Modelling the Human Placental Interface In Vitro—A Review
Abstract
:1. Introduction
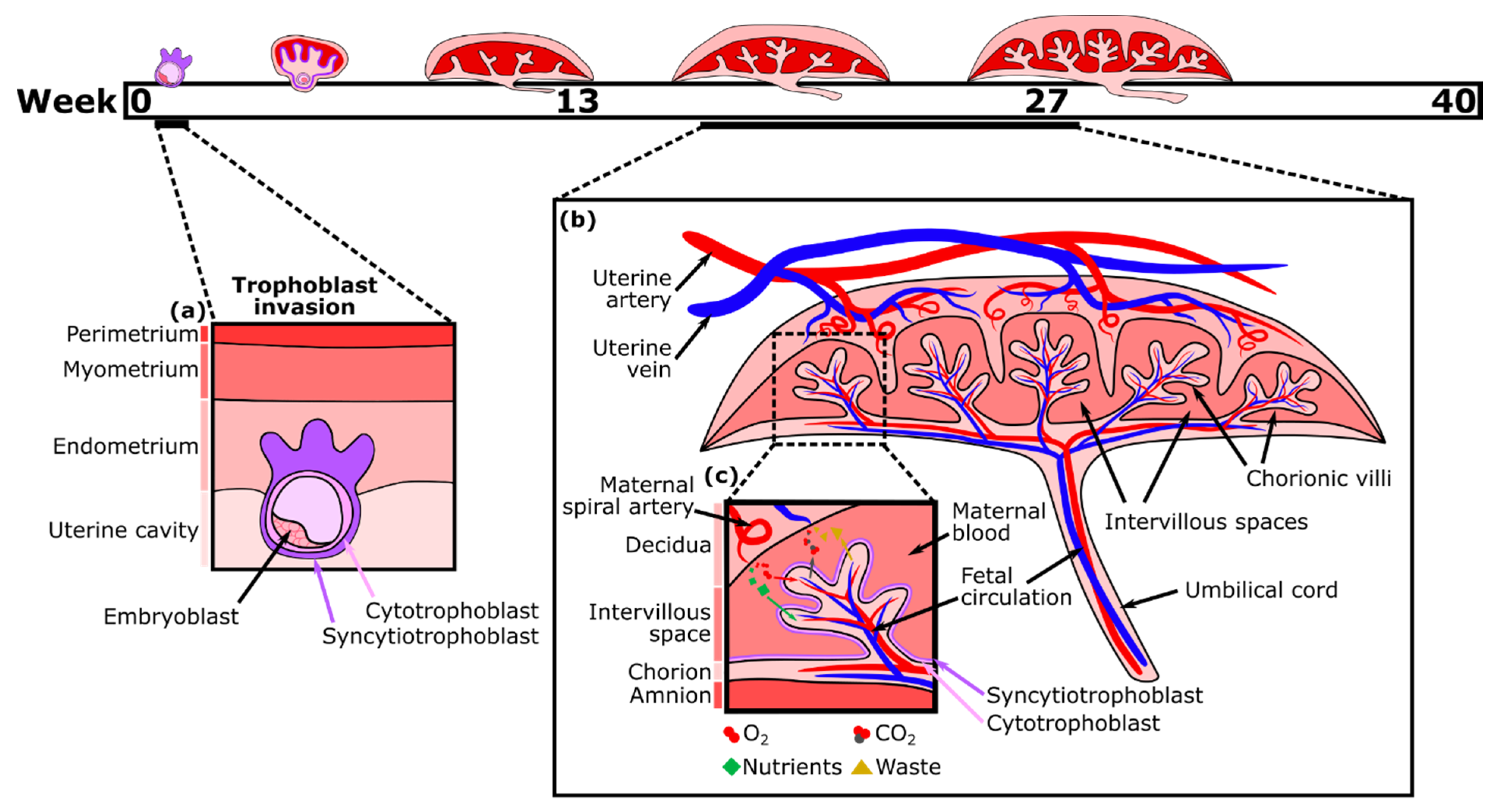
2. Development and Functions of the Human Placenta
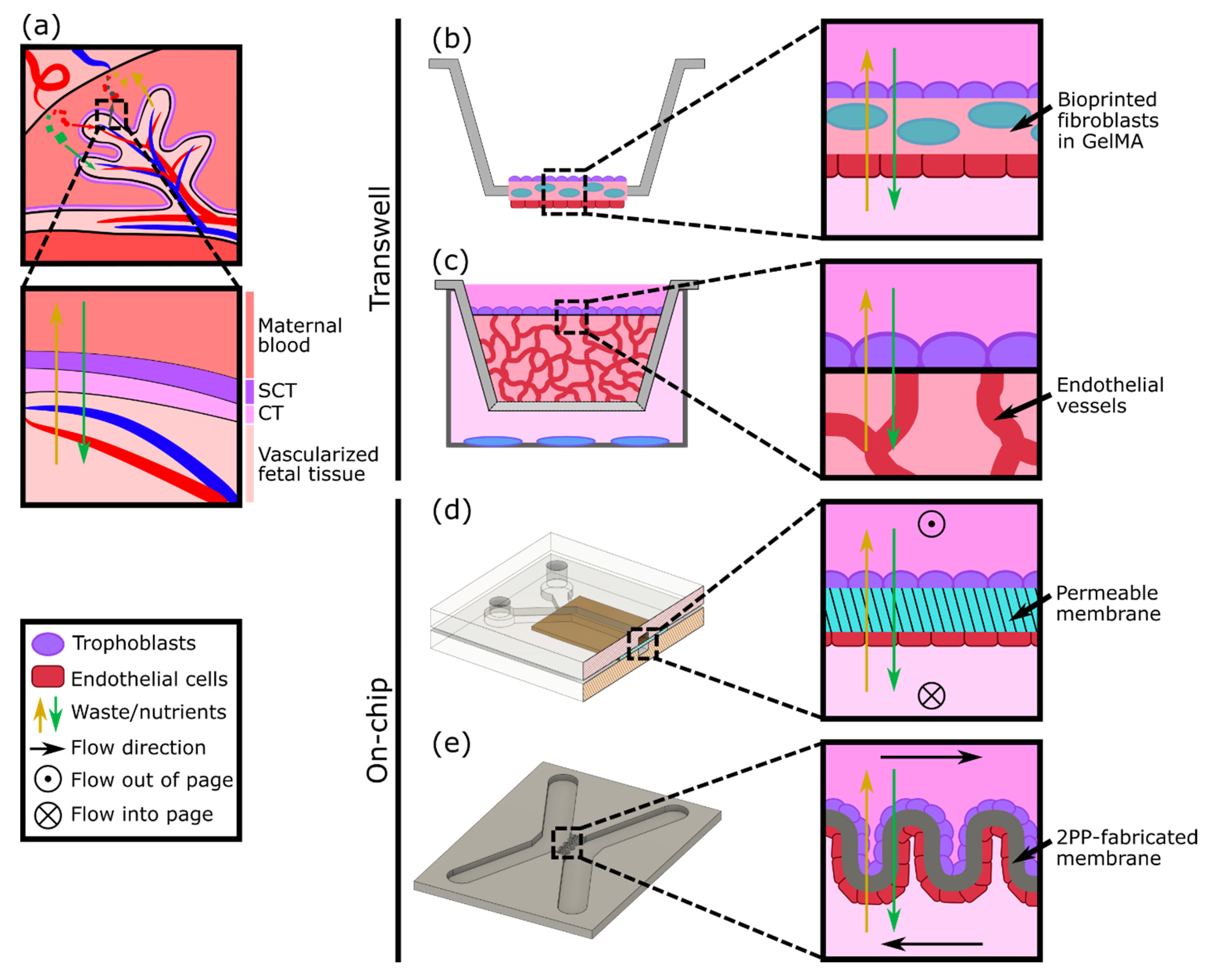
3. In Vitro Models of the Placental Barrier
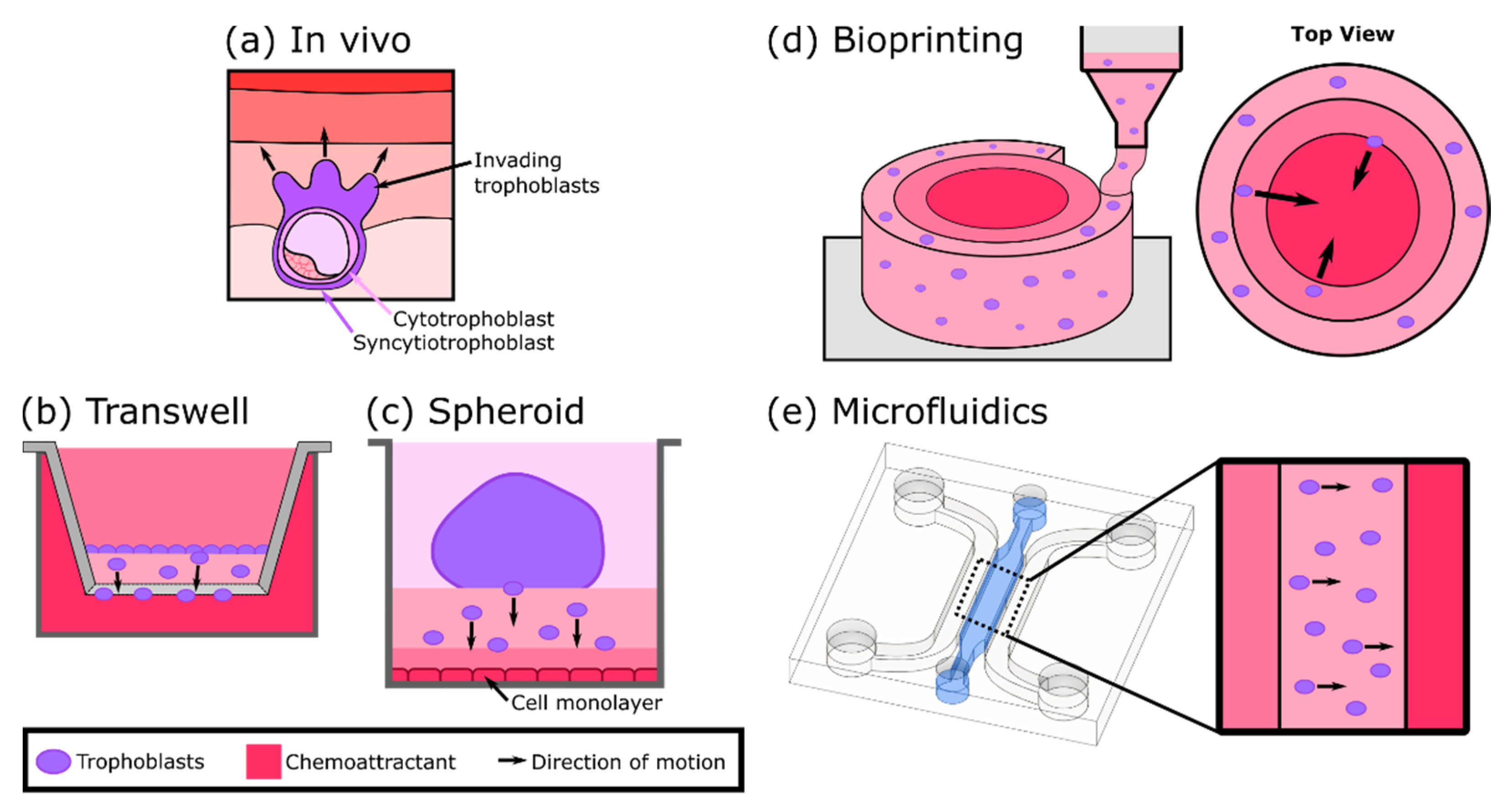
4. In Vitro Models of Trophoblast Invasion
5. Three-Dimensional Models to Study Placental Dysfunction, Infections, and Maternal-Fetal Toxicology
6. Engineering an Ideal Human Placenta-on-a-Chip
7. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Maltepe, E.; Fisher, S.J. Placenta: The Forgotten Organ. Annu. Rev. Cell Dev. Biol. 2015, 31, 523–552. [Google Scholar] [CrossRef]
- Higgins, L.E.; De Castro, N.R.; Addo, N.; Wareing, M.; Greenwood, S.; Jones, R.L.; Sibley, C.P.; Johnstone, E.; Heazell, A. Placental Features of Late-Onset Adverse Pregnancy Outcome. PLoS ONE 2015, 10, e0129117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visiedo, F.; Bugatto, F.; Prado, R.Q.; Cózar-Castellano, I.; Bartha, J.L.; Perdomo, G. Glucose and Fatty Acid Metabolism in Placental Explants from Pregnancies Complicated With Gestational Diabetes Mellitus. Reprod. Sci. 2015, 22, 798–801. [Google Scholar] [CrossRef]
- Gonzales, S.K.; Badell, M.; Cottrell, H.; Rimawi, B.; Deepak, V.; Sidell, N.; Rajakumar, A. Villous explants from preeclamptic placentas induce sFlt1 in PBMCs: An ex vivo co-culture study. Pregnancy Hypertens. 2018, 12, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, P.L. Animal Models to Study Placental Development and Function throughout Normal and Dysfunctional Human Pregnancy. Semin. Reprod. Med. 2016, 34, 011–016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, A.; Prieto, D.M.M.; Pastuschek, J.; Fröhlich, K.; Markert, U.R. Only humans have human placentas: Molecular differences between mice and humans. J. Reprod. Immunol. 2015, 108, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.; Filis, P.; Soffientini, U.; Bellingham, M.; O’Shaughnessy, P.J.; Fowler, P.A. Placental transporter localization and expression in the Human: The importance of species, sex, and gestational age differences. Biol. Reprod. 2017, 96, 733–742. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, M.L.; Oyen, M.L. Bioengineering Approaches for Placental Research. Ann. Biomed. Eng. 2021, 1–14. [Google Scholar] [CrossRef]
- Turco, M.Y.; Moffett, A. Development of the human placenta. Development 2019, 146, dev163428. [Google Scholar] [CrossRef] [Green Version]
- Huppertz, B.; Weiss, G.; Moser, G. Trophoblast invasion and oxygenation of the placenta: Measurements versus presumptions. J. Reprod. Immunol. 2014, 101–102, 74–79. [Google Scholar] [CrossRef]
- Knöfler, M.; Haider, S.; Saleh, L.; Pollheimer, J.; Gamage, T.K.J.B.; James, J. Human placenta and trophoblast development: Key molecular mechanisms and model systems. Cell. Mol. Life Sci. 2019, 76, 3479–3496. [Google Scholar] [CrossRef] [Green Version]
- Tetro, N.; Moushaev, S.; Rubinchik-Stern, M.; Eyal, S. The Placental Barrier: The Gate and the Fate in Drug Distribution. Pharm. Res. 2018, 35, 71. [Google Scholar] [CrossRef]
- Manning, J.M.; Manning, L.R.; Dumoulin, A.; Padovan, J.C.; Chait, B. Embryonic and Fetal Human Hemoglobins: Structures, Oxygen Binding, and Physiological Roles. Prokaryotic Cytoskelet. 2020, 94, 275–296. [Google Scholar] [CrossRef]
- Burton, G.J.; Fowden, A.L. The placenta: A multifaceted, transient organ. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.K.; Gupta, R.C. Chapter 68—Placental Toxicity. In Reproductive and Developmental Toxicology, 2nd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 1301–1325. ISBN 9780128042397. [Google Scholar]
- Leber, A.; Zenclussen, M.L.; Teles, A.; Brachwitz, N.; Casalis, P.; El-Mousleh, T.; Jensen, F.; Woidacki, K.; Zenclussen, A. Pregnancy: Tolerance and Suppression of Immune Responses. In Suppression and Regulation of Immune Responses: Methods and Protocols; Cuturi, M.C., Anegon, I., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 397–417. ISBN 9781607618690. [Google Scholar]
- Simister, N.E. Placental transport of immunoglobulin G. Vaccine 2003, 21, 3365–3369. [Google Scholar] [CrossRef]
- Srisuparp, S.; Strakova, Z.; Fazleabas, A.T. The Role of Chorionic Gonadotropin (CG) in Blastocyst Implantation. Arch. Med. Res. 2001, 32, 627–634. [Google Scholar] [CrossRef]
- Evain-Brion, D.; Malassine, A. Human placenta as an endocrine organ. Growth Horm. IGF Res. 2003, 13, S34–S37. [Google Scholar] [CrossRef]
- Pattillo, R.A.; Gey, G.O. The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Res. 1968, 28, 1231–1236. [Google Scholar] [PubMed]
- Kohler, P.O.; Bridson, W.E. Isolation of Hormone-Producing Clonal Lines of Human Choriocarcinoma1. J. Clin. Endocrinol. Metab. 1971, 32, 683–687. [Google Scholar] [CrossRef]
- Graham, C.H.; Hawley, T.S.; Hawley, R.G.; MacDougall, J.R.; Kerbel, R.S.; Khoo, N.; Lala, P.K. Establishment and Characterization of First Trimester Human Trophoblast Cells with Extended Lifespan. Exp. Cell Res. 1993, 206, 204–211. [Google Scholar] [CrossRef]
- Rothbauer, M.; Patel, N.; Gondola, H.; Siwetz, M.; Huppertz, B.; Ertl, P. A comparative study of five physiological key parameters between four different human trophoblast-derived cell lines. Sci. Rep. 2017, 7, 5892. [Google Scholar] [CrossRef]
- Almeida, M.P.O.; Ferro, E.A.V.; Briceño, M.P.; Oliveira, M.C.; Barbosa, B.F.; Silva, N.M. Susceptibility of human villous (BeWo) and extravillous (HTR-8/SVneo) trophoblast cells to Toxoplasma gondii infection is modulated by intracellular iron availability. Parasitol. Res. 2019, 118, 1559–1572. [Google Scholar] [CrossRef]
- Widhalm, R.; Ellinger, I.; Granitzer, S.; Forsthuber, M.; Bajtela, R.; Gelles, K.; Hartig, P.-Y.; Hengstschläger, M.; Zeisler, H.; Salzer, H.; et al. Human placental cell line HTR-8/SVneo accumulates cadmium by divalent metal transporters DMT1 and ZIP14. Metallomics 2020, 12, 1822–1833. [Google Scholar] [CrossRef] [PubMed]
- Kreuder, A.-E.; Bolaños-Rosales, A.; Palmer, C.; Thomas, A.; Geiger, M.-A.; Lam, T.; Amler, A.-K.; Markert, U.R.; Lauster, R.; Kloke, L. Inspired by the human placenta: A novel 3D bioprinted membrane system to create barrier models. Sci. Rep. 2020, 10, 15606. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, A.; Gilmore, C.; Sood, A.; Matsusaki, M.; Collett, G.; Tannetta, D.; Sargent, I.L.; McGarvey, J.; Halemani, N.D.; Hanley, J.; et al. In vitro placenta barrier model using primary human trophoblasts, underlying connective tissue and vascular endothelium. Biomaterials 2019, 192, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Blundell, C.; Tess, E.R.; Schanzer, A.S.R.; Coutifaris, C.; Su, E.J.; Parry, S.; Huh, D. A microphysiological model of the human placental barrier. Lab Chip 2016, 16, 3065–3073. [Google Scholar] [CrossRef] [PubMed]
- Pemathilaka, R.L.; Caplin, J.D.; Aykar, S.S.; Montazami, R.; Hashemi, N.N. Placenta-on-a-Chip: In Vitro Study of Caffeine Transport across Placental Barrier Using Liquid Chromatography Mass Spectrometry. Glob. Chall. 2019, 3, 1800112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandt, D.; Gruber, P.; Markovic, M.; Tromayer, M.; Rothbauer, M.; Kratz, S.R.A.; Ali, F.; Van Hoorick, J.; Holnthoner, W.; Mühleder, S.; et al. Fabrication of placental barrier structures within a microfluidic device utilizing two-photon polymerization. Int. J. Bioprint. 2018, 4, 144. [Google Scholar] [CrossRef]
- Huppertz, B. Traditional and New Routes of Trophoblast Invasion and Their Implications for Pregnancy Diseases. Int. J. Mol. Sci. 2019, 21, 289. [Google Scholar] [CrossRef] [Green Version]
- Lacroix, M.-C.; Guibourdenche, J.; Fournier, T.; Laurendeau, I.; Igout, A.; Goffin, V.; Pantel, J.; Tsatsaris, V.; Evain-Brion, D. Stimulation of Human Trophoblast Invasion by Placental Growth Hormone. Endocrinology 2005, 146, 2434–2444. [Google Scholar] [CrossRef] [Green Version]
- Desforges, M.; Harris, L.K.; Aplin, J.D. Elastin-derived peptides stimulate trophoblast migration and invasion: A positive feedback loop to enhance spiral artery remodelling. Mol. Hum. Reprod. 2015, 21, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Li, P.; Wang, Y.; Yan, H. Hypoxia-induced expression of CXCR4 favors trophoblast cell migration and invasion via the activation of HIF-1α. Int. J. Mol. Med. 2018, 42, 1508–1516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bojić-Trbojević, Ž.; Krivokuća, M.J.; Vilotić, A.; Kolundžić, N.; Stefanoska, I.; Zetterberg, F.; Nilsson, U.J.; Leffler, H.; Vićovac, L. Human trophoblast requires galectin-3 for cell migration and invasion. Sci. Rep. 2019, 9, 2136. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Stelzl, P.; Zhaing, Y.; Porter, J.; Liu, H.; Liao, A.; Aldo, P.B.; Mor, G. Novel 3D in vitro models to evaluate trophoblast migration and invasion. Am. J. Reprod. Immunol. 2019, 81, e13076. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-Y.; Guo, T.; Cabrera-Luque, J.; Arumugasaamy, N.; Bracaglia, L.; Garcia-Vivas, A.; Santoro, M.; Baker, H.; Fisher, J.; Kim, P. Placental basement membrane proteins are required for effective cytotrophoblast invasion in a three-dimensional bioprinted placenta model. J. Biomed. Mater. Res. Part A 2018, 106, 1476–1487. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Illsley, N.P.; Chang, R.C. 3D Bioprinted GelMA Based Models for the Study of Trophoblast Cell Invasion. Sci. Rep. 2019, 9, 18854. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.-Y.; Eranki, A.; Placone, J.K.; Rhodes, K.R.; Aranda-Espinoza, H.; Fernandes, R.; Fisher, J.P.; Kim, P.C.W. Development of a 3D Printed, Bioengineered Placenta Model to Evaluate the Role of Trophoblast Migration in Preeclampsia. ACS Biomater. Sci. Eng. 2016, 2, 1817–1826. [Google Scholar] [CrossRef]
- Abbas, Y.; Oefner, C.M.; Polacheck, W.; Gardner, L.; Farrell, L.; Sharkey, A.; Kamm, R.; Moffett, A.; Oyen, M.L. A microfluidics assay to study invasion of human placental trophoblast cells. J. R. Soc. Interface 2017, 14, 20170131. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Gingrich, J.; Veiga-Lopez, A. A 3-dimensional microfluidic platform for modeling human extravillous trophoblast invasion and toxicological screening. Lab Chip 2021, 21, 546–557. [Google Scholar] [CrossRef]
- Armant, D.; Fritz, R.; Kilburn, B.; Kim, Y.; Nien, J.K.; Maihle, N.; Romero, R.; Leach, R. Reduced expression of the epidermal growth factor signaling system in preeclampsia. Placenta 2015, 36, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Turowski, G.; Vogel, M. Re-view and view on maturation disorders in the placenta. APMIS 2018, 126, 602–612. [Google Scholar] [CrossRef]
- Weckman, A.M.; Ngai, M.; Wright, J.; McDonald, C.R.; Kain, K.C. The Impact of Infection in Pregnancy on Placental Vascular Development and Adverse Birth Outcomes. Front. Microbiol. 2019, 10, 1924. [Google Scholar] [CrossRef] [Green Version]
- Koren, G.; Ornoy, A. The role of the placenta in drug transport and fetal drug exposure. Expert Rev. Clin. Pharmacol. 2018, 11, 373–385. [Google Scholar] [CrossRef]
- Mathiesen, L.; Buerki-Thurnherr, T.; Pastuschek, J.; Aengenheister, L.; Knudsen, L.E. Fetal exposure to environmental chemicals; insights from placental perfusion studies. Placenta 2021, 106, 58–66. [Google Scholar] [CrossRef]
- Haase, K.; Gillrie, M.R.; Hajal, C.; Kamm, R.D. Pericytes Contribute to Dysfunction in a Human 3D Model of Placental Microvasculature through VEGF-Ang-Tie2 Signaling. Adv. Sci. 2019, 6, 1900878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coyne, C.B.; Lazear, H.M. Zika virus—Reigniting the TORCH. Nat. Rev. Genet. Microbiol. 2016, 14, 707–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutts, J.C.; Agius, P.A.; Lin, Z.; Powell, R.; Moore, K.; Draper, B.; Simpson, J.A.; Fowkes, F.J.I. Pregnancy-specific malarial immunity and risk of malaria in pregnancy and adverse birth outcomes: A systematic review. BMC Med. 2020, 18, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McConkey, C.A.; Delorme-Axford, E.; Nickerson, C.A.; Kim, K.S.; Sadovsky, Y.; Boyle, J.P.; Coyne, C.B. A three-dimensional culture system recapitulates placental syncytiotrophoblast development and microbial resistance. Sci. Adv. 2016, 2, e1501462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Yin, F.; Wang, H.; Wang, L.; Yuan, J.; Qin, J. Placental Barrier-on-a-Chip: Modeling Placental Inflammatory Responses to Bacterial Infection. ACS Biomater. Sci. Eng. 2018, 4, 3356–3363. [Google Scholar] [CrossRef]
- Richardson, L.S.; Kim, S.; Han, A.; Menon, R. Modeling ascending infection with a feto-maternal interface organ-on-chip. Lab Chip 2020, 20, 4486–4501. [Google Scholar] [CrossRef]
- Green, E.S.; Arck, P.C. Pathogenesis of preterm birth: Bidirectional inflammation in mother and fetus. Semin. Immunopathol. 2020, 42, 413–429. [Google Scholar] [CrossRef]
- McBride, W. Thalidomide and congenital abnormalities. Lancet 1961, 278, 1358. [Google Scholar] [CrossRef]
- Blundell, C.; Yi, Y.-S.; Ma, L.; Tess, E.R.; Farrell, M.J.; Georgescu, A.; Aleksunes, L.; Huh, D. Placental Drug Transport-on-a-Chip: A Microengineered In Vitro Model of Transporter-Mediated Drug Efflux in the Human Placental Barrier. Adv. Healthc. Mater. 2018, 7, 1700786. [Google Scholar] [CrossRef]
- Dugershaw, B.B.; Aengenheister, L.; Hansen, S.S.K.; Hougaard, K.S.; Buerki-Thurnherr, T. Recent insights on indirect mechanisms in developmental toxicity of nanomaterials. Part. Fibre Toxicol. 2020, 17, 31. [Google Scholar] [CrossRef]
- Yin, F.; Zhu, Y.; Zhang, M.; Yu, H.; Chen, W.; Qin, J. A 3D human placenta-on-a-chip model to probe nanoparticle exposure at the placental barrier. Toxicol. In Vitro 2019, 54, 105–113. [Google Scholar] [CrossRef]
- Kaur, G.; Dufour, J.M. Cell lines. Spermatogenesis 2012, 2, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Petroff, M.G.; Phillips, T.A.; Ka, H.; Pace, J.L.; Hunt, J.S. Isolation and Culture of Term Human Trophoblast Cells. In Placenta and Trophoblast: Methods and Protocols; Soares, M.J., Hunt, J.S., Eds.; Humana Press: Totowa, NJ, USA, 2006; Volume 1, pp. 203–217. ISBN 9781592599837. [Google Scholar]
- Ilic, D.; Kapidzic, M.; Genbacev, O. Isolation of Human Placental Fibroblasts. Curr. Protoc. Stem Cell Biol. 2008, 5, 1C.6.1–1C.6.17. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Schust, D.J. Isolation, purification and in vitro differentiation of cytotrophoblast cells from human term placenta. Reprod. Biol. Endocrinol. 2015, 13, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelekanos, R.A.; Sardesai, V.S.; Futrega, K.; Lott, W.B.; Kuhn, M.; Doran, M.R. Isolation and Expansion of Mesenchymal Stem/Stromal Cells Derived from Human Placenta Tissue. J. Vis. Exp. 2016, 10, e54204. [Google Scholar] [CrossRef] [Green Version]
- Papait, A.; Vertua, E.; Magatti, M.; Ceccariglia, S.; De Munari, S.; Silini, A.R.; Sheleg, M.; Ofir, R.; Parolini, O. Mesenchymal Stromal Cells from Fetal and Maternal Placenta Possess Key Similarities and Differences: Potential Implications for Their Applications in Regenerative Medicine. Cells 2020, 9, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, R.M.; Ezashi, T.; Sheridan, M.A.; Yang, Y. Specification of trophoblast from embryonic stem cells exposed to BMP4. Biol. Reprod. 2018, 99, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Beltcheva, M.; Gontarz, P.; Zhang, B.; Popli, P.; Fischer, L.A.; Khan, S.A.; Park, K.-M.; Yoon, E.-J.; Xing, X.; et al. Derivation of trophoblast stem cells from naïve human pluripotent stem cells. eLife 2020, 9, e52504. [Google Scholar] [CrossRef]
- Horii, M.; Bui, T.; Touma, O.; Cho, H.Y.; Parast, M.M. An Improved Two-Step Protocol for Trophoblast Differentiation of Human Pluripotent Stem Cells. Curr. Protoc. Stem Cell Biol. 2019, 50, e96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheridan, M.; Yang, Y.; Jain, A.; Lyons, A.S.; Yang, P.; Brahmasani, S.R.; Dai, A.; Tian, Y.; Ellersieck, M.R.; Tuteja, G.; et al. Early onset preeclampsia in a model for human placental trophoblast. Proc. Natl. Acad. Sci. USA 2019, 116, 4336–4345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horii, M.; Morey, R.; Bui, T.; Touma, O.; Nelson, K.K.; Cho, H.-Y.; Rishik, H.; Laurent, L.C.; Parast, M.M. Modeling preeclampsia using human induced pluripotent stem cells. Sci. Rep. 2021, 11, 5877. [Google Scholar] [CrossRef] [PubMed]
- Urbanczyk, M.; Layland, S.L.; Schenke-Layland, K. The role of extracellular matrix in biomechanics and its impact on bioengineering of cells and 3D tissues. Matrix Biol. 2020, 85–86, 1–14. [Google Scholar] [CrossRef]
- Karaman, E.; Arslan, H.; Çetin, O.; Şahin, H.G.; Bora, A.; Yavuz, A.; Elasan, S.; Akbudak, I. Comparison of placental elasticity in normal and pre-eclamptic pregnant women by acoustic radiation force impulse elastosonography. J. Obstet. Gynaecol. Res. 2016, 42, 1464–1470. [Google Scholar] [CrossRef]
- Eroğlu, H.; Tolunay, H.E.; Tonyalı, N.V.; Orgul, G.; Şahin, D.; Yucel, A. Comparison of placental elasticity in normal and intrauterine growth retardation pregnancies by ex vivo strain elastography. Arch. Gynecol. Obstet. 2020, 302, 109–115. [Google Scholar] [CrossRef]
- Ma, Z.; Sagrillo-Fagundes, L.; Mok, S.; Vaillancourt, C.; Moraes, C. Mechanobiological regulation of placental trophoblast fusion and function through extracellular matrix rigidity. Sci. Rep. 2020, 10, 5837. [Google Scholar] [CrossRef] [Green Version]
- Turco, M.Y.; Gardner, L.; Kay, R.G.; Hamilton, R.; Prater, M.; Hollinshead, M.S.; McWhinnie, A.; Esposito, L.; Fernando, R.; Skelton, H.; et al. Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nat. Cell Biol. 2018, 564, 263–267. [Google Scholar] [CrossRef] [Green Version]
- Morley, L.C.; Beech, D.J.; Walker, J.J.; Simpson, N.A.B. Emerging concepts of shear stress in placental development and function. Mol. Hum. Reprod. 2019, 25, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Demir, R.; Seval, Y.; Huppertz, B. Vasculogenesis and angiogenesis in the early human placenta. Acta Histochem. 2007, 109, 257–265. [Google Scholar] [CrossRef]
- Haase, K.; Kamm, R.D. Advances in on-chip vascularization. Regen. Med. 2017, 12, 285–302. [Google Scholar] [CrossRef] [Green Version]
- Zulu, M.; Martinez, F.O.; Gordon, S.; Gray, C.M. The Elusive Role of Placental Macrophages: The Hofbauer Cell. J. Innate Immun. 2019, 11, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.R.; Appios, A.; Zhao, X.; Dutkiewicz, R.; Donde, M.; Lee, C.Y.; Naidu, P.; Lee, C.; Cerveira, J.; Liu, B.; et al. Phenotypic and functional characterization of first-trimester human placental macrophages, Hofbauer cells. J. Exp. Med. 2021, 218, e20200891. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.J.; Iqbal, K.; Kozai, K. Hypoxia and Placental Development. Birth Defects Res. 2017, 109, 1309–1329. [Google Scholar] [CrossRef]
- Lan, K.-C.; Lai, Y.-J.; Cheng, H.-H.; Tsai, N.-C.; Su, Y.-T.; Tsai, C.-C.; Hsu, T.-Y. Levels of sex steroid hormones and their receptors in women with preeclampsia. Reprod. Biol. Endocrinol. 2020, 18, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, B.C.; Levine, R.J.; Karumanchi, S.A. Pathogenesis of Preeclampsia. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 173–192. [Google Scholar] [CrossRef]
- Harmon, A.C.; Cornelius, D.; Amaral, L.M.; Faulkner, J.L.; Cunningham, M.W., Jr.; Wallace, K.; LaMarca, B. The role of inflammation in the pathology of preeclampsia. Clin. Sci. 2016, 130, 409–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Mosavati, B.; Oleinikov, A.V.; Du, E. Biosensors for Detection of Human Placental Pathologies: A Review of Emerging Technologies and Current Trends. Transl. Res. 2019, 213, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Schuller, P.; Rothbauer, M.; Kratz, S.R.; Höll, G.; Taus, P.; Schinnerl, M.; Genser, J.; Bastús, N.G.; Moriones, O.H.; Puntes, V.; et al. A lab-on-a-chip system with an embedded porous membrane-based impedance biosensor array for nanoparticle risk assessment on placental Bewo trophoblast cells. Sens. Actuators B Chem. 2020, 312, 127946. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherubini, M.; Erickson, S.; Haase, K. Modelling the Human Placental Interface In Vitro—A Review. Micromachines 2021, 12, 884. https://doi.org/10.3390/mi12080884
Cherubini M, Erickson S, Haase K. Modelling the Human Placental Interface In Vitro—A Review. Micromachines. 2021; 12(8):884. https://doi.org/10.3390/mi12080884
Chicago/Turabian StyleCherubini, Marta, Scott Erickson, and Kristina Haase. 2021. "Modelling the Human Placental Interface In Vitro—A Review" Micromachines 12, no. 8: 884. https://doi.org/10.3390/mi12080884
APA StyleCherubini, M., Erickson, S., & Haase, K. (2021). Modelling the Human Placental Interface In Vitro—A Review. Micromachines, 12(8), 884. https://doi.org/10.3390/mi12080884





