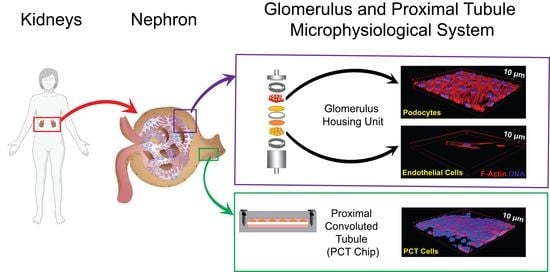
Modelling Renal Filtration and Reabsorption Processes in a Human Glomerulus and Proximal Tubule Microphysiological System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Simulating and Validating Model in COMSOL MultiPhysics®
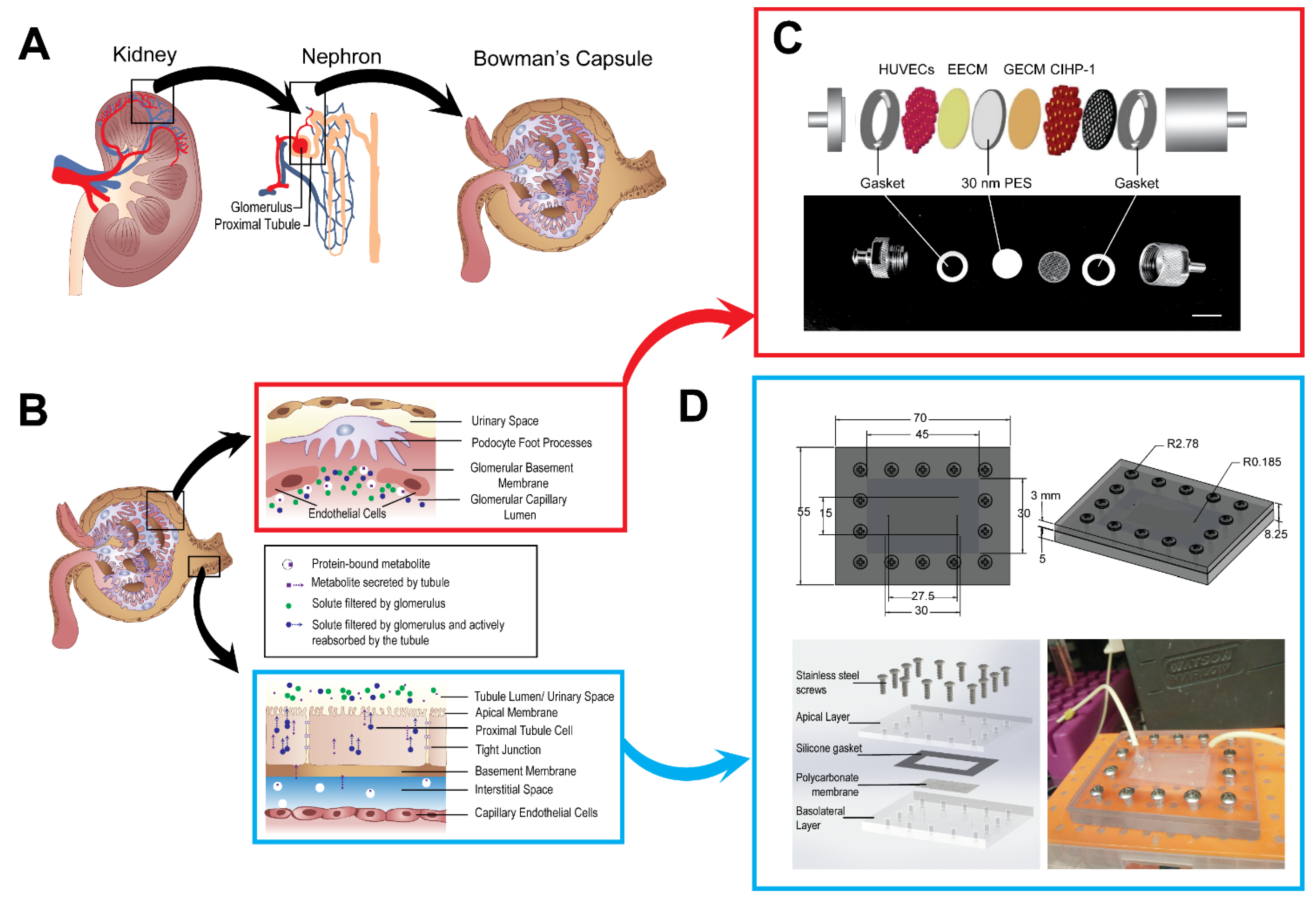
2.2. Fabrication of the Microphysiological System
2.3. Cell Culture
2.4. Cell Viability and Proliferation Assay
2.5. CIHP-1, HUVECs, and HK-2 Adhesion on Porous Membranes
2.6. Assembly of the Housing Unit and the Microfluidic Device
2.7. MPS Cellular Models
2.8. Volume Flow Assay
2.9. FITC-Human Serum Albumin Flow Assay
2.10. Fluorescent Staining and Image Processing
2.11. Infinity Glucose Assay
2.12. Statistical Analysis
3. Results
3.1. A Glomerular and Proximal Tubular Microphysiological System
3.2. Flow Characterization of Microphysiological System
3.3. Recapitulation of Glomerulus and Proximal Tubule Functions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woodcock, J.; Woosley, R. The FDA critical path initiative and its influence on new drug development. Annu. Rev. Med. 2008, 59, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wehling, M. Translational science in medicine. Int. J. Pharm. Med. 2006, 20, 303–310. [Google Scholar] [CrossRef]
- Giffin, R.; Robinson, S.; Olson, S. Accelerating the Development of Biomarkers for Drug Safety: Workshop Summary; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Wilmer, M.J.; Ng, C.P.; Lanz, H.L.; Vulto, P.; Suter-Dick, L.; Masereeuw, R. Kidney-on-a-chip technology for drug-induced nephrotoxicity screening. Trends Biotechnol. 2016, 34, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Awdishu, L.; Mehta, R.L. The 6R’s of drug induced nephrotoxicity. BMC Nephrol. 2017, 18, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.J.; Wu, M. Microfluidics for mammalian cell chemotaxis. Ann. Biomed. Eng. 2012, 40, 1316–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakolish, C.; Weber, E.J.; Kelly, E.J.; Himmelfarb, J.; Mouneimne, R.; Grimm, F.A.; House, J.S.; Wade, T.; Han, A.; Chiu, W.A.; et al. Technology transfer of the microphysiological systems: A case study of the human proximal tubule tissue chip. Sci. Rep. 2018, 8, 14882. [Google Scholar] [CrossRef]
- Sakolish, C.M.; Esch, M.B.; Hickman, J.J.; Shuler, M.L.; Mahler, G.J. Modeling barrier tissues in vitro: Methods, achievements, and challenges. EBioMedicine 2016, 5, 30–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, J.A.; Grandhi, T.S.P.; Davis, M.; Gautier, J.-C.; Hariparsad, N.; Keller, D.; Sura, R.; Van Vleet, T.R. A pharmaceutical industry perspective on microphysiological kidney systems for evaluation of safety for new therapies. Lab Chip 2020, 20, 468–476. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ-on-a-chip: Recent breakthroughs and future prospects. Biomed. Eng. Online 2020, 19, 9. [Google Scholar] [CrossRef] [Green Version]
- Sakolish, C.M.; Philip, B.; Mahler, G.J. A human proximal tubule-on-a-chip to study renal disease and toxicity. Biomicrofluidics 2019, 13, 014107. [Google Scholar] [CrossRef]
- Musah, S.; Mammoto, A.; Ferrante, T.C.; Jeanty, S.S.; Hirano-Kobayashi, M.; Mammoto, T.; Roberts, K.; Chung, S.; Novak, R.; Ingram, M.; et al. Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat. Biomed. Eng. 2017, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sciancalepore, A.G.; Sallustio, F.; Girardo, S.; Passione, L.G.; Camposeo, A.; Mele, E.; Di Lorenzo, M.; Costantino, V.; Schena, F.P.; Pisignano, D. A bioartificial renal tubule device embedding human renal stem/progenitor cells. PLoS ONE 2014, 9, e87496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, M.E.; Rodrigues, M.T.; Domingues, R.M.; Reis, R.L. Tissue engineering and regenerative medicine: New trends and directions—A year in review. Tissue Eng. Part B Rev. 2017, 23, 211–224. [Google Scholar] [CrossRef]
- Van Midwoud, P.M.; Janse, A.; Merema, M.T.; Groothuis, G.M.; Verpoorte, E. Comparison of biocompatibility and adsorption properties of different plastics for advanced microfluidic cell and tissue culture models. Anal. Chem. 2012, 84, 3938–3944. [Google Scholar] [CrossRef]
- Homan, K.A.; Kolesky, D.B.; Skylar-Scott, M.A.; Herrmann, J.; Obuobi, H.; Moisan, A.; Lewis, J.A. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci. Rep. 2016, 6, 34845. [Google Scholar] [CrossRef] [Green Version]
- Jansen, J.; Fedecostante, M.; Wilmer, M.; Van den Heuvel, L.; Hoenderop, J.; Masereeuw, R. Biotechnological challenges of bioartificial kidney engineering. Biotechnol. Adv. 2014, 32, 1317–1327. [Google Scholar] [CrossRef]
- Ng, C.P.; Zhuang, Y.; Lin, A.W.H.; Teo, J.C.M. A fibrin-based tissue-engineered renal proximal tubule for bioartificial kidney devices: Development, characterization and in vitro transport study. Int. J. Tissue Eng. 2012, 2013, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Tourovskaia, A.; Fauver, M.; Kramer, G.; Simonson, S.; Neumann, T. Tissue-engineered microenvironment systems for modeling human vasculature. Exp. Biol. Med. 2014, 239, 1264–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, K.-J.; Mehr, A.P.; Hamilton, G.A.; McPartlin, L.A.; Chung, S.; Suh, K.-Y.; Ingber, D.E. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr. Biol. 2013, 5, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Marieb, E.N.; Hoehn, K. Human Anatomy and Physiology; Pearson Education: London, UK, 2007. [Google Scholar]
- Koeppen, B.M.; Stanton, B.A. Renal Physiology E-Book: Mosby Physiology Monograph Series; Elsevier Health Sciences: Maryland Heights, MO, USA, 2012. [Google Scholar]
- Zanetti, F. Kidney-on-a-chip. In Organ-on-a-Chip; Elsevier: Amsterdam, The Netherlands, 2020; pp. 233–253. [Google Scholar]
- Bonventre, J.V.; Vaidya, V.S.; Schmouder, R.; Feig, P.; Dieterle, F. Next-generation biomarkers for detecting kidney toxicity. Nat. Biotechnol. 2010, 28, 436–440. [Google Scholar] [CrossRef] [Green Version]
- Sakolish, C.; Chen, Z.; Dalaijamts, C.; Mitra, K.; Liu, Y.; Fulton, T.; Wade, T.L.; Kelly, E.J.; Rusyn, I.; Chiu, W.A. Predicting tubular reabsorption with a human kidney proximal tubule tissue-on-a-chip and physiologically-based modeling. Toxicol. Vitr. 2020, 63, 104752. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.J.; Chapron, A.; Chapron, B.D.; Voellinger, J.L.; Lidberg, K.A.; Yeung, C.K.; Wang, Z.; Yamaura, Y.; Hailey, D.W.; Neumann, T.; et al. Development of a microphysiological model of human kidney proximal tubule function. Kidney Int. 2016, 90, 627–637. [Google Scholar] [CrossRef] [Green Version]
- Feher, J.J. Quantitative Human Physiology: An Introduction; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Sakolish, C.M.; Mahler, G.J. A novel microfluidic device to model the human proximal tubule and glomerulus. RSC Adv. 2017, 7, 4216–4225. [Google Scholar] [CrossRef] [Green Version]
- Fuster, M.M.; Wang, L. Endothelial heparan sulfate in angiogenesis. Prog. Mol. Biol. Transl. Sci. 2010, 93, 179–212. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Lote, C.J.; Lote, C.J. Principles of Renal Physiology; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Raghavan, V.; Rbaibi, Y.; Pastor-Soler, N.M.; Carattino, M.D.; Weisz, O.A. Shear stress-dependent regulation of apical endocytosis in renal proximal tubule cells mediated by primary cilia. Proc. Natl. Acad. Sci. USA 2014, 111, 8506–8511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabbisetti, V.S.; Waikar, S.S.; Antoine, D.J.; Smiles, A.; Wang, C.; Ravisankar, A.; Ito, K.; Sharma, S.; Ramadesikan, S.; Lee, M.; et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J. Am. Soc. Nephrol. 2014, 25, 2177–2186. [Google Scholar] [CrossRef]
- Jansen, J.; Fedecostante, M.; Wilmer, M.; Peters, J.; Kreuser, U.; Van Den Broek, P.; Mensink, R.; Boltje, T.; Stamatialis, D.; Wetzels, J.; et al. Bioengineered kidney tubules efficiently excrete uremic toxins. Sci. Rep. 2016, 6, 26715. [Google Scholar] [CrossRef]
- McQuarrie, E.P.; Shakerdi, L.; Jardine, A.G.; Fox, J.G.; Mackinnon, B. Fractional excretions of albumin and IgG are the best predictors of progression in primary glomerulonephritis. Nephrol. Dial. Transplant. 2011, 26, 1563–1569. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Corbelli, A.; Watanabe, S.; Armelloni, S.; Ikehata, M.; Parazzi, V.; Pignatari, C.; Giardino, L.; Mattinzoli, D.; Lazzari, L.; et al. Three-dimensional podocyte–endothelial cell co-cultures: Assembly, validation, and application to drug testing and intercellular signaling studies. Eur. J. Pharm. Sci. 2016, 86, 1–12. [Google Scholar] [CrossRef]
- Xinaris, C.; Benedetti, V.; Rizzo, P.; Abbate, M.; Corna, D.; Azzollini, N.; Conti, S.; Unbekandt, M.; Davies, J.A.; Morigi, M.; et al. In vivo maturation of functional renal organoids formed from embryonic cell suspensions. J. Am. Soc. Nephrol. 2012, 23, 1857–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xinaris, C.; Benedetti, V.; Novelli, R.; Abbate, M.; Rizzo, P.; Conti, S.; Tomasoni, S.; Corna, D.; Pozzobon, M.; Cavallotti, D.; et al. Functional human podocytes generated in organoids from amniotic fluid stem cells. J. Am. Soc. Nephrol. 2016, 27, 1400–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caulfield, J.P.; Farquhar, M.G. The permeability of glomerular capillaries to graded dextrans: Identification of the basement membrane as the primary filtration barrier. J. Cell Biol. 1974, 63, 883–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venturoli, D.; Rippe, B. Ficoll and dextran vs. globular proteins as probes for testing glomerular permselectivity: Effects of molecular size, shape, charge, and deformability. Am. J. Physiol. Ren. Physiol. 2005, 288, F605–F613. [Google Scholar] [CrossRef] [PubMed]
- Abrahamson, D.R.; Hudson, B.G.; Stroganova, L.; Borza, D.-B.; John, P.L.S. Cellular origins of type IV collagen networks in developing glomeruli. J. Am. Soc. Nephrol. 2009, 20, 1471–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John, P.L.S.; Abrahamson, D.R. Glomerular endothelial cells and podocytes jointly synthesize laminin-1 and-11 chains. Kidney Int. 2001, 60, 1037–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauer, S.M. Structural-functional correlations of diabetic nephropathy. Kidney Int. 1994, 45, 612–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavenstadt, H.; Kriz, W.; Kretzler, M. Cell biology of the glomerular podocyte. Physiol. Rev. 2003, 83, 253–307. [Google Scholar] [CrossRef] [Green Version]





| Inlet Mass Flow Rate (10−7 kg/s) | Tubing ID Diameter (mm) | Filtrate Output Length (mm) | Bloodstream Output Length (mm) |
|---|---|---|---|
| 2.66 | 0.25 | 457 | 622 |
| 5.15 | 474 | 660 | |
| 6.67 | 0.51 | 491 | 698 |
| 7.55 | 508 | 736 | |
| 10.1 | 525 | 774 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.Y.; Mahler, G.J. Modelling Renal Filtration and Reabsorption Processes in a Human Glomerulus and Proximal Tubule Microphysiological System. Micromachines 2021, 12, 983. https://doi.org/10.3390/mi12080983
Zhang SY, Mahler GJ. Modelling Renal Filtration and Reabsorption Processes in a Human Glomerulus and Proximal Tubule Microphysiological System. Micromachines. 2021; 12(8):983. https://doi.org/10.3390/mi12080983
Chicago/Turabian StyleZhang, Stephanie Y., and Gretchen J. Mahler. 2021. "Modelling Renal Filtration and Reabsorption Processes in a Human Glomerulus and Proximal Tubule Microphysiological System" Micromachines 12, no. 8: 983. https://doi.org/10.3390/mi12080983
APA StyleZhang, S. Y., & Mahler, G. J. (2021). Modelling Renal Filtration and Reabsorption Processes in a Human Glomerulus and Proximal Tubule Microphysiological System. Micromachines, 12(8), 983. https://doi.org/10.3390/mi12080983