High-Throughput Cell Concentration Using A Piezoelectric Pump in Closed-Loop Viscoelastic Microfluidics
Abstract
:1. Introduction
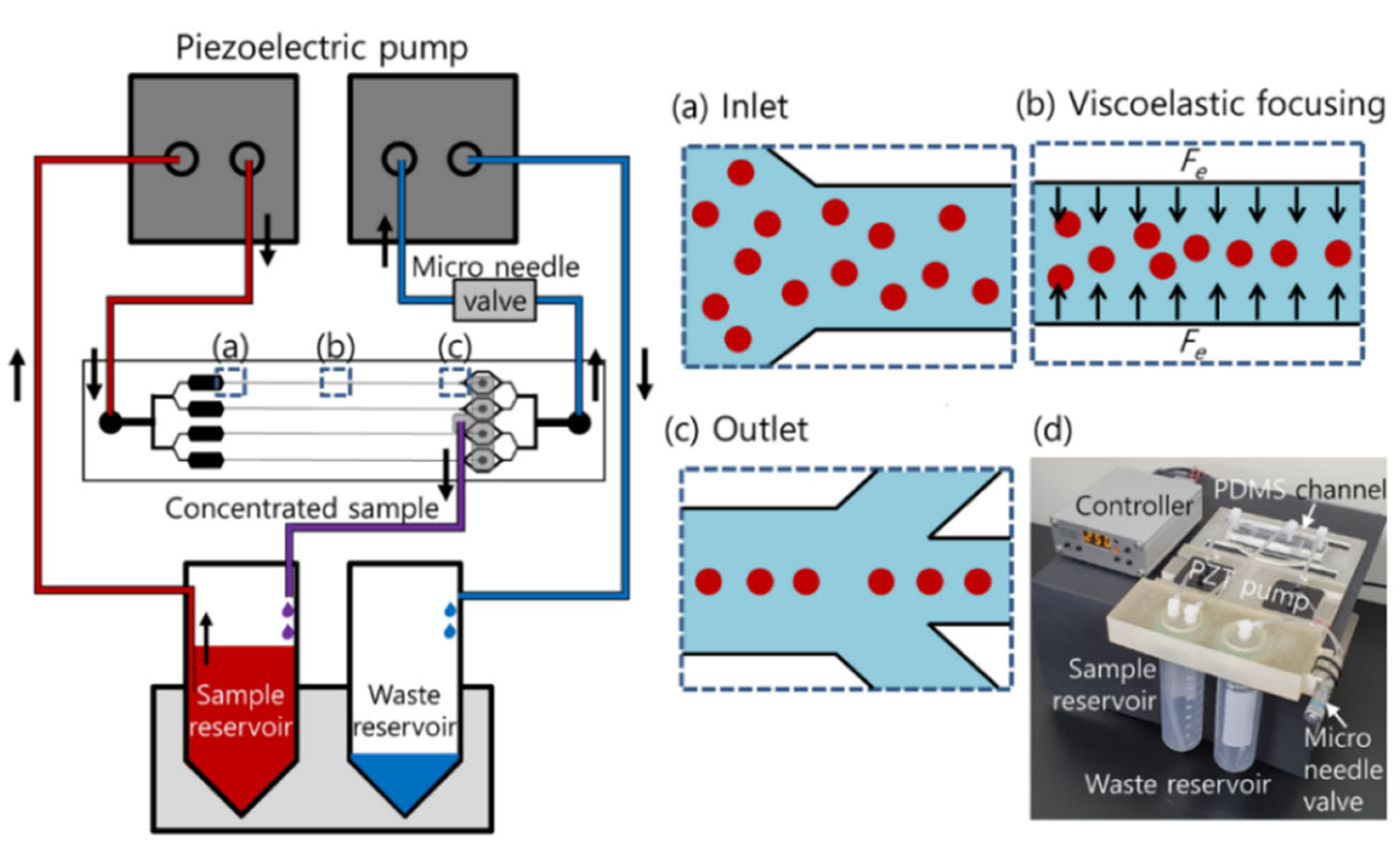
2. Materials and Methods
2.1. Device Fabrication
2.2. Cell Culture
2.3. Sample Preparation
2.4. Experimental Procedure
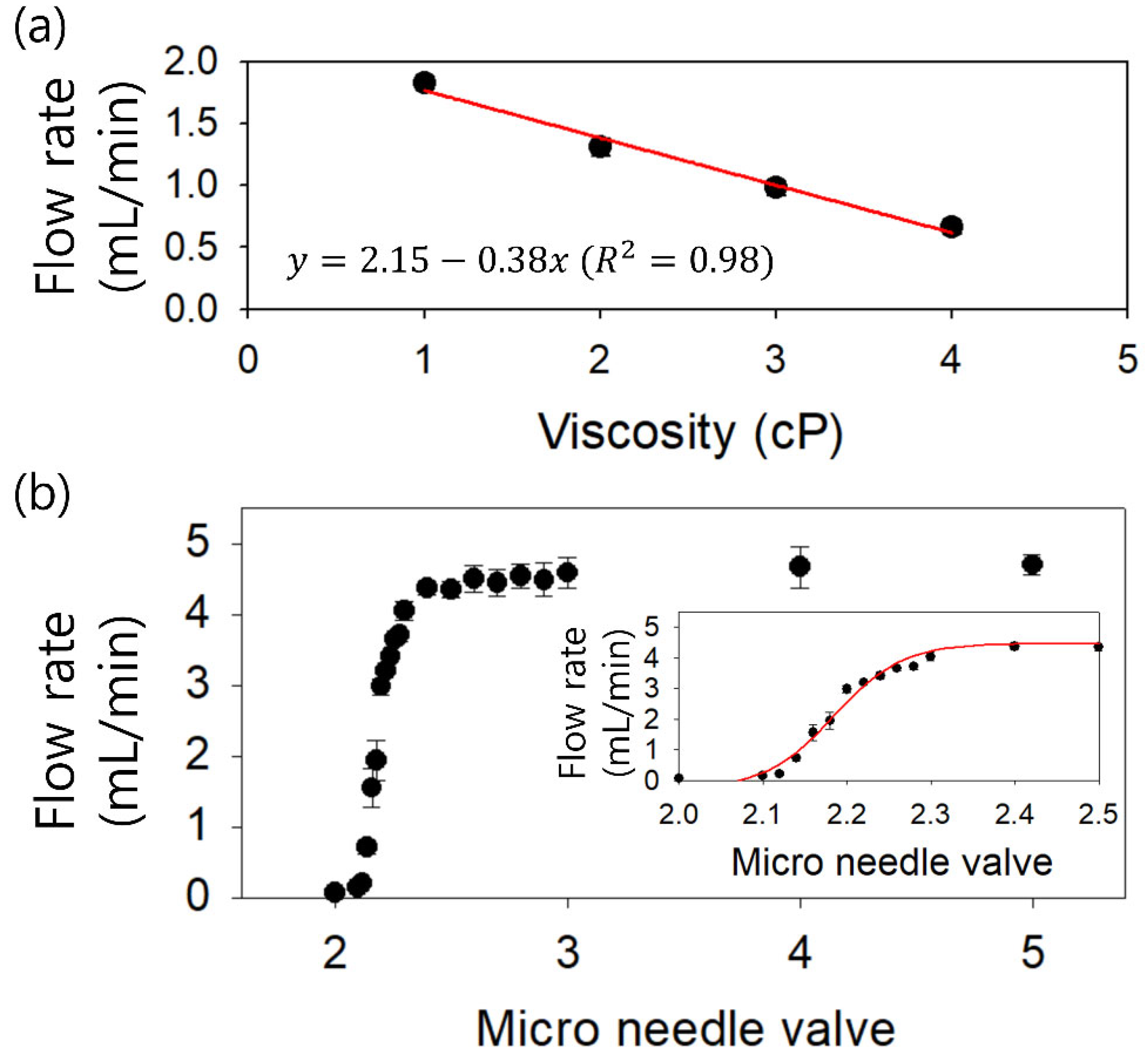
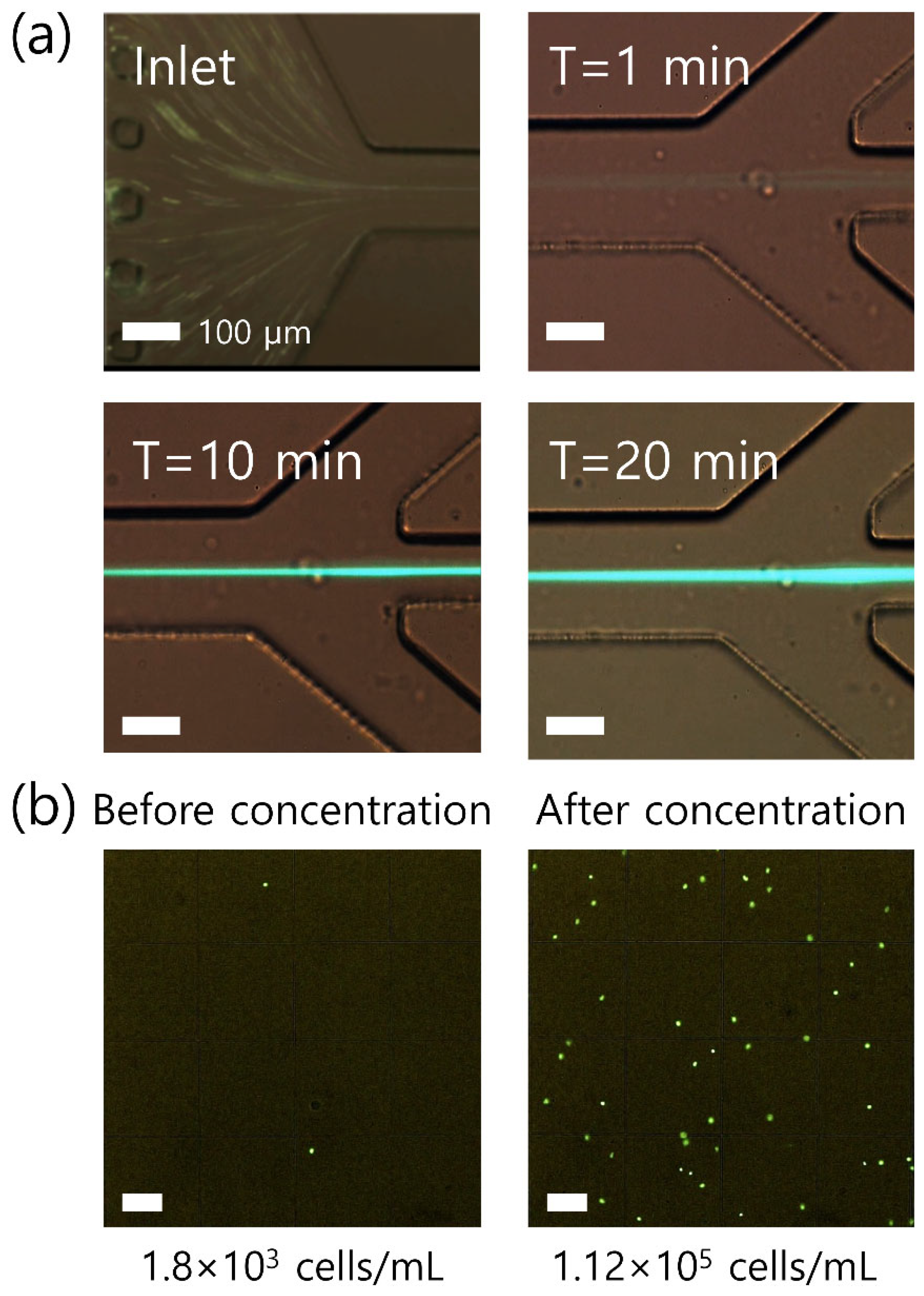
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warrick, J.; Casavant, B.; Frisk, M.; Beebe, D. A Microfluidic Cell Concentrator. Anal. Chem. 2010, 82, 8320–8326. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.I.C.; Sinskey, T.J.; Gerner, R.E.; De Filippi, R.P. Effect of centrifugation on the viability of Burkitt lymphoma cells. Biotechnol. Bioeng. 1968, 10, 641–649. [Google Scholar] [CrossRef]
- Yang, J.; Hooper, W.C.; Phillips, D.J.; Tondella, M.L.; Talkington, D.F. Centrifugation of Human Lung Epithelial Carcinoma A549 Cells Up-Regulates Interleukin-1β Gene Expression. Clin. Vaccine Immunol. 2002, 9, 1142–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dürr, M.; Kentsch, J.; Müller, T.; Schnelle, T.; Stelzle, M. Microdevices for manipulation and accumulation of micro-and nanoparticles by dielectrophoresis. Electrophoresis 2002, 24, 722–731. [Google Scholar] [CrossRef]
- Zhu, J.; Xuan, X. Dielectrophoretic focusing of particles in a microchannel constriction using DC-biased AC flectric fields. Electrophoresis 2009, 30, 2668–2675. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Gu, Y.; Li, P.; Ding, X.; Wang, L.; McCoy, J.P.; Levine, S.J.; Huang, T.J. Continuous enrichment of low-abundance cell samples using standing surface acoustic waves (SSAW). Lab Chip 2014, 14, 924–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobsson, O.; Oh, S.S.; Antfolk, M.; Eisenstein, M.; Laurell, T.; Soh, H.T. Thousand-Fold Volumetric Concentration of Live Cells with a Recirculating Acoustofluidic Device. Anal. Chem. 2015, 87, 8497–8502. [Google Scholar] [CrossRef]
- Zalis, M.C.; Reyes, J.F.; Augustsson, P.; Holmqvist, S.; Roybon, L.; Laurell, T.; Deierborg, T. Label-free concentration of viable neurons, hESCs and cancer cells by means of acoustophoresis. Integr. Biol. 2016, 8, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Seki, M. Hydrodynamic filtration for on-chip particle concentration and classification utilizing microfluidics. Lab Chip 2005, 5, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- D’Avino, G.; Maffettone, P.; Greco, F.; Hulsen, M. Viscoelasticity-induced migration of a rigid sphere in confined shear flow. J. Non-Newton. Fluid Mech. 2010, 165, 466–474. [Google Scholar] [CrossRef]
- Leshansky, A.M.; Bransky, A.; Korin, N.; Dinnar, U. Tunable nonlinear viscoelastic “focusing” in a microfluidic device. Phys. Rev. Lett. 2007, 98, 234501. [Google Scholar] [CrossRef]
- Nam, J.; Jang, W.S.; Hong, D.H.; Lim, C.S. Viscoelastic Separation and Concentration of Fungi from Blood for Highly Sensitive Molecular Diagnostics. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.; Kim, J.M. Elasto-inertial particle focusing under the viscoelastic flow of DNA solution in a square channel. Biomicrofluidics 2016, 10, 024111. [Google Scholar] [CrossRef]
- Liu, C.; Xue, C.; Hu, G. Sheathless separation of particles and cells by viscoelastic effects in straight rectangular micro-channels. Procedia Eng. 2015, 126, 721–724. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Guoqing, H.; Tian, F.; Yang, N.; Yanping, D.; Ding, Y.; Wei, J.; Hu, G.; Nie, G.; Sun, J. Field-Free Isolation of Exosomes from Extracellular Vesicles by Microfluidic Viscoelastic Flows. ACS Nano 2017, 11, 6968–6976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, J.; Lim, H.; Kim, D.; Jung, H.; Shin, S. Continuous separation of microparticles in a microfluidic channel via the elasto-inertial effect of non-Newtonian fluid. Lab Chip 2012, 12, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Namgung, B.; Lim, C.T.; Bae, J.-E.; Leo, H.L.; Cho, K.S.; Kim, S. Microfluidic device for sheathless particle focusing and separation using a viscoelastic fluid. J. Chromatogr. A 2015, 1406, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Shin, Y.; Tan, J.K.S.; Lim, Y.B.; Lim, C.T.; Kim, S. High-throughput malaria parasite separation using a viscoelastic fluid for ultrasensitive PCR detection. Lab Chip 2016, 16, 2086–2092. [Google Scholar] [CrossRef]
- Nam, J.; Tan, J.; Khoo, B.L.; Namgung, B.; Leo, H.L.; Lim, C.T.; Kim, S. Hybrid capillary-inserted microfluidic device for sheathless particle focusing and separation in viscoelastic flow. Biomicrofluidics 2015, 9, 064117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, J.; Jee, H.; Jang, W.S.; Yoon, J.; Park, B.G.; Lee, S.J.; Lim, C.S. Sheathless Shape-Based Separation of Candida Albicans Using a Viscoelastic Non-Newtonian Fluid. Micromachines 2019, 10, 817. [Google Scholar] [CrossRef] [Green Version]
- Nam, J.; Jang, W.S.; Lim, C.S. Non-electrical powered continuous cell concentration for enumeration of residual white blood cells in WBC-depleted blood using a viscoelastic fluid. Talanta 2019, 197, 12–19. [Google Scholar] [CrossRef]
- Choi, K.; Ryu, H.; Siddle, K.J.; Piantadosi, A.; Freimark, L.; Park, D.J.; Sabeti, P.; Han, J. Negative Selection by Spiral Inertial Microfluidics Improves Viral Recovery and Sequencing from Blood. Anal. Chem. 2018, 90, 4657–4662. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.; Choi, K.; Qu, Y.; Kwon, T.; Lee, J.; Han, J. Patient-Derived Airway Secretion Dissociation Technique to Isolate and Concentrate Immune Cells Using Closed-Loop Inertial Microfluidics. Anal. Chem. 2017, 89, 5549–5556. [Google Scholar] [CrossRef] [Green Version]
- Mu, C.; Zhang, Z.; Lin, M.; Dai, Z.; Cao, X. Development of a highly effective multi-stage surface acoustic wave SU-8 microfluidic concentrator. Sens. Actuators B Chem. 2015, 215, 77–85. [Google Scholar] [CrossRef]
- Li, Z.; Mak, S.Y.; Sauret, A.; Shum, H.C. Syringe-pump-induced fluctuation in all-aqueous microfluidic system implications for flow rate accuracy. Lab Chip 2014, 14, 744–749. [Google Scholar] [CrossRef] [Green Version]
- Yobas, L.; Tang, K.-C.; Yong, S.-E.; Ong, E.K.-Z. A disposable planar peristaltic pump for lab-on-a-chip. Lab Chip 2008, 8, 660–662. [Google Scholar] [CrossRef]
- Zhao, B.; Cui, X.; Ren, W.; Xu, F.; Liu, M.; Ye, Z.-G. A Controllable and Integrated Pump-enabled Microfluidic Chip and Its Application in Droplets Generating. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Cazorla, P.-H.; Fuchs, O.; Cochet, M.; Maubert, S.; Le Rhun, G.; Robert, P.; Fouillet, Y.; Defay, E. Piezoelectric Micro-pump with PZT Thin Film for Low Consumption Microfluidic Devices. Procedia Eng. 2014, 87, 488–491. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y. Guan Performance Analysis of a Microfluidic Pump Based on Combined Actuation of the Piezoelectric Effect and Liquid Crystal Backflow Effect. Micromachines 2019, 10, 584. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.; Back, S.M.; Hwang, M.H.; Lee, D.-H.; Choi, H.; Nam, J. Sheathless High-Throughput Circulating Tumor Cell Separation Using Viscoelastic non-Newtonian Fluid. Micromachines 2019, 10, 462. [Google Scholar] [CrossRef] [Green Version]
- Trenkle, F.; Haeberle, S.; Zengerle, R. Normally-closed peristaltic micropump with re-usable actuator and disposable fluidic chip. Procedia Chem. 2009, 1, 1515–1518. [Google Scholar] [CrossRef] [Green Version]
- Seo, K.W.; Byeon, H.J.; Huh, H.K.; Lee, S.J. Particle migration and single-line particle focusing in microscale pipe flow of viscoelastic fluids. RSC Adv. 2014, 4, 3512–3520. [Google Scholar] [CrossRef] [Green Version]
- Tehrani, M.A. An experimental study of particle migration in pipe flow of viscoelastic fluids. J. Rheol. 1996, 40, 1057–1077. [Google Scholar] [CrossRef]
- Kim, B.; You, D.; Kim, Y.-J.; Oh, I.; Choi, S. Motorized smart pipette for handheld operation of a microfluidic blood plasma separator. Sens. Actuators B Chem. 2018, 267, 581–588. [Google Scholar] [CrossRef]
- Zhou, J.; Giridhar, P.V.; Kasper, S.; Papautsky, I. Modulation of aspect ratio for complete separation in an inertial microfluidic channel. Lab Chip 2013, 13, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Papautsky, I. Fundamentals of inertial focusing in microchannels. Lab Chip 2013, 13, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Inman, B.A.; Etienne, W.; Rubin, R.; Owusu, R.A.; Oliveira, T.R.; Rodriques, D.B.; Maccarini, P.F.; Stauffer, P.R.; Mashal, A.; Dewhirst, M.W. The impact of temperature and urinary constituents on urine viscosity and its relevance to bladder hyper-thermia treatment. Int. J. Hyperth. 2013, 29, 206–210. [Google Scholar] [CrossRef]
- Brunzel, N.A. Body Fluid Analysis: Manual Hemocytometer Counts and Differential Slide Preparation. In Fundamentals of Urine and Body Fluid Analysis, 4th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Harmening, D. Body Fluid Examination: The Qualitative, Quantitative, and Morphologic Analysis of Serous, Cerebrospinal, and Synovial Fluids. In Clinical Hematology and Fundamentals of Hemostasis, 5th ed.; F. A. Davis Company: Philadelphia, PA, USA, 2009. [Google Scholar]
- Nam, J.; Lim, H.; Kim, D.; Shin, S. Separation of platelets from whole blood using standing surface acoustic waves in a microchannel. Lab Chip 2011, 11, 3361–3364. [Google Scholar] [CrossRef] [Green Version]
- Parks, J.W.; Olson, M.A.; Kim, J.; Ozcelik, D.; Cai, H.; Carrion, R.; Patterson, J.L.; Mathies, R.A.; Hawkins, A.R.; Schmidt, H. Integration of programmable microfluidics and on-chip fluorescence detection for biosensing applications. Biomicrofluidics 2014, 8, 054111. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Li, Q.; Hu, X.; Lo, Y. Microfluidic cytometers with integrated on-chip optical systems for red blood cell and platelet counting. Biomicrofluidics 2016, 10, 064119. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-S.; Ma, Y.-D.; Chen, C.; Shiesh, S.-C.; Lee, G.-B. An integrated microfluidic system for on-chip enrichment and quantification of circulating extracellular vesicles from whole blood. Lab Chip 2019, 19, 3305–3315. [Google Scholar] [CrossRef] [PubMed]
- Reichel, F.; Mauer, J.; Nawaz, A.A.; Gompper, G.; Guck, J.; Fedosov, D.A. High-Throughput Microfluidic Characterization of Erythrocyte Shapes and Mechanical Variability. Biophys. J. 2019, 117, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Pinho, D.; Muñoz-Sánchez, B.; Anes, C.; Vega, E.; Lima, R. Flexible PDMS microparticles to mimic RBCs in blood particulate analogue fluids. Mech. Res. Commun. 2019, 100, 103399. [Google Scholar] [CrossRef] [Green Version]
- Fredrickson, C.K.; Fan, Z.H. Macro-to-micro interfaces for microfluidic devices. Lab Chip 2004, 4, 526–533. [Google Scholar] [CrossRef]
- Han, S.I. Microfluidic Interface Technology Based on Stereolithography for Glass-Based Lab-on-a-Chips. In Microfluidic Diagnostics: Methods and Protocols; Jenkins, G., Mansfield, C.D., Eds.; Springer: New York, NY, USA, 2013; pp. 169–184. [Google Scholar]
- Pfreundt, A.; Andersen, K.B.; Dimaki, M.; E Svendsen, W. An easy-to-use microfluidic interconnection system to create quick and reversibly interfaced simple microfluidic devices. J. Micromech. Microeng. 2015, 25, 115010. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-N.; Fu, L.-M. Micropumps and biomedical applications—A review. Microelectron. Eng. 2018, 195, 121–138. [Google Scholar] [CrossRef]
- Menaker, J.; Martin, I.B.K.; Hirshon, J.M. Marked elevation of cerebrospinal fluid white blood cell count: An unusual case of Streptococcus pneumoniae meningitis, differential diagnosis, and a brief review of current epidemiology and treatment recommendations. J. Emerg. Med. 2005, 29, 37–41. [Google Scholar] [CrossRef]
- Neumann, B.; Steinberg, T.; Lee, D.-H.; Kress, J.; Kufner, M.; Schlachetzki, F.; Weinfurtner, G.; Linker, R.; Angstwurm, K. Reactive increase of cerebrospinal fluid white blood cell count after lumbar puncture: Fact or fiction? J. Neurol. Sci. 2020, 414, 116876. [Google Scholar] [CrossRef]
- Allan, D.; Keeney, M.; Howson-Jan, K.; Popma, J.; Weir, K.; Bhatia, M.; Sutherland, D.R.; Chin-Yee, I.H.; Allan, D. Number of viable CD34+ cells reinfused predicts engraftment in autologous hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002, 29, 967–972. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.; Liu, J.; Yang, X.; Zhang, Q.; Yang, W.; Zhang, H.; Liu, L. Microfluidic-based cancer cell separation using active and passive mechanisms. Microfluid. Nanofluid. 2020, 24, 1–19. [Google Scholar] [CrossRef]
- Khoo, B.L.; Warkiani, M.E.; Tan, D.S.-W.; Bhagat, A.A.S.; Irwin, D.; Lau, D.P.; Lim, A.S.T.; Lim, K.H.; Krisna, S.S.; Lim, W.-T.; et al. Clinical Validation of an Ultra High-Throughput Spiral Microfluidics for the Detection and Enrichment of Viable Circulating Tumor Cells. PLoS ONE 2014, 9, e99409. [Google Scholar] [CrossRef] [Green Version]
- Warkiani, M.E.; Khoo, B.L.; Tan, D.S.-W.; Bhagat, A.A.S.; Lim, W.-T.; Yap, Y.S.; Lee, S.C.; Soo, R.A.; Han, J.; Lim, C.T. An ultra-high-throughput spiral microfluidic biochip for the enrichment of circulating tumor cells. Analyst 2014, 139, 3245–3255. [Google Scholar] [CrossRef] [PubMed]



| Flow Resistance (Pa·s·m−3) | 60 V | 100 V | 150 V | 200 V | 250 V |
|---|---|---|---|---|---|
| 4.96 × 1011 | Q = 0.20 | Q = 0.49 | Q = 1.08 | Q = 1.64 | Q = 2.82 |
| Re = 10.12 | Re = 24.80 | Re = 54.66 | Re = 83.00 | Re = 142.7 | |
| Wi = 0.92 | Wi = 2.26 | Wi = 5 | Wi = 7.59 | Wi = 13.05 | |
| El = 0.09 | El = 0.009 | El = 0.09 | El = 0.09 | El = 0.09 | |
| 9.92 × 1011 | Q = 0.19 | Q = 0.27 | Q = 0.44 | Q = 0.72 | Q = 1.01 |
| Re = 9.61 | Re = 13.66 | Re = 22.26 | Re = 36.44 | Re = 51.11 | |
| Wi = 0.87 | Wi = 1.25 | Wi = 2.03 | Wi = 3.33 | Wi = 4.67 | |
| El = 0.09 | El = 0.09 | El = 0.09 | El = 0.09 | El = 0.09 | |
| 1.48 × 1012 | Q = 0.11 | Q = 0.19 | Q = 0.28 | Q = 0.42 | Q = 0.59 |
| Re = 3.18 | Re = 9.61 | Re = 14.17 | Re = 21.25 | Re = 29.86 | |
| Wi = 0.29 | Wi = 0.87 | Wi = 1.29 | Wi = 1.94 | Wi = 2.73 | |
| El = 0.09 | El = 0.09 | El = 0.09 | El = 0.09 | El = 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lim, H.; Jee, H.; Choo, S.; Yang, M.; Park, S.; Lee, K.; Park, H.; Lim, C.; Nam, J. High-Throughput Cell Concentration Using A Piezoelectric Pump in Closed-Loop Viscoelastic Microfluidics. Micromachines 2021, 12, 677. https://doi.org/10.3390/mi12060677
Kim J, Lim H, Jee H, Choo S, Yang M, Park S, Lee K, Park H, Lim C, Nam J. High-Throughput Cell Concentration Using A Piezoelectric Pump in Closed-Loop Viscoelastic Microfluidics. Micromachines. 2021; 12(6):677. https://doi.org/10.3390/mi12060677
Chicago/Turabian StyleKim, Jeeyong, Hyunjung Lim, Hyunseul Jee, Seunghee Choo, Minji Yang, Sungha Park, Kyounghwa Lee, Hyoungsook Park, Chaeseung Lim, and Jeonghun Nam. 2021. "High-Throughput Cell Concentration Using A Piezoelectric Pump in Closed-Loop Viscoelastic Microfluidics" Micromachines 12, no. 6: 677. https://doi.org/10.3390/mi12060677






