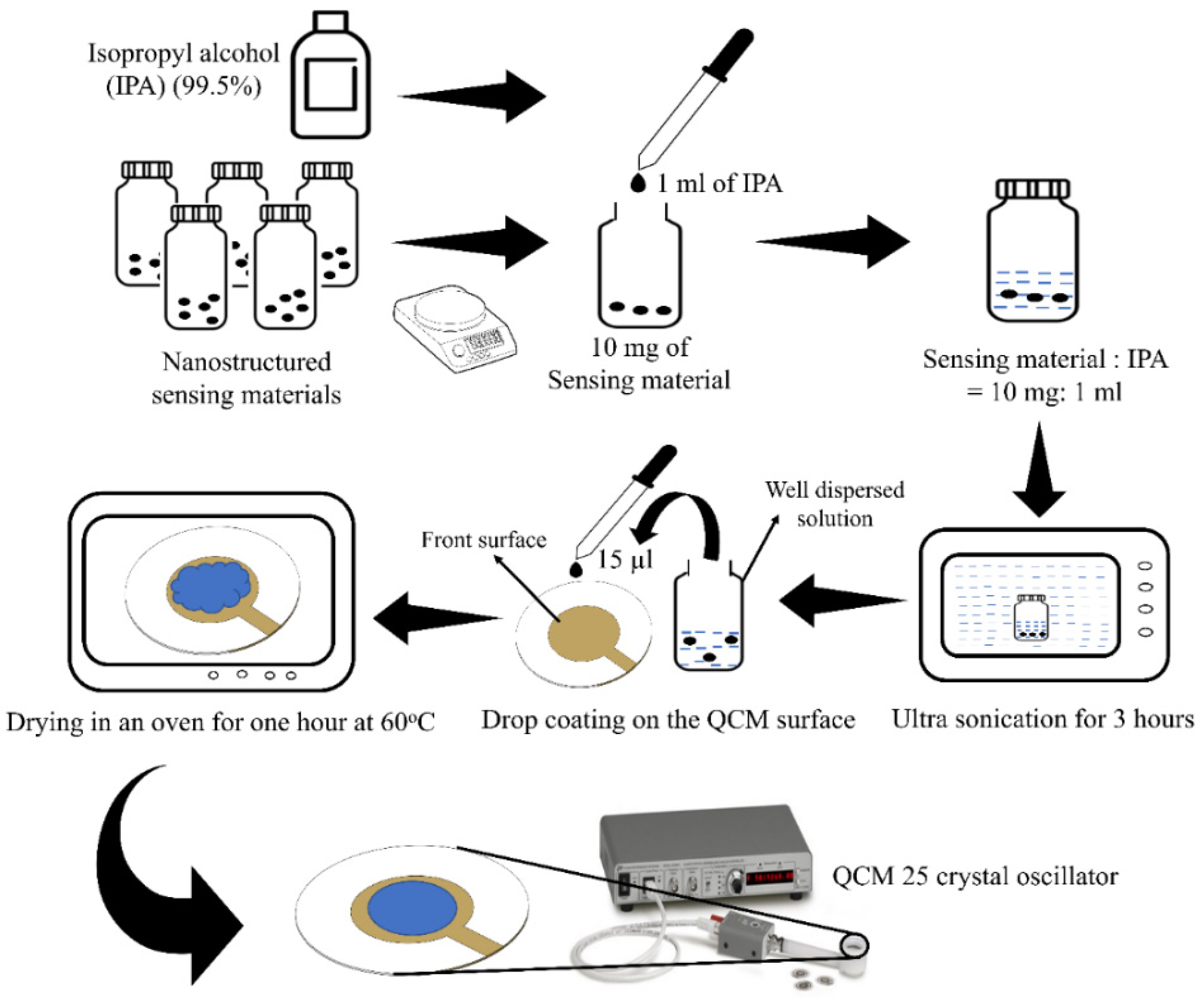
2.1. Fabrication of Composite Sensing Materials
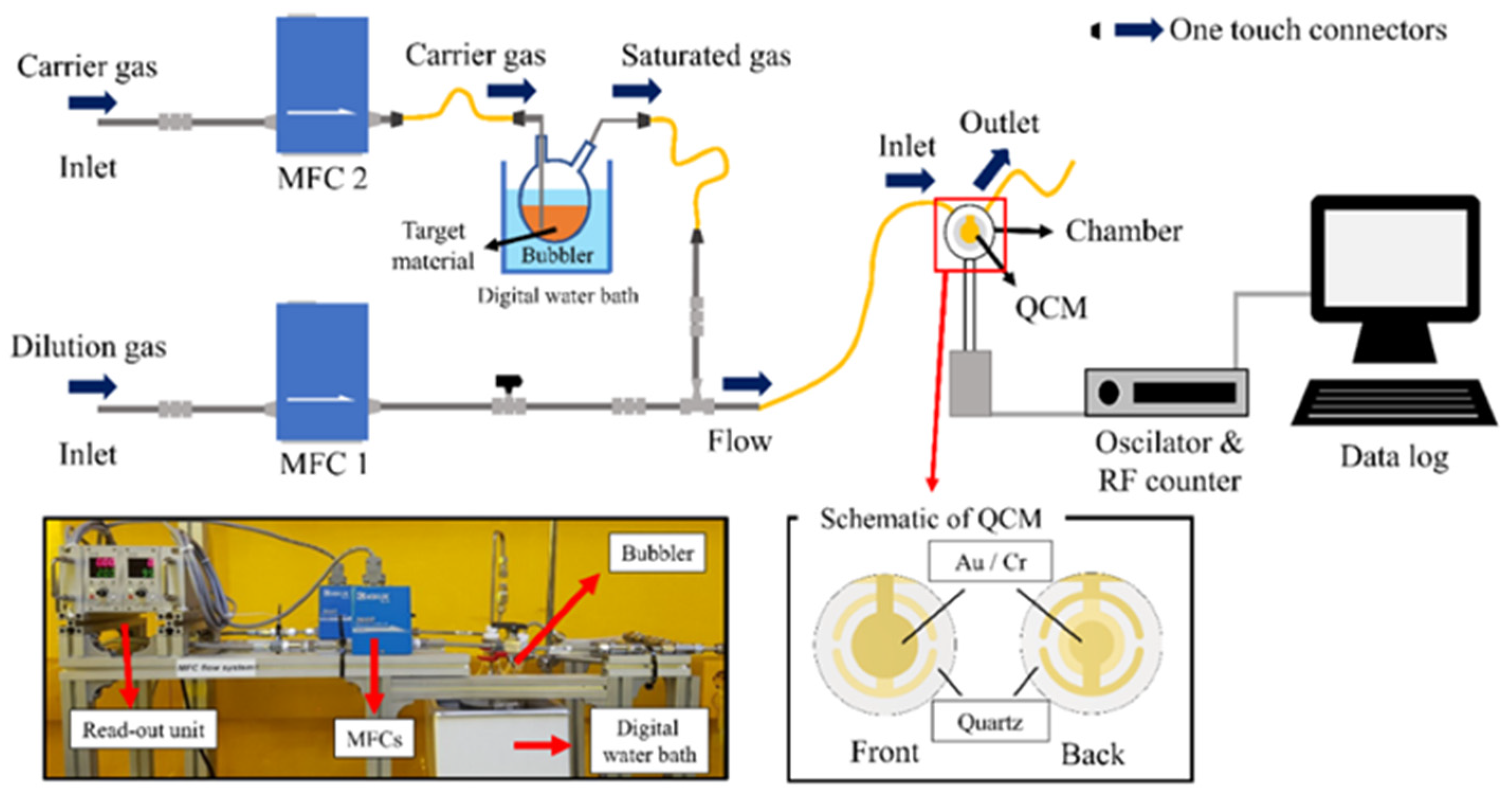
2.2. Characterization Apparatus and Selectivity Conditions
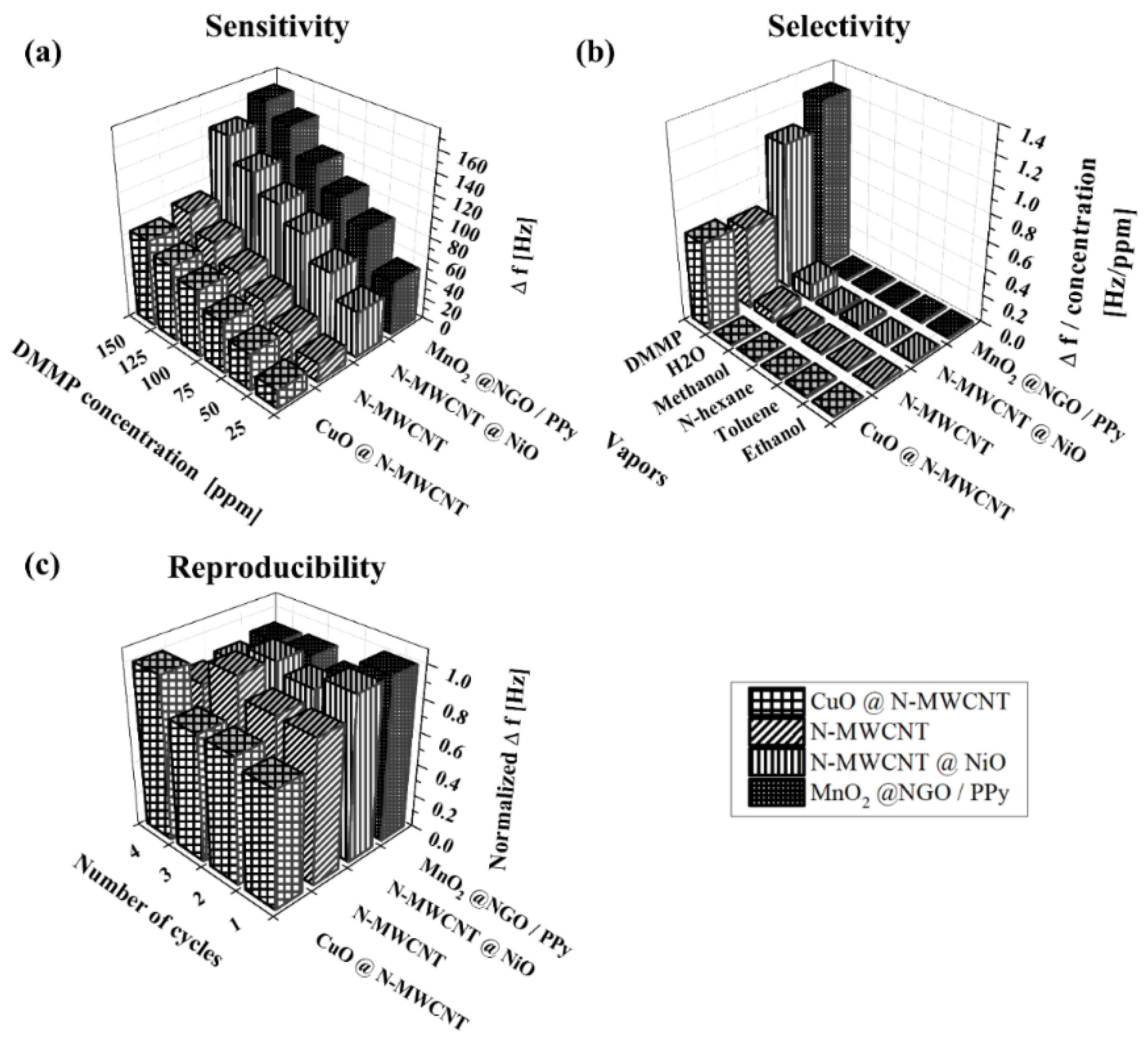
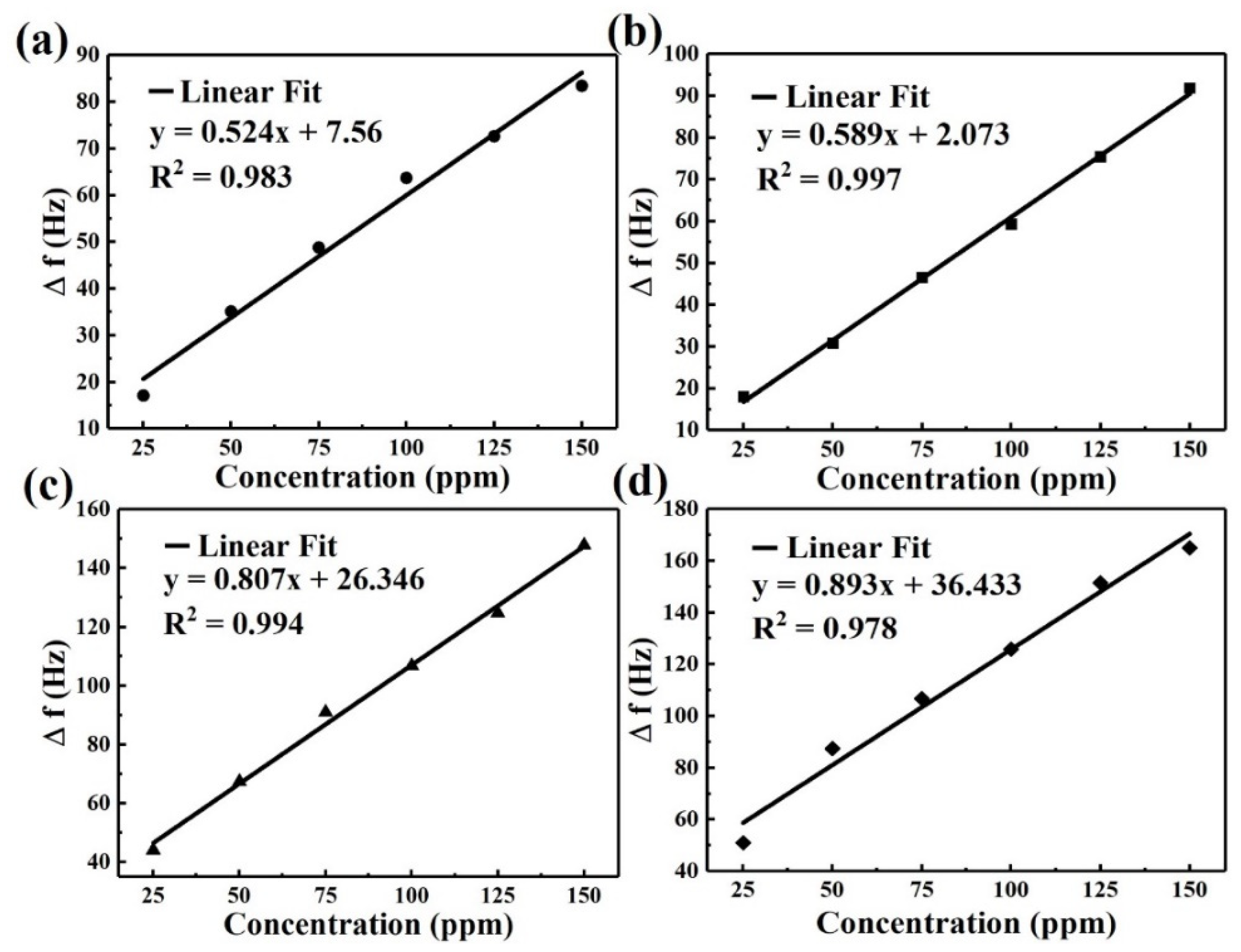
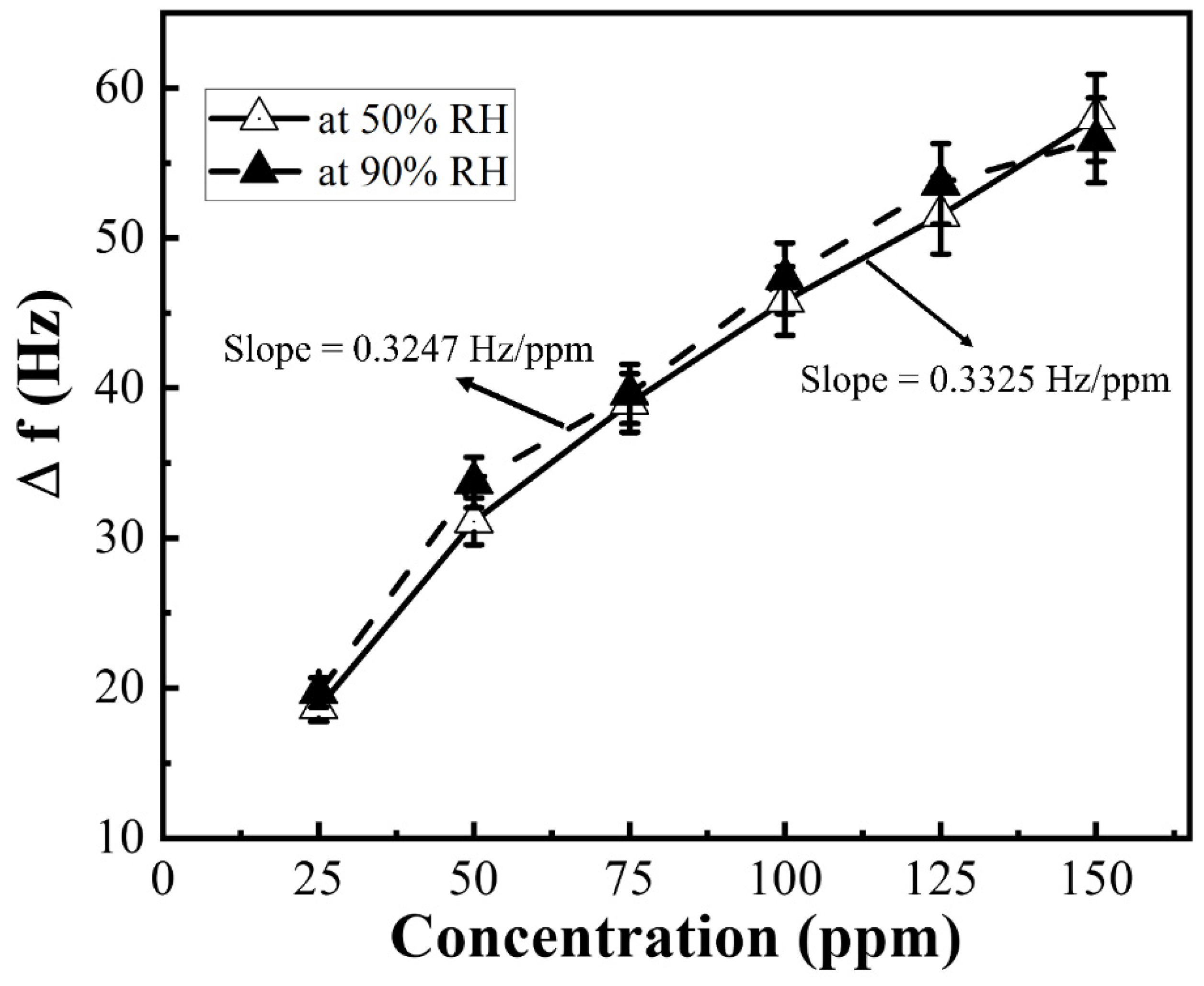
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chauhan, S.; D’Cruz, R.; Faruqi, S.; Singh, K.K.; Varma, S.; Singh, M.; Karthik, V. Chemical warfare agents. Environ. Toxicol. Pharmacol. 2008, 26, 113–122. [Google Scholar] [CrossRef]
- Lavoie, J.; Srinivasan, S.; Nagarajan, R. Using cheminformatics to find simulants for chemical warfare agents. J. Hazard. Mater. 2011, 194, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Y.; Loke, W.K.; Tan, Y.T.; Nguyen, N.T. A lab-on-a-chip for detection of nerve agent sarin in blood. Lab Chip 2008, 8, 885–891. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yang, Z.; Hou, Z.; Xu, D.; Wei, L.; Kong, E.S.W.; Zhang, Y. Flexible gas sensors with assembled carbon nanotube thin films for DMMP vapor detection. Sens. Actuators B Chem. 2010, 150, 708–714. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Z.; Yang, Z.; Chen, X.; Xu, D.; Zhang, Y. Gas sensors based on deposited single-walled carbon nanotube networks for DMMP detection. Nanotechnology 2009, 20, 345502. [Google Scholar] [CrossRef]
- Du, X.; Ying, Z.; Jiang, Y.; Liu, Z.; Yang, T.; Xie, G. Synthesis and evaluation of a new polysiloxane as SAW sensor coatings for DMMP detection. Sens. Actuators B Chem. 2008, 134, 409–413. [Google Scholar] [CrossRef]
- Wen, W.; Shitang, H.; Shunzhou, L.; Minghua, L.; Yong, P. Enhanced sensitivity of SAW gas sensor coated molecularly imprinted polymer incorporating high frequency stability oscillator. Sens. Actuators B Chem. 2007, 125, 422–427. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Kim, J.-G.; Kim, J.-H.; Yun, J.; Jang, W.J. Detection of dimethyl methylphosphonate (DMMP) using polyhedral poligomeric silsesquioxane (POSS). J. Nanosci. Nanotechnol. 2018, 18, 6565–6569. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Jiang, Y.; Du, X.; Xie, G.; Yu, J.; Wang, H. PVDF coated quartz crystal microbalance sensor for DMMP vapor detection. Sens. Actuators B Chem. 2007, 125, 167–172. [Google Scholar] [CrossRef]
- Novak, J.P.; Snow, E.S.; Houser, E.J.; Park, D.; Stepnowski, J.L.; McGill, R.A. Nerve agent detection using networks of single-walled carbon nanotubes. Appl. Phys. Lett. 2003, 83, 4026–4028. [Google Scholar] [CrossRef]
- Wang, F.; Gu, H.; Swager, T.M. Carbon nanotube/polythiophene chemiresistive sensors for chemical warfare agents. J. Am. Chem. Soc. 2008, 130, 5392–5393. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, H.F.; Thundat, T. Nerve agents detection using a Cu2+/L-cysteine bilayer-coated microcantilever. J. Am. Chem. Soc. 2003, 125, 1124–1125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Feng, W.; Wang, C.; Hu, X.; Li, X.; Sun, P.; Shimanoe, K.; Yamazoe, N.; Lu, G. Porous ZnO/ZnCo2O4 hollow spheres: Synthesis, characterization, and applications in gas sensing. J. Mater. Chem. A 2014, 2, 17683–17690. [Google Scholar] [CrossRef]
- Pei, Z.; Ma, X.; Ding, P.; Zhang, W.; Luo, Z.; Li, G. Study of a QCM dimethyl methylphosphonate sensor based on a zno-modified nanowire-structured manganese dioxide film. Sensors 2010, 10, 8275–8290. [Google Scholar] [CrossRef] [Green Version]
- Abad, J.M.; Pariente, F.; Hernandez, L.; Abruna, H.D.; Lorenzo, E. Determination of organophosphorus and carbamate pesticides using a piezoelectric biosensor. Anal. Chem. 1998, 70, 2848–2855. [Google Scholar] [CrossRef]
- Nivens, D.E.; Palmer, R.J., Jr.; White, D.C. Continuous nondestructive monitoring of microbial biofilms: A review of analytical techniques. J. Ind. Microbiol. Biotechnol. 1995, 15, 263–276. [Google Scholar] [CrossRef]
- Huang, X.; Bai, Q.; Hu, J.; Hou, D. A practical model of quartz crystal microbalance in actual applications. Sensors 2017, 17, 1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dirri, F.; Palomba, E.; Longobardo, A.; Zampetti, E.; Saggin, B.; Scaccabarozzi, D. A review of quartz crystal microbalances for space applications. Sens. Actuators A Phys. 2019, 287, 48–75. [Google Scholar] [CrossRef]
- Kim, J.; Kim, E.; Kim, J.; Kim, J.-H.; Ha, S.; Song, C.; Jang, W.J.; Yun, J. Four-channel Monitoring System with Surface Acoustic Wave Sensors for Detection of Chemical Warfare Agents. J. Nanosci. Nanotechnol. 2020, 20, 7151–7157. [Google Scholar] [CrossRef] [PubMed]
- Bannov, A.G.; Popov, M.V.; Brester, A.E.; Kurmashov, P.B. Recent advances in ammonia gas sensors based on carbon nanomaterials. Micromachines 2021, 12, 186. [Google Scholar] [CrossRef]
- Pathak, P.; Park, S.; Cho, H.J. A carbon nanotube-metal oxide hybrid material for visible-blind flexible UV-Sensor. Micromachines 2020, 11, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Xia, Y. Multiple-walled nanotubes made of metals. Adv. Mater. 2004, 16, 264–268. [Google Scholar] [CrossRef]
- Sharifi, T.; Nitze, F.; Barzegar, H.R.; Tai, C.W.; Mazurkiewicz, M.; Malolepszy, A.; Stobinski, L.; Wågberg, T. Nitrogen doped multi walled carbon nanotubes produced by CVD-correlating XPS and Raman spectroscopy for the study of nitrogen inclusion. Carbon 2012, 50, 3535–3541. [Google Scholar] [CrossRef]
- Sayago, I.; Matatagui, D.; Fernández, M.J.; Fontecha, J.L.; Jurewicz, I.; Garriga, R.; Muñoz, E. Graphene oxide as sensitive layer in Love-wave surface acoustic wave sensors for the detection of chemical warfare agent simulants. Talanta 2016, 148, 393–400. [Google Scholar] [CrossRef]
- Tiwari, D.C.; Sharma, R.; Vyas, K.D.; Boopathi, M.; Singh, V.V.; Pandey, P. Electrochemical incorporation of copper phthalocyanine in conducting polypyrrole for the sensing of DMMP. Sens. Actuators B Chem. 2010, 151, 256–264. [Google Scholar] [CrossRef]
- Yoo, R.; Kim, J.; Song, M.J.; Lee, W.; Noh, J.S. Nano-composite sensors composed of single-walled carbon nanotubes and polyaniline for the detection of a nerve agent simulant gas. Sens. Actuators B Chem. 2015, 209, 444–448. [Google Scholar] [CrossRef]
- Segal, S.R.; Suib, S.L.; Tang, X.; Satyapal, S. Photoassisted decomposition of dimethyl methylphosphonate over amorphous manganese oxide catalysts. Chem. Mater. 1999, 11, 1687–1695. [Google Scholar] [CrossRef]
- Ramesh, S.; Yadav, H.M.; Karuppasamy, K.; Vikraman, D.; Kim, H.S.; Kim, J.H.; Kim, H.S. Fabrication of manganese oxide@nitrogen doped graphene oxide/polypyrrole (MnO2@NGO/PPy) hybrid composite electrodes for energy storage devices. J. Mater. Res. Technol. 2019, 8, 4227–4238. [Google Scholar] [CrossRef]
- Ramesh, S.; Karuppasamy, K.; Yadav, H.M.; Lee, J.J.; Kim, H.S.; Kim, H.S.; Kim, J.H. Ni(OH)2-decorated nitrogen doped MWCNT nanosheets as an efficient electrode for high performance supercapacitors. Sci. Rep. 2019, 9, 6034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramesh, S.; Kathalingam, A.; Karuppasamy, K.; Kim, H.S.; Kim, H.S. Nanostructured CuO/Co2O4@ nitrogen doped MWCNT hybrid composite electrode for high-performance supercapacitors. Compos. Part B Eng. 2019, 166, 74–85. [Google Scholar] [CrossRef]
- QCM200 Quartz Crystal Microbalance Digital Controller. Available online: https://www.thinksrs.com/downloads/pdfs/manuals/QCM200m.pdf (accessed on 27 April 2021).
- Hersee, S.D.; Ballingall, J.M. The operation of metalorganic bubblers at reduced pressure. J. Vac. Sci. Technol. Vac. Surf. Film 1990, 8, 800–804. [Google Scholar] [CrossRef]
- Butrow, A.B.; Buchanan, J.H.; Tevault, D.E. Vapor pressure of organophosphorus nerve agent simulant compounds. J. Chem. Eng. Data 2009, 54, 1876–1883. [Google Scholar] [CrossRef]
- Ambrose, D.; Sprake, C.H.S. Thermodynamic properties of organic oxygen compounds XXV. Vapour pressures and normal boiling temperatures of aliphatic alcohols. J. Chem. Thermodyn. 1970, 2, 631–645. [Google Scholar] [CrossRef]
- Williamham, C.B.; Taylor, W.J.; Pignocco, J.M.; Rossini, F.D. Vapor pressures and boiling points of some paraffin, alkylcyclopentane, alkylcyclohexane, and alkylbenzene hydrocarbons. J. Res. Natl. Bur. Stand. 1945, 35, 219–244. [Google Scholar] [CrossRef]
- Yaws, C.L.; Yang, H.C. To estimate vapor pressure easily. Hydrocarb. Process. 1989, 49, 795–802. [Google Scholar]
- Kim, Y.S.; Ha, S.C.; Yang, H.; Kim, Y.T. Gas sensor measurement system capable of sampling volatile organic compounds (VOCs) in wide concentration range. Sens. Actuators B Chem. 2007, 122, 211–218. [Google Scholar] [CrossRef]
- Wang, L.; Dou, H.; Lou, Z.; Zhang, T. Encapsuled nanoreactors (Au@SnO2): A new sensing material for chemical sensors. Nanoscale 2013, 5, 2686–2691. [Google Scholar] [CrossRef]
- Wei, L.; Shi, D.; Ye, P.; Dai, Z.; Chen, H.; Chen, C.; Wang, J.; Zhang, L.; Xu, D.; Wang, Z. Hole doping and surface functionalization of single-walled carbon nanotube chemiresistive sensors for ultrasensitive and highly selective organophosphor vapor detection. Nanotechnology 2011, 22, 425501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghighi, E.; Zeinali, S. Nanoporous MIL-101(Cr) as a sensing layer coated on a quartz crystal microbalance (QCM) nanosensor to detect volatile organic compounds (VOCs). RSC Adv. 2019, 9, 24460–24470. [Google Scholar] [CrossRef] [Green Version]
- Shaik, M.; Rao, V.K.; Ramana, G.V.; Halder, M.; Gutch, P.K.; Pandey, P.; Jain, R. p-Hexafluoroisopropanol phenyl functionalized graphene for QCM based detection of dimethyl methylphosphonate, a simulant of the nerve agent sarin. RSC Adv. 2018, 8, 8240–8245. [Google Scholar] [CrossRef] [Green Version]
- Kösemen, A.; Öztürk, S.; Şen, Z.; Kösemen, Z.A.; Harbeck, M.; Öztürk, Z.Z. Volatile organic compounds and dimethyl methyl phosphonate (DMMP) sensing properties of the metal oxide functionalized QCM transducers at room temperature. J. Electrochem. Soc. 2017, 164, B657. [Google Scholar] [CrossRef]
- Ramesh, S.; Lee, Y.-J.; Msolli, S.; Kim, J.-G.; Kim, H.S.; Kim, J.-H. Synthesis of a Co3O4@ gold/MWCNT/polypyrrole hybrid composite for DMMP detection in chemical sensors. RSC Adv. 2017, 7, 50912–50919. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Zhang, K.; Zhou, H.; Fan, G.; Wang, Y.; Li, G.; Hu, R. A wireless-electrodeless quartz crystal microbalance with dissipation DMMP sensor. Sens. Actuators B Chem. 2018, 261, 408–417. [Google Scholar] [CrossRef]
- Ji, X.; Yao, W.; Hu, Y.; Ren, N.; Zhou, J.; Huang, Y.; Tang, Y. Adsorption and desorption characteristics of nanozeolites as adsorbent for Dimethyl Methylphosphonate. Sens. Mater. 2011, 23, 303–313. [Google Scholar]
- Zhao, Y.; He, J.; Yang, M.; Gao, S.; Zuo, G.; Yan, C.; Cheng, Z. Single crystal WO3 nanoflakes as quartz crystal microbalance sensing layer for ultrafast detection of trace sarin simulant. Anal. Chim. Acta 2009, 654, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Kim, G.-H.; Jung, D.; Kim, J.-H. Dimethyl Methylphosphonate detection using a quartz crystal microbalance as chemical warfare sensor. Nanosci. Nanotechnol. Lett. 2015, 7, 1015–1018. [Google Scholar] [CrossRef]
- Procek, M.; Stolarczyk, A.; Pustelny, T.; Maciak, E. A Study of a QCM Sensor Based on TiO2 Nanostructures for the Detection of NO2 and Explosives Vapours in Air. Sensors 2015, 15, 9563–9581. [Google Scholar] [CrossRef] [PubMed]
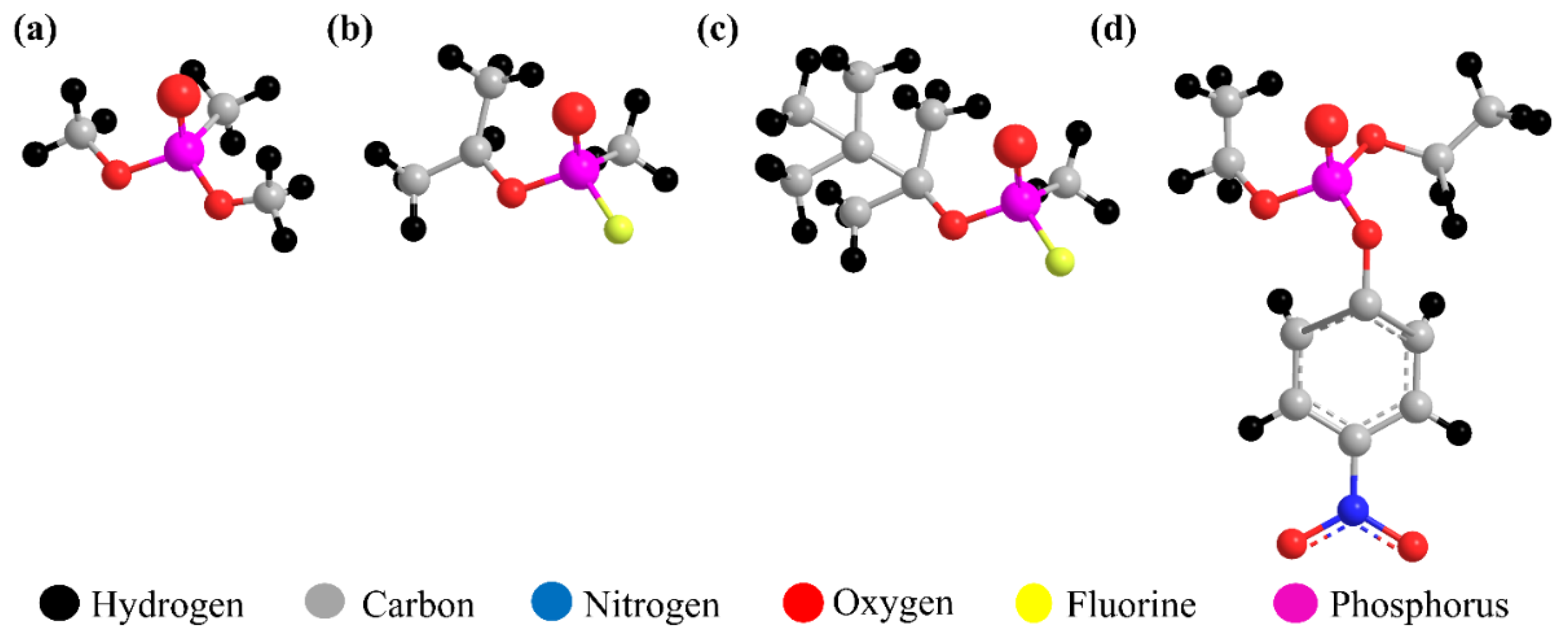


| Vapors | Equation | Unit | A | B | C | Temp. Range [K] |
|---|---|---|---|---|---|---|
| DMMP | (2) | T (K), P (Pa) | 22.31900 | 4340.00 | −51.700 | 263.2–453.8 |
| Ethanol | (3) | T (K), P (kPa) | 7.24739 | 1599.04 | −46.391 | 292.8–366.6 |
| N-hexane | (3) | T (°C), P (mmHg) | 6.87776 | 1171.53 | 224.366 | 286.18–342.7 |
| Water | (3) | T (°C), P (mmHg) | 8.07131 | 1730.63 | 233.426 | 274.0–373.0 |
| Toluene | (3) | T (°C), P (mmHg) | 6.95464 | 1344.80 | 219.482 | 279.0–409.0 |
| Methanol | (3) | T (°C), P (mmHg) | 8.07240 | 1574.99 | 238.870 | 257.0–364.0 |
| Sensing Nanocomposite Film | Response (Hz) | Standard Deviation (Hz) |
|---|---|---|
| CuO@N-MWCNT | 12.6 | 1.71 |
| N-MWCNT | 19.02 | 1.21 |
| N-MWCNT@NiO | 47.40 | 2.38 |
| MnO2@NGO/PPy | 47.29 | 2.69 |
| Sensor Characteristics | DMMP Concentration (ppm) | |||||
|---|---|---|---|---|---|---|
| Nanocomposites | 25 | 50 | 75 | 100 | 125 | |
| Response time (s) τ90% | CuO@N-MWCNT | ~121 | ~115 | ~108 | ~104 | ~102 |
| N-MWCNT | ~130 | ~100 | ~100 | ~101 | ~100 | |
| N-MWCNT@NiO | ~117 | ~104 | ~106 | ~103 | ~96 | |
| MnO2@NGO/PPy | ~136 | ~101 | ~103 | ~99 | ~123 | |
| Recovery time (s) τ90% | CuO@N-MWCNT | ~155 | ~195 | ~113 | ~139 | ~143 |
| N-MWCNT | ~193 | ~142 | ~135 | ~132 | ~170 | |
| N-MWCNT@NiO | ~206 | ~142 | ~150 | ~139 | ~144 | |
| MnO2@NGO/PPy | ~142 | ~123 | ~117 | ~118 | ~113 | |
| Ref. | Materials | Concentration (ppm) | Response (Hz) | Response Time (s) | Recovery Times (s) |
|---|---|---|---|---|---|
| [41] | HFIPP-GR | 5 | 71 ± 4 | T80 < 108 | T80 = 600 |
| [42] | V2O5 coated ZnO nanorods | 15 | ~40 | T80 < 300 | T80 > 900 |
| [43] | Co3O4@gold/MWCNT/polypyrrole | 60 | 90 | T98 = 60 | T98 = 493 |
| [44] | In2O3-Au | 50 | <80 | <100 | ~200 |
| [45] | Zeolite Socony Mobil-5 | 20 | ~55 | T80 < 100 | - |
| [46] | WO3 nanoflake | 3.91 | <160 | 30 | 73 |
| [47] | Polyvinylidene fluoride | 150 | ~50 | ~60 | ~60 |
| This work | MnO2@NGO/PPy | 50 | 87 | T90 = 101 | T90 = 123 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lama, S.; Kim, J.; Ramesh, S.; Lee, Y.-J.; Kim, J.; Kim, J.-H. Highly Sensitive Hybrid Nanostructures for Dimethyl Methyl Phosphonate Detection. Micromachines 2021, 12, 648. https://doi.org/10.3390/mi12060648
Lama S, Kim J, Ramesh S, Lee Y-J, Kim J, Kim J-H. Highly Sensitive Hybrid Nanostructures for Dimethyl Methyl Phosphonate Detection. Micromachines. 2021; 12(6):648. https://doi.org/10.3390/mi12060648
Chicago/Turabian StyleLama, Sanjeeb, Jinuk Kim, Sivalingam Ramesh, Young-Jun Lee, Jihyun Kim, and Joo-Hyung Kim. 2021. "Highly Sensitive Hybrid Nanostructures for Dimethyl Methyl Phosphonate Detection" Micromachines 12, no. 6: 648. https://doi.org/10.3390/mi12060648





