Dielectrophoresis as a Tool to Reveal the Potential Role of Ion Channels and Early Electrophysiological Changes in Osteoarthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Theory
2.2. Chemicals
2.3. Cell Culture
2.4. OA-Induced Cell Model
2.5. Pharmacological Treatment
2.6. Sample Preparation
2.7. Dielectrophoretic Experiments and Analysis
3. Results and Discussion
3.1. Role of Potassium Ions in Chondrocyte Electrophysiology
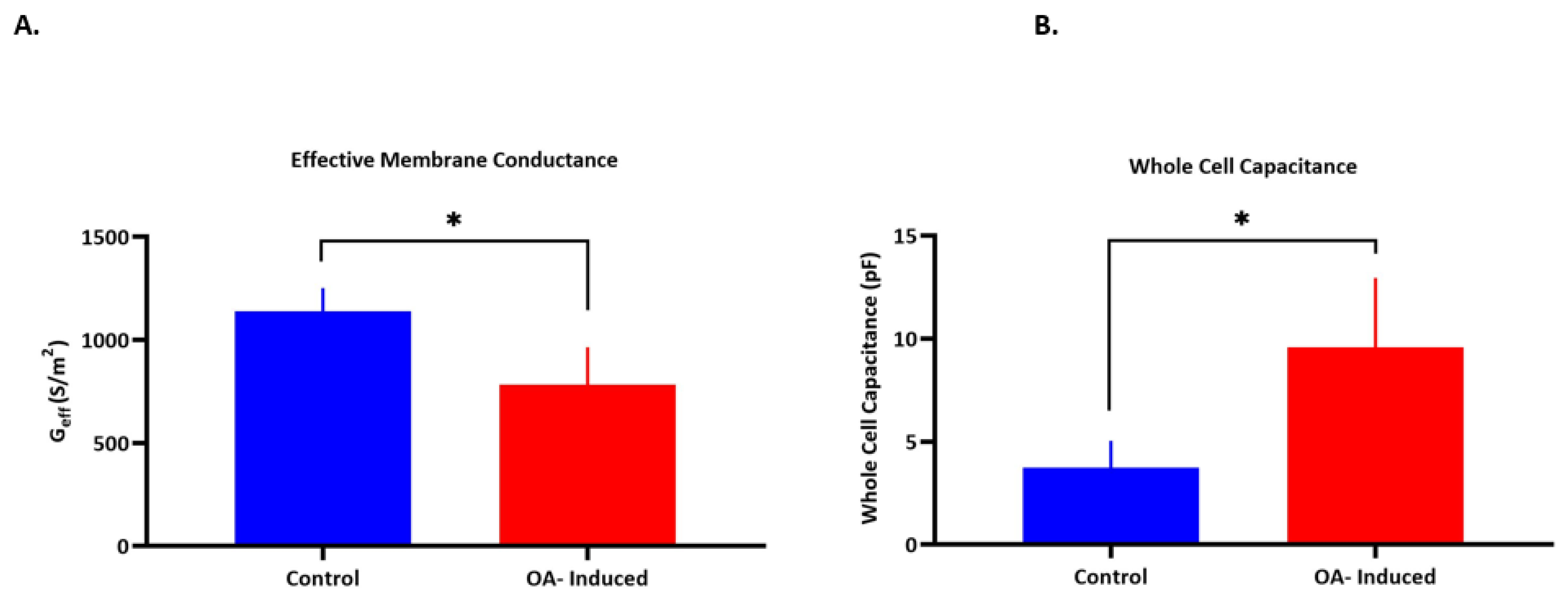
3.2. Electrophysiological Differences between Healthy and Arthritic Chondrocytes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Clark, R.B.; Kondo, C.; Belke, D.D.; Giles, W.R. Two-pore domain K + channels regulate membrane potential of isolated human articular chondrocytes. J. Physiol. 2011, 589, 5071–5089. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, H.; Nohe, A. Role of chondrocytes in cartilage formation, progression of osteoarthritis and cartilage regeneration. J. Dev. Biol. 2015, 3, 177–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matta, C.; Zákány, R.; Mobasheri, A. Voltage-Dependent Calcium Channels in Chondrocytes: Roles in Health and Disease. Curr. Rheumatol. Rep. 2015, 17, 43. [Google Scholar] [CrossRef] [Green Version]
- Vina, E.R.; Kwoh, C.K. Epidemiology of Osteoarthritis: Literature Update Ernest. Physiol. Behav. 2018, 30, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Kang, S.; Pei, S.; Sang, C.; Huang, Y. MiR93-5p inhibits chondrocyte apoptosis in osteoarthritis by targeting lncRNA CASC2. BMC Musculoskelet. Disord. 2020, 21, 1–7. [Google Scholar] [CrossRef]
- Roseti, L.; Desando, G.; Cavallo, C.; Petretta, M.; Grigolo, B. Articular Cartilage Regeneration in Osteoarthritis. Cells 2019, 8, 1305. [Google Scholar] [CrossRef] [Green Version]
- Bertram, K.L.; Banderali, U.; Tailor, P.; Krawetz, R.J.; Bertram, K.L.; Banderali, U.; Tailor, P.; Krawetz, J.R. Ion channel expression and function in normal and osteoarthritic human synovial fluid progenitor cells. Channels 2016, 10, 148–157. [Google Scholar] [CrossRef]
- Hall, A.C. The Role of Chondrocyte Morphology and Volume in Controlling Phenotype—Implications for Osteoarthritis, Cartilage Repair, and Cartilage Engineering. Curr. Rheumatol. Rep. 2019, 21, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldonado, M.; Nam, J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed. Res. Int. 2013, 2013, 284873. [Google Scholar] [CrossRef] [Green Version]
- Maleckar, M.M.; Clark, R.B.; Votta, B.; Giles, W.R. The resting potential and K+ currents in Primary Human articular chondrocytes. Front. Physiol. 2018, 9, 1–21. [Google Scholar] [CrossRef]
- Lewis, R.; Asplin, K.E.; Bruce, G.; Dart, C.; Mobasheri, A.; Barrett-Jolley, R. The role of the membrane potential in chondrocyte volume regulation. J. Cell. Physiol. 2011, 226, 2979–2986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, K.A.; Mulhall, H.J.; Labeed, F.H.; Lewis, M.P.; Hoettges, K.F.; Kalavrezos, N.; McCaul, J.; Liew, C.; Porter, S.; Fedele, S.; et al. A dielectrophoretic method of discrimination between normal oral epithelium, and oral and oropharyngeal cancer in a clinical setting. Analyst 2015, 140, 5198–5204. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Graham, K.A.; Johannessen, A.C.; Costea, D.E.; Labeed, H.F. Human oral cancer cells with increasing tumorigenic abilities exhibit higher effective membrane capacitance. Integr. Biol. 2014, 6, 545–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, R.; Feetham, C.H.; Gentles, L.; Penny, J.; Tregilgas, L.; Tohami, W.; Mobasheri, A.; Barrett-Jolley, R. Benzamil sensitive ion channels contribute to volume regulation in canine chondrocytes. Br. J. Pharmacol. 2013, 168, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.; Barrett-Jolley, R. Changes in membrane receptors and ion channels as potential biomarkers for osteoarthritis. Front. Physiol. 2015, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Thiel, G.; Moroni, A.; Blanc, G.; van Etten, J.L. Potassium ion channels: Could they have evolved from viruses? Plant Physiol. 2013, 162, 1215–1224. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yamamura, H.; Imaizumi, Y.; Clark, R.B.; Giles, W.R. K+ and Ca2+ Channels Regulate Ca2+ Signaling in Chondrocytes: An Illustrated Review. Cells 2020, 9, 1577. [Google Scholar] [CrossRef]
- Staunton, C.A.; Lewis, R.; Barrett-Jolley, R. Ion channels and osteoarthritic pain: Potential for novel analgesics. Curr. Pain Headache Rep. 2013, 17, 17. [Google Scholar] [CrossRef] [Green Version]
- Barrett-Jolley, R.; Lewis, R.; Fallman, R.; Mobasheri, A. The emerging chondrocyte channelome. Front. Physiol. 2010, 1, 135. [Google Scholar] [CrossRef] [Green Version]
- Budd, E.; Nalesso, G.; Mobasheri, A. Extracellular genomic biomarkers of osteoarthritis. Expert Rev. Mol. Diagn. 2018, 18, 55–74. [Google Scholar] [CrossRef]
- Mobasheri, A.; Matta, C.; Uzielienè, I.; Budd, E.; Martín-Vasallo, P.; Bernotiene, E. The chondrocyte channelome: A narrative review. Jt. Bone Spine 2019, 86, 29–35. [Google Scholar] [CrossRef]
- Lewis, R.; May, H.; Mobasheri, A.; Barrett-Jolley, R. Chondrocyte channel transcriptomics: Do microarray data fit with expression and functional data? Channels 2013, 7, 459–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, R.; Feetham, C.H.; Barrett-Jolley, R. Biochemistry Cell Volume Regulation in Chondrocytes. Cell. Physiol. Biochem. 2011, 28, 1111–1122. [Google Scholar] [CrossRef]
- Stacey, M.W.; Sabuncu, A.C.; Beskok, A. Biochimica et Biophysica Acta Dielectric characterization of costal cartilage chondrocytes. BBA Gen. Subj. 2014, 1840, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Musumeci, G.; Castrogiovanni, P.; Trovato, F.M.; Weinberg, A.M.; Al-Wasiyah, M.K.; Alqahtani, M.H.; Mobasheri, A. Biomarkers of chondrocyte apoptosis and autophagy in osteoarthritis. Int. J. Mol. Sci. 2015, 16, 20560–20575. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.Y.; Jin, Y.; Ma, X.H.; Wang, C.Y.; Guo, Y.; Zhou, D. The potential role of mechanically sensitive ion channels in the physiology, injury, and repair of articular cartilage. J. Orthop. Surg. 2020, 28, 1–8. [Google Scholar] [CrossRef]
- Sánchez, J.C.; López-zapata, D.F. Effects of osmotic challenges on membrane potential in human articular chondrocytes from healthy and osteoarthritic cartilage. Biorheology 2010, 47, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Wohlrab, D.H.W.; Vocke, M.; Klapperstück, T. Effects of potassium and anion channel blockers on the cellular response of human osteoarthritic chondrocytes. J. Orthop. Sci. 2004, 9, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.H.; Volders, P.G.A.; Kohl, P.; Prinzen, F.W.; Zaza, A.; Kääb, S.; Oto, A.; Schotten, U. Opportunities and challenges of current electrophysiology research: A plea to establish “translational electrophysiology” curricula. Europace 2015, 17, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Henslee, E.A. Review: Dielectrophoresis in cell characterization. Electrophoresis 2020, 41, 1915–1930. [Google Scholar] [CrossRef]
- Drexler, S.; Wann, A.; Vincent, T.L. Are cellular mechanosensors potential therapeutic targets in osteoarthritis? Int. J. Clin. Rheumtol. 2014, 9, 155–167. [Google Scholar] [CrossRef]
- Chen, C.C.; Cang, C.; Fenske, S.; Butz, E.; Chao, Y.K.; Biel, M.; Ren, D.; Wahl-Schott, C.; Grimm, C. Patch-clamp technique to characterize ion channels in enlarged individual endolysosomes. Nat. Protoc. 2017, 12, 1639–1658. [Google Scholar] [CrossRef] [PubMed]
- Yajuan, X.; Xin, L.; Zhiyuan, L. A Comparison of the Performance and Application Differences Between Manual and Automated Patch-Clamp Techniques. Curr. Chem. Genom. 2013, 6, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Henslee, E.A.; Crosby, P.; Kitcatt, S.J.; Parry, J.S.W.; Bernardini, A.; Abdallat, R.G.; Braun, G.; Fatoyinbo, H.O.; Harrison, E.J.; Edgar, R.S.; et al. Rhythmic potassium transport regulates the circadian clock in human red blood cells. Nat. Commun. 2017, 8, 1978. [Google Scholar] [CrossRef]
- Rahman, N.A.; Ibrahim, F.; Yafouz, B. Dielectrophoresis for biomedical sciences applications: A review. Sensors 2017, 17, 449. [Google Scholar] [CrossRef] [Green Version]
- Fam, T.K.; Klymchenko, A.S.; Collot, M. Recent advances in fluorescent probes for lipid droplets. Materials 2018, 11, 1768. [Google Scholar] [CrossRef] [Green Version]
- Haandbæk, N.; Bürgel, S.C.; Heer, F.; Hierlemann, A. Characterization of subcellular morphology of single yeast cells using high frequency microfluidic impedance cytometer. Lab Chip. 2014, 14, 369–377. [Google Scholar] [CrossRef]
- Ronald, R. Pethig, Dielectrophoresis: Theory, Methodology and Biological Applications; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Hughes, M.P. Fifty years of dielectrophoretic cell separation technology. Biomicrofluidics 2016, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoettges, K.F.; Henslee, E.A.; Serrano, R.M.T.; Jabr, R.I.; Abdallat, R.G.; Beale, A.D.; Waheed, A.; Camelliti, P.; Fry, C.H.; van der Veen, D.R.; et al. Ten–Second Electrophysiology: Evaluation of the 3DEP Platform for high-speed, high-accuracy cell analysis. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Mulhall, H.J.; Labeed, F.H.; Kazmi, B.; Costea, D.E.; Hughes, M.P.; Lewis, M.P. Cancer, pre-cancer and normal oral cells distinguished by dielectrophoresis. Anal. Bioanal. Chem. 2011, 401, 2455–2463. [Google Scholar] [CrossRef] [Green Version]
- Chin, S.; Hughes, M.P.; Coley, H.M.; Labeed, F.H. Rapid assessment of early biophysical changes in K562 cells during apoptosis determined using dielectrophoresis. Int. J. Nanomed. 2006, 1, 333–337. [Google Scholar]
- Hoettges, K.F.; Hübner, Y.; Broche, L.M.; Ogin, S.L.; Kass, G.E.N.; Hughes, M.P. Dielectrophoresis-activated multiwell plate for label-free high-throughput drug assessment. Anal. Chem. 2008, 80, 2063–2068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broche, L.M.; Hoettges, K.F.; Ogin, S.L.; Kass, G.E.N.; Hughes, M.P. Rapid, automated measurement of dielectrophoretic forces using DEP-activated microwells. Electrophoresis 2011, 32, 2393–2399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mobasheri, A.; Lewis, R.; Ferreira-Mendes, A.; Rufino, A.; Dart, C.; Barrett-Jolley, R. Potassium channels in articular chondrocytes. Channels 2012, 6, 416–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulhall, H.J.; Cardnell, A.; Hoettges, K.F.; Labeed, F.H.; Hughes, M.P. Apoptosis progression studied using parallel dielectrophoresis electrophysiological analysis and flow cytometry. Integr. Biol. 2015, 7, 1396–1401. [Google Scholar] [CrossRef]
- Sabuncu, A.C.; Asmar, A.J.; Stacey, M.W.; Beskok, A. Differential dielectric responses of chondrocyte and Jurkat cells in electromanipulation buffers. Electrophoresis 2015, 36, 1499–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pethig, R.; Bressler, V.; Carswell-crumpton, C.; Foster-haje, L.; García-ojeda, M.E.; Lee, R.S.; Lock, G.M.; Talary, M.S.; Tate, K.M. A Biosystems, Dielectrophoretic studies of the activation of human T lymphocytes using a newly developed cell profiling system. Electrophoresis 2002, 23, 2057–2063. [Google Scholar] [CrossRef]
- Pethig, R.; Talary, M.S. Dielectrophoretic detection of membrane morphology changes in Jurkat T-cells undergoing etoposide-induced apoptosis. IET Nanobiotechnol. 2007, 1, 2–9. [Google Scholar] [CrossRef]
- Wilson, J.R.; Duncan, N.A.; Giles, W.R.; Clark, R.B. A voltage-dependent K+ current contributes to membrane potential of acutely isolated canine articular chondrocytes. J. Physiol. 2004, 557, 93–104. [Google Scholar] [CrossRef]
- Mobasheri, A.; Gent, T.C.; Womack, M.D.; Carter, S.D.; Clegg, P.D.; Barrett-Jolley, R. Quantitative analysis of voltage-gated potassium currents from primary equine (Equus caballus) and elephant (Loxodonta africana) articular chondrocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Golowasch, J.; Nadim, F. Capacitance, Membrane. In Encyclopedia of Computational Neuroscience; Jaeger, D., Jung, R., Eds.; Springer: New York, NY, USA, 2013; pp. 1–5. [Google Scholar] [CrossRef]
- Mobasheri, A.; Lewis, R.; Maxwell, J.E.J.; Hill, C.; Womack, M.; Barrett-Jolley, R. Characterization of a stretch-activated potassium channel in chondrocytes. J. Cell. Physiol. 2010, 223, 511–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, R.B.; Hatano, N.; Kondo, C.; Belke, D.D.; Barry, S.; Kumar, S.; Votta, B.J.; Giles, W.R.; Clark, R.B.; Hatano, N.; et al. Voltage-gated K currents in mouse articular chondrocytes regulate membrane potential Voltage-gated K + currents in mouse articular chondrocytes regulate membrane potential. Channels 2010, 4, 179–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponce, A. Cellular Physiology and Biochemistr y Biochemistry Expression of Voltage Dependent Potassium Currents in Freshly Dissociated Rat Articular Chondrocytes. Cell. Physiol. Biochem. 2006, 18, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Kadir, L.A.; Stacey, M.; Barrett-Jolley, R. Emerging roles of the membrane potential: Action beyond the action potential. Front. Physiol. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Duncan, L.; Shelmerdine, H.; Hughes, M.P.; Coley, H.M.; Hübner, Y.; Labeed, F.H. Dielectrophoretic analysis of changes in cytoplasmic ion levels due to ion channel blocker action reveals underlying differences between drug-sensitive and multidrug-resistant leukaemic cells. Phys. Med. Biol. 2008, 53, N1–N7. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Mobasheri, R.; Francis, M.J.O.; Trujillo, E.; de la Rosa, D.A.; Martín-Vasallo, P. Ion transport in chondrocytes: Membrane transporters involved in intracellular ion homeostasis and the regulation of cell volume, free [Ca2+] and pH. Histol. Histopathol. 1998, 13, 893–910. [Google Scholar] [CrossRef]
- Jeremiasse, B.; Matta, C.; Fellows, C.R.; Boocock, D.J.; Smith, J.R.; Liddell, S.; Lafeber, F.; van Spil, W.E.; Mobasheri, A. Alterations in the chondrocyte surfaceome in response to pro-inflammatory cytokines. BMC Mol. Cell Biol. 2020, 21, 47. [Google Scholar] [CrossRef]
- Matta, C.; Boocock, D.J.; Fellows, C.R.; Miosge, N.; Dixon, J.E.; Liddell, S.; Smith, J.; Mobasheri, A. Molecular phenotyping of the surfaceome of migratory chondroprogenitors and mesenchymal stem cells using biotinylation, glycocapture and quantitative LC-MS/MS proteomic analysis. Sci. Rep. 2019, 9, 9018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, W.; Zhao, Y.; Liu, L.; Wang, Y.; Li, W.J.; Lee, G.B. Determination of Cell Membrane Capacitance and Conductance via Optically Induced Electrokinetics. Biophys. J. 2017, 113, 1531–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, A.; Hughes, M.P.; Mulhall, H.J.; Oreffo, R.O.C.; Labeed, F.H.; Ismail, A.; Hughes, M.P.; Mulhall, H.J.; Oreffo, R.O.; Labeed, F.H. Characterization of human skeletal stem and bone cell populations using dielectrophoresis. J. Tissue Eng. Regen. Med. 2015, 9, 162–168. [Google Scholar] [CrossRef] [Green Version]
- Kachroo, U.; Livingston, A.; Vinod, E.; Sathishkumar, S.; Boopalan, P.R.J.V.C. Comparison of Electrophysiological Properties and Gene Expression between Human Chondrocytes and Chondroprogenitors Derived from Normal and Osteoarthritic Cartilage. Cartilage 2020, 11, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Matta, C.; Lewis, R.; Barrett-Jolley, R.; Labeed, F.H.; Hughes, M.P.; Uribe, M.C.; May, S.; Miosge, N.; Mobsheri, A. Ion channel expression and function in a chondrogenic progenitor cell line derived from osteoarthritic cartilage, Osteoarthr. Cartilage 2016, 24, S141. [Google Scholar] [CrossRef] [Green Version]
- Wohlrab, H.W.; Wohlrab, D.; Reichel, H.J. Is the proliferation of human chondrocytes regulated by ionic channels? J. Orthop. Sci. 2001, 6, 155–159. [Google Scholar] [CrossRef] [PubMed]

| r (μm) | Membrane Conductance | Membrane Capacitance | Cytoplasm Conductivity | |
|---|---|---|---|---|
| (Geff) (S/m2) | (Ceff) (mF/m2) | (σcyt) (S/m) | ||
| a-Control | 6.40 (±0.12) | 8571 (±1010) | 14.5 (±0.01) | 0.26 (±0.01) |
| b-Chronic | 6.58 (±0.18) | 7857 (±1190) | 13.9 (±1.22) | 0.29 (±0.03) |
| c-Acute | 6.47 (±0.07) | 6191 (±738) * | 10.3 (±1.47) * | 0.27 (±0.05) |
| r (μm) | Membrane Conductance | Membrane Capacitance | Cytoplasm Conductivity | |
|---|---|---|---|---|
| (Geff) (S/m2) | (Ceff) (mF/m2) | (σcyt) (S/m) | ||
| a-Control | 6.3 (±0.82) | 1139 (±112) | 7.51 (±2.6) | 0.22 (±0.06) |
| b-OA-induced | 8.5 (±0.97) * | 782 (±183) * | 6.92 (±3.5) | 0.24 (±0.06) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdallat, R.; Kruchek, E.; Matta, C.; Lewis, R.; Labeed, F.H. Dielectrophoresis as a Tool to Reveal the Potential Role of Ion Channels and Early Electrophysiological Changes in Osteoarthritis. Micromachines 2021, 12, 949. https://doi.org/10.3390/mi12080949
Abdallat R, Kruchek E, Matta C, Lewis R, Labeed FH. Dielectrophoresis as a Tool to Reveal the Potential Role of Ion Channels and Early Electrophysiological Changes in Osteoarthritis. Micromachines. 2021; 12(8):949. https://doi.org/10.3390/mi12080949
Chicago/Turabian StyleAbdallat, Rula, Emily Kruchek, Csaba Matta, Rebecca Lewis, and Fatima H. Labeed. 2021. "Dielectrophoresis as a Tool to Reveal the Potential Role of Ion Channels and Early Electrophysiological Changes in Osteoarthritis" Micromachines 12, no. 8: 949. https://doi.org/10.3390/mi12080949






