Numerical Analysis of the Heterogeneity Effect on Electroosmotic Micromixers Based on the Standard Deviation of Concentration and Mixing Entropy Index
Abstract
:1. Introduction
2. Governing Equations
- The solution is a Newtonian incompressible fluid.
- No chemical reaction occurs between the species or between the species and the microchannel wall.
- The electrolyte solution is symmetric, meaning that the positive and negative valence number (z) of ions is equal.
- Gravitational and buoyancy effects are negligible.
- The steady-state of the equations are extracted.
2.1. Poisson–Boltzmann Equation
2.2. Nernst–Planck Equations
2.3. Navier–Stokes Equations
2.4. Species Concentration Equation
2.5. Helmholtz–Smoluchowski Modeling
2.6. Concentration Evaluation Criteria
2.7. Mixing Entropy Index
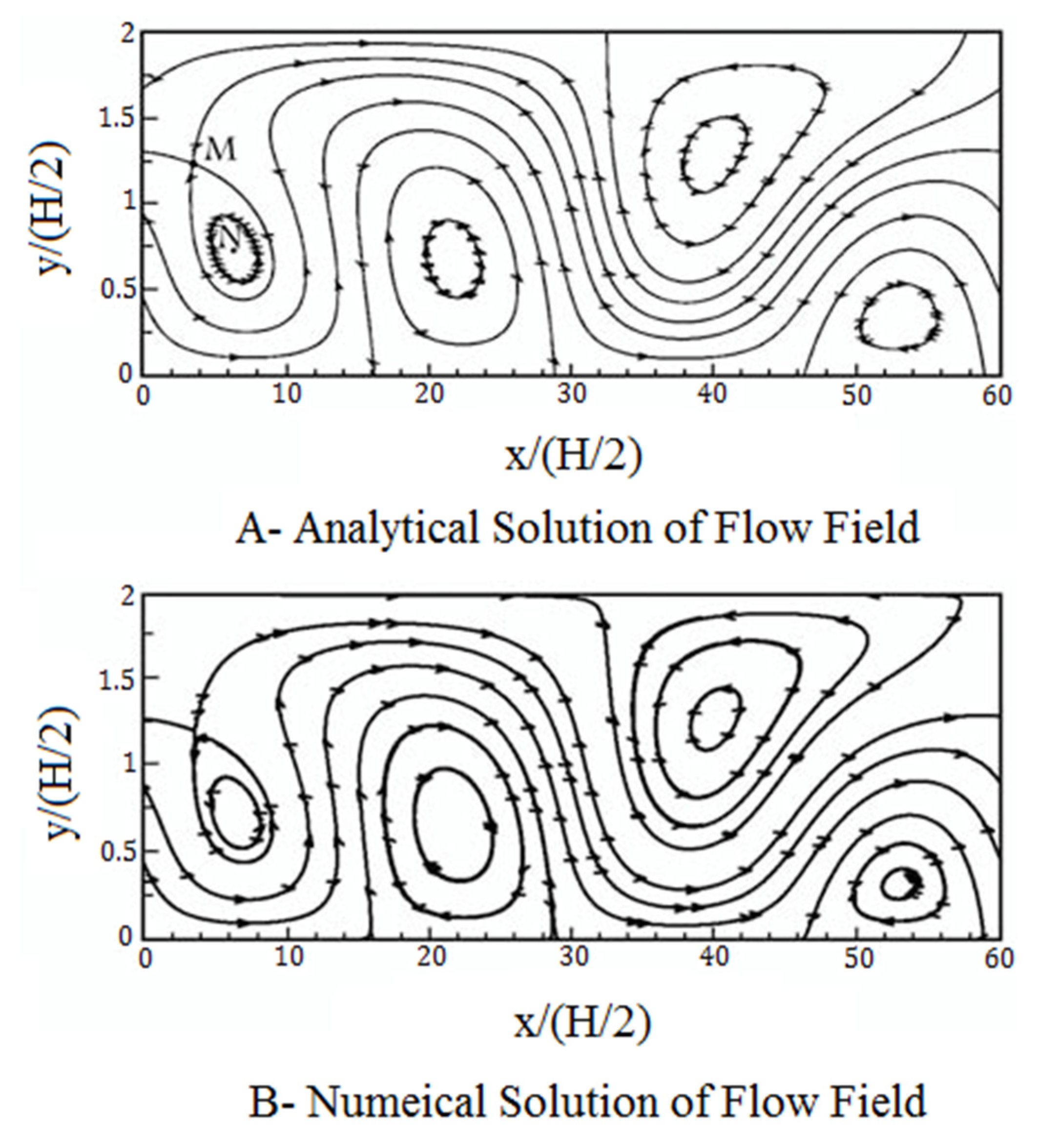
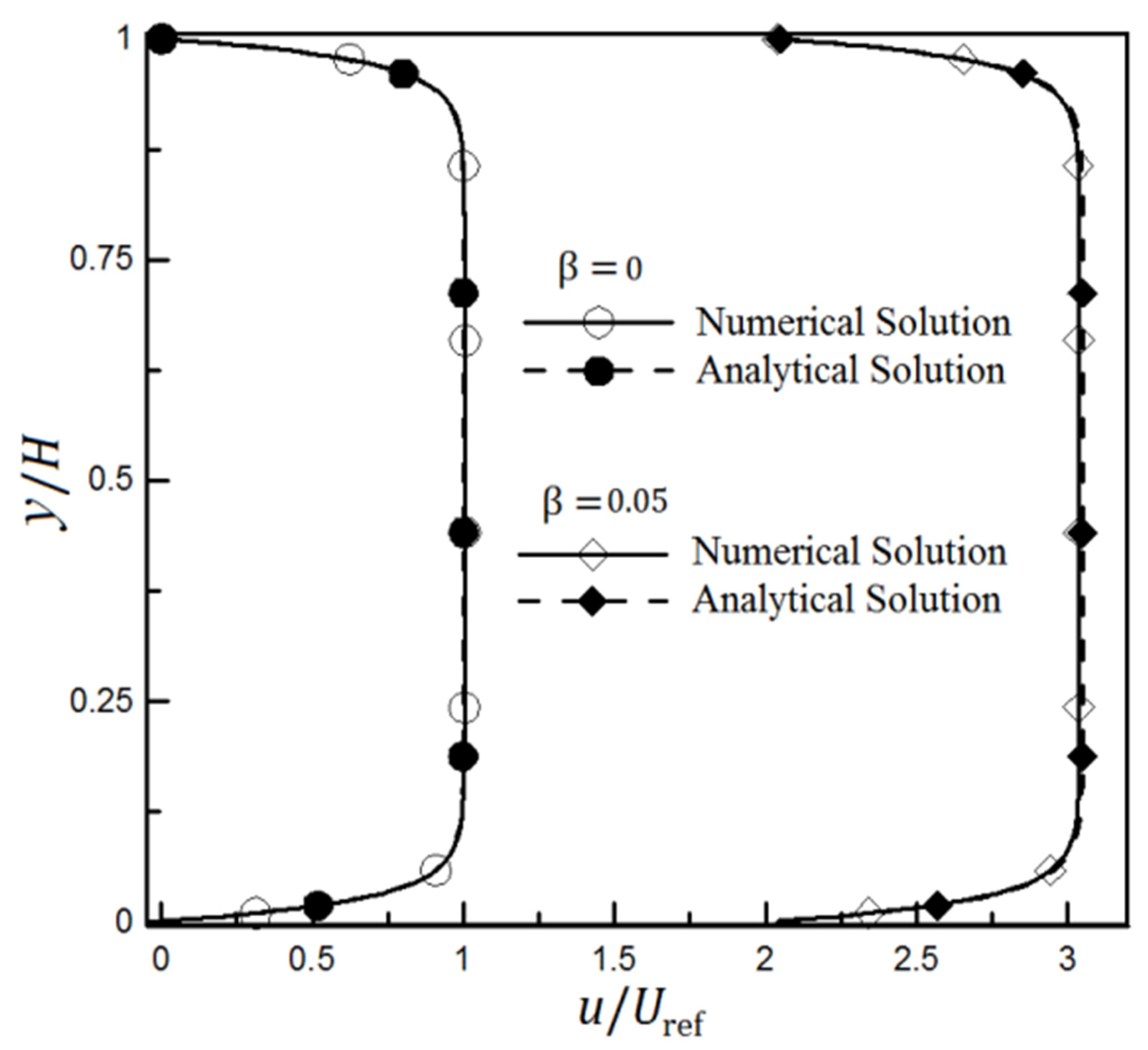
3. Numerical Method and Validation
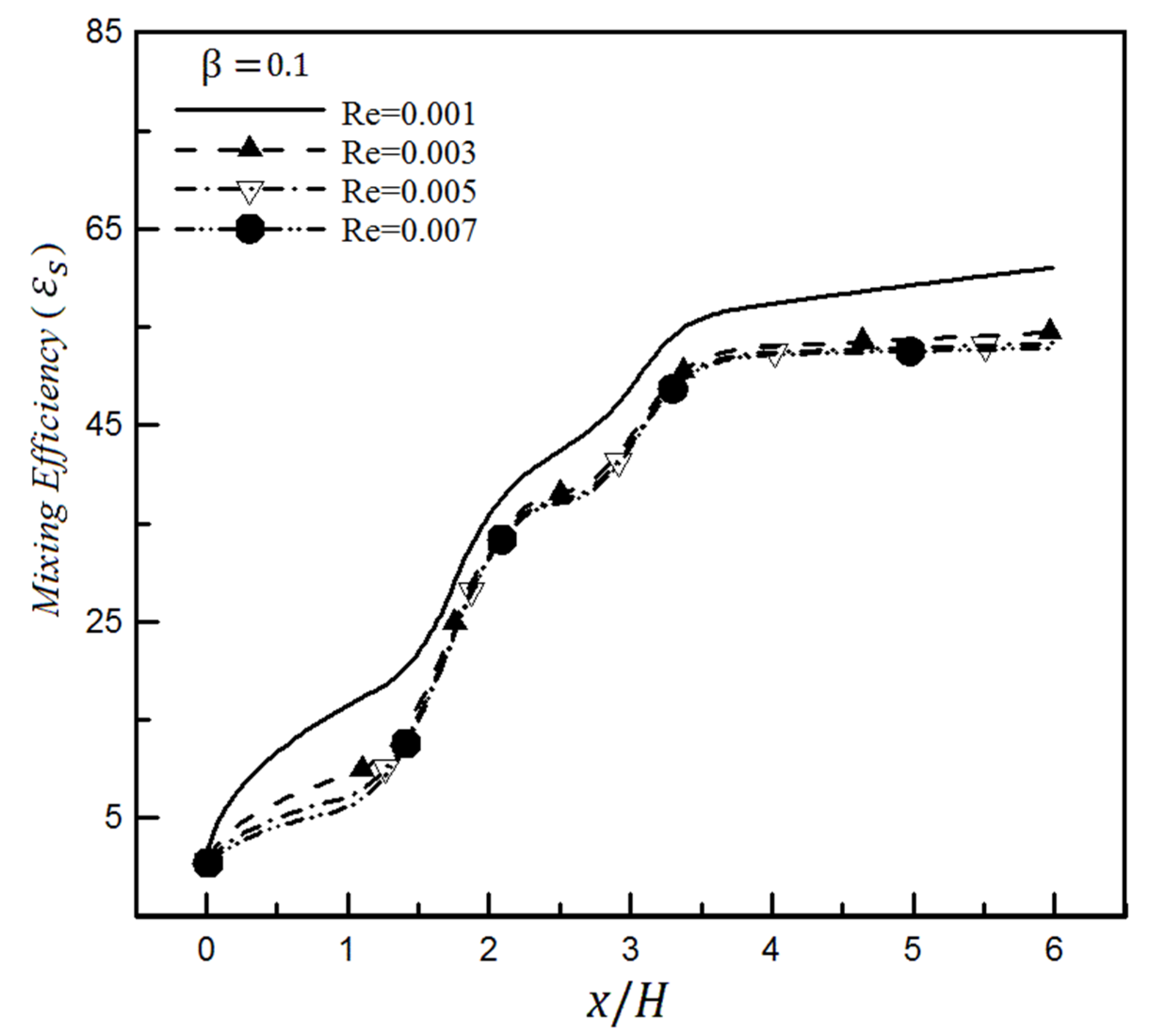
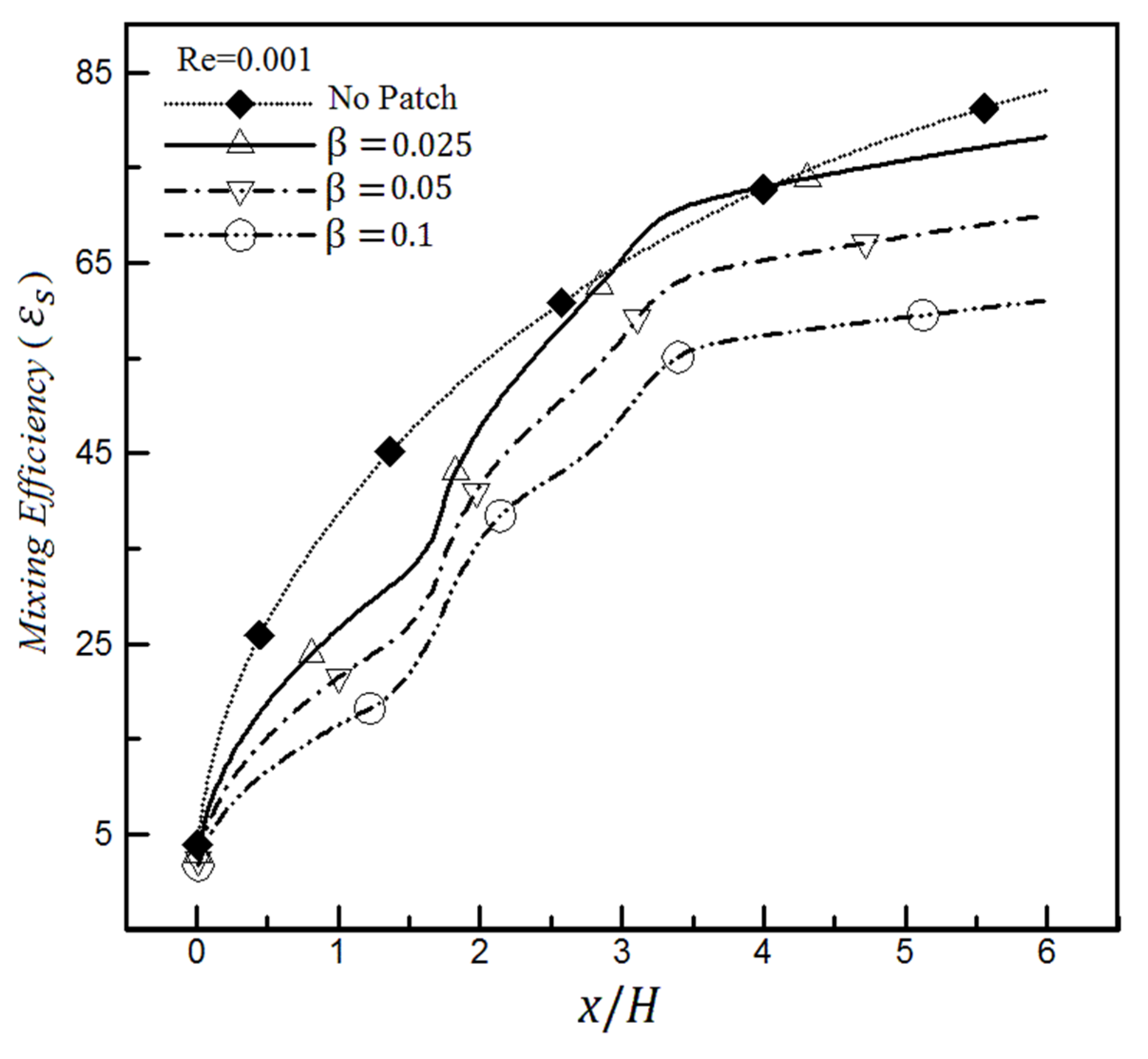
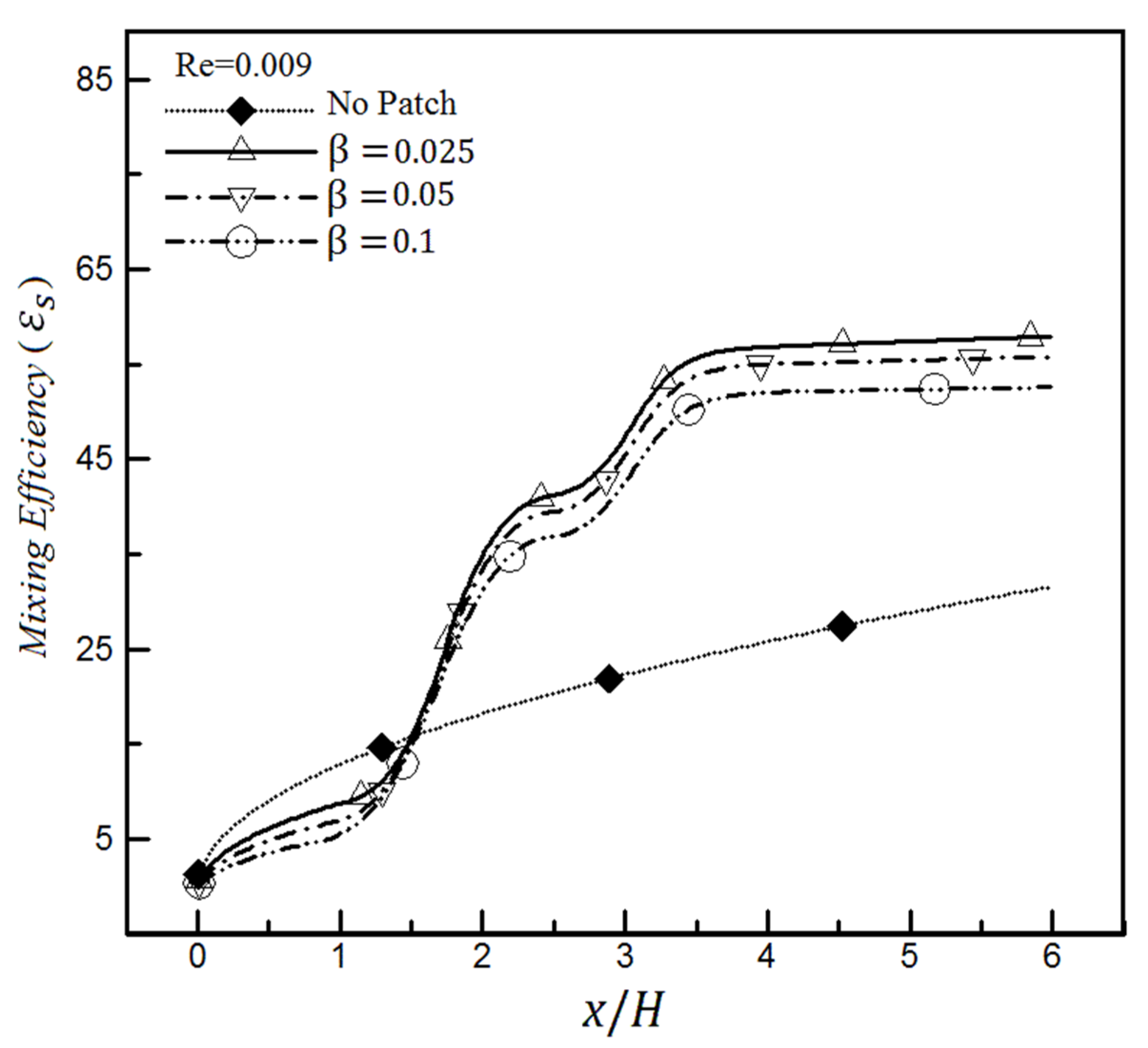
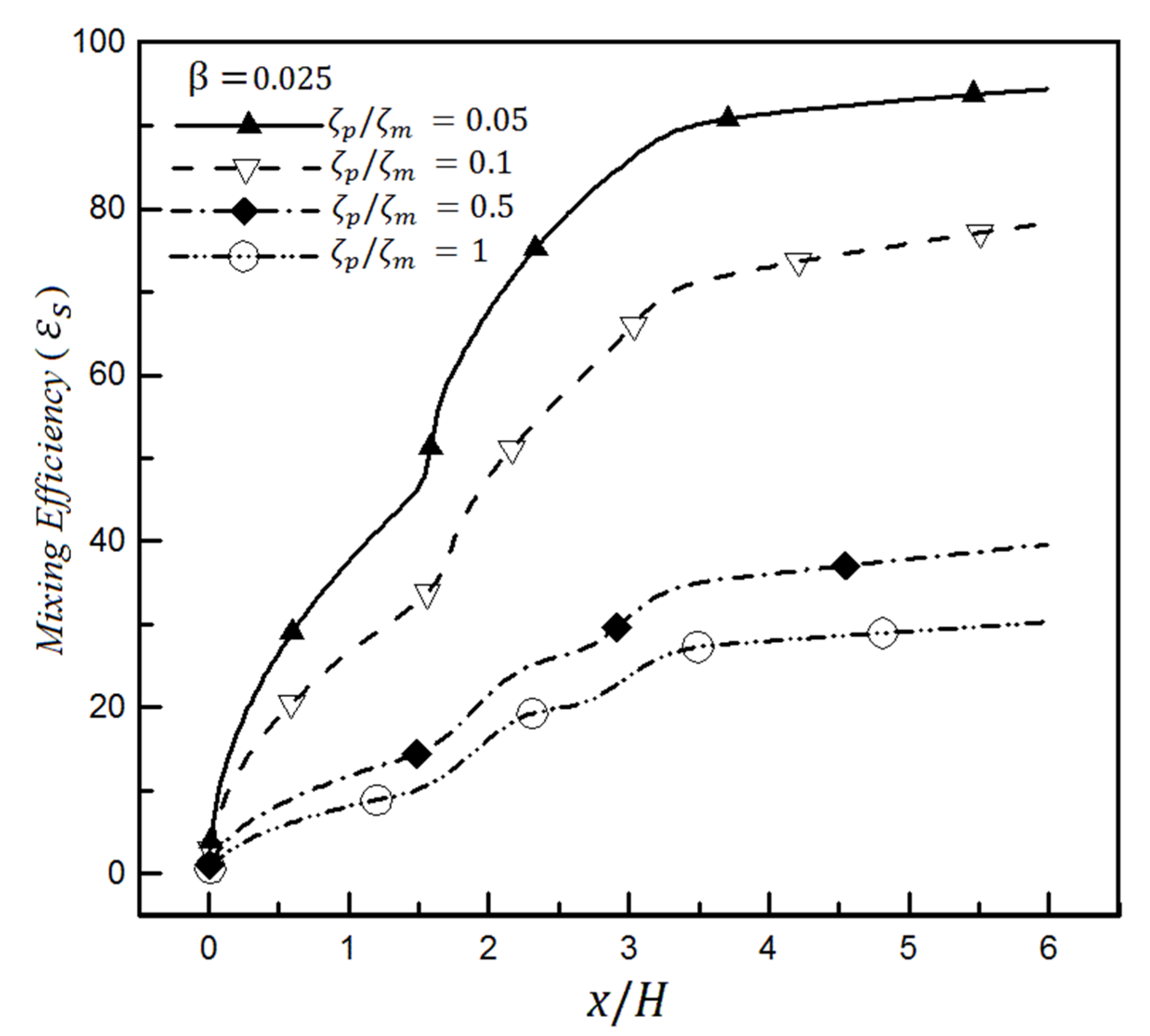
4. Results
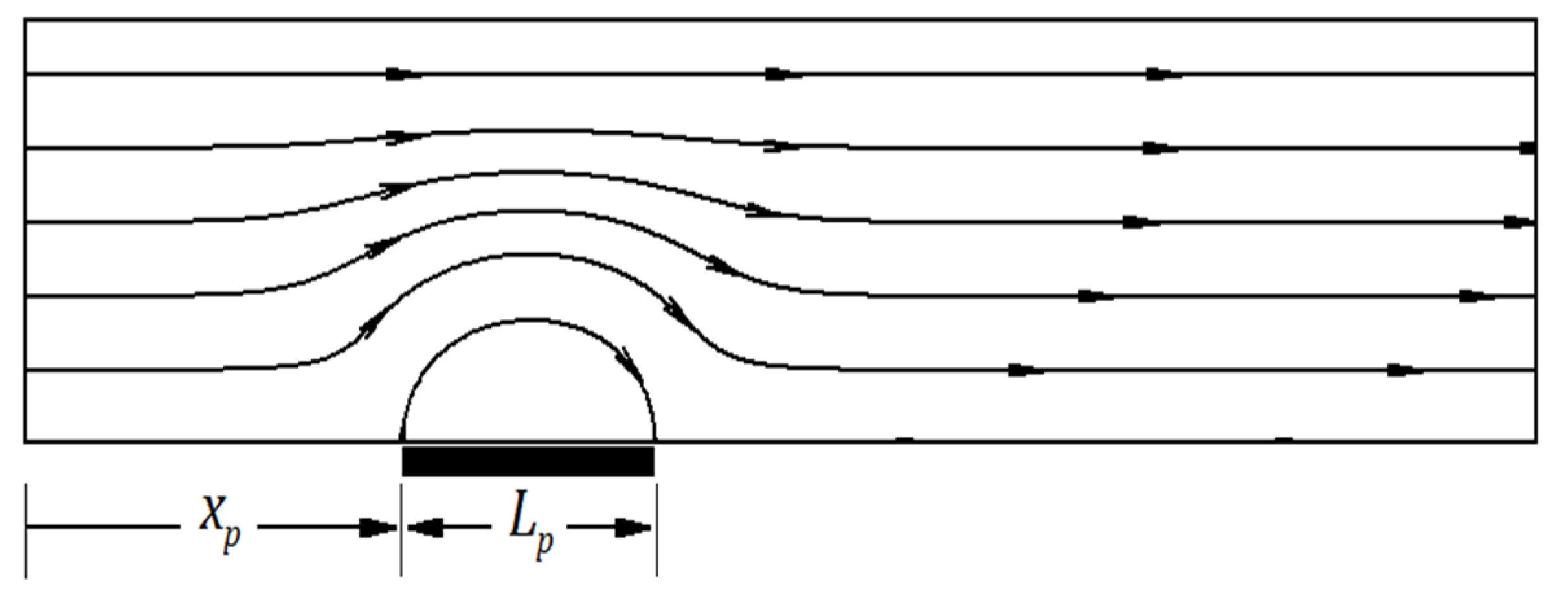
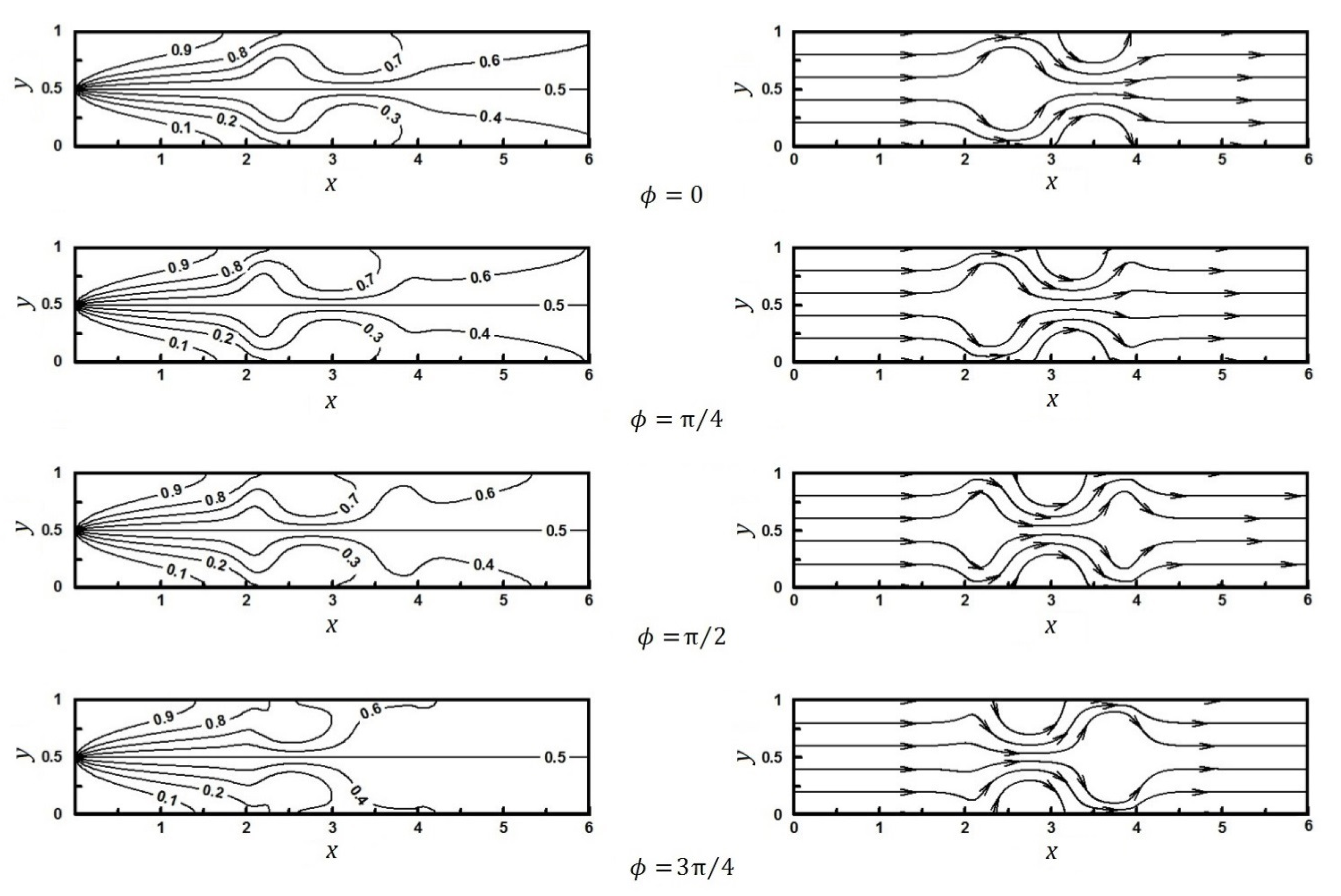
4.1. The Effect of Heterogeneity Patch
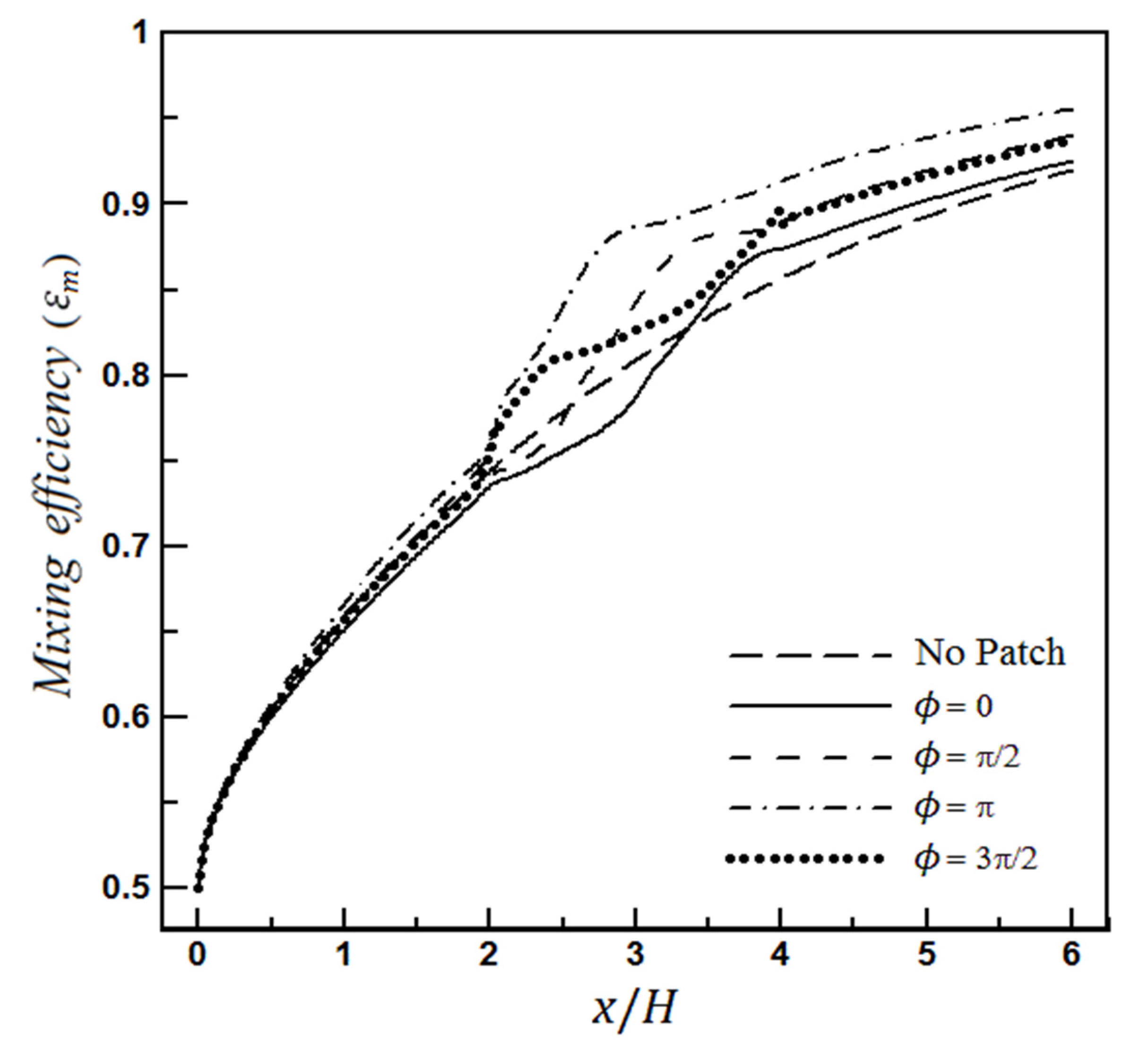
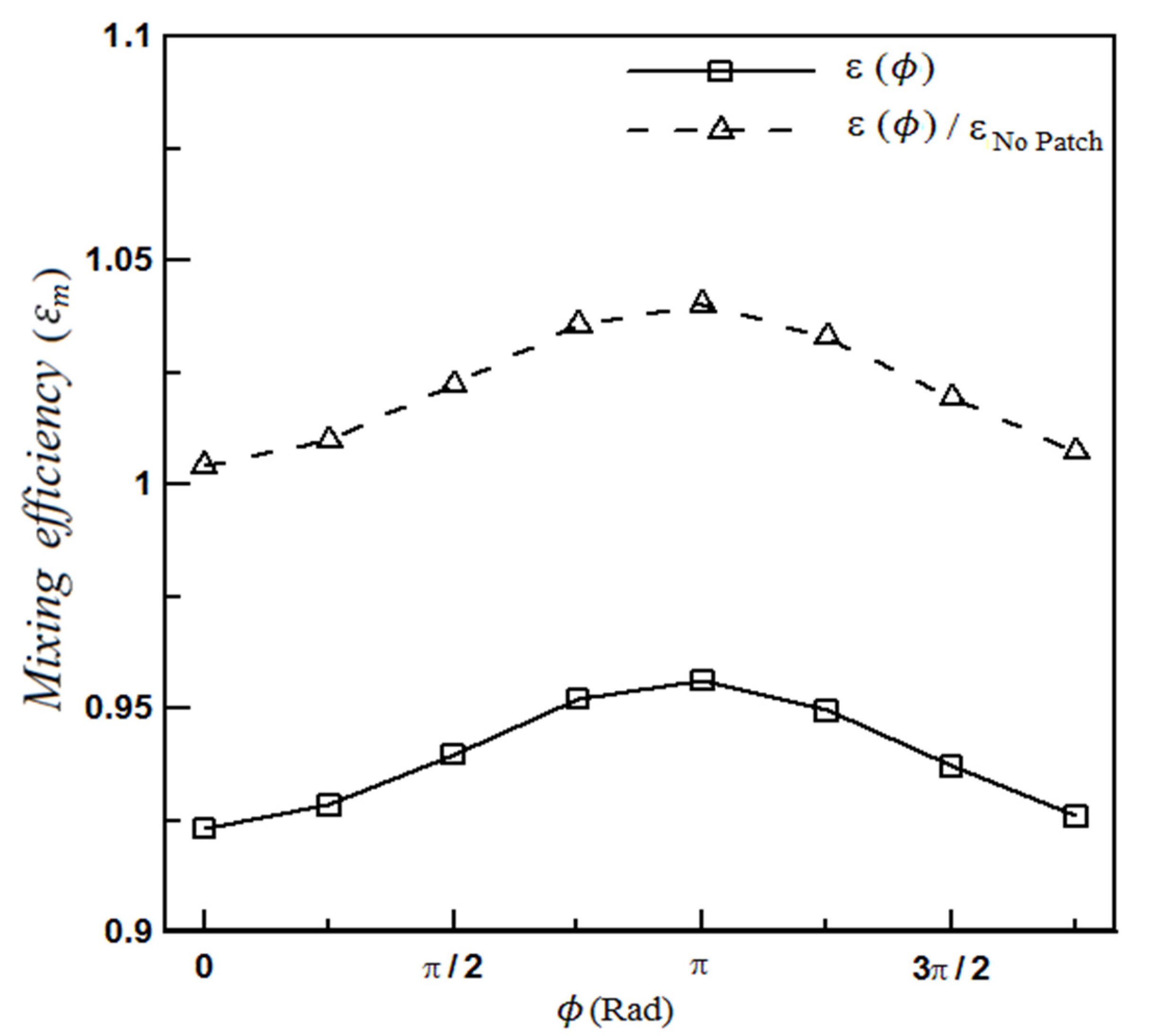
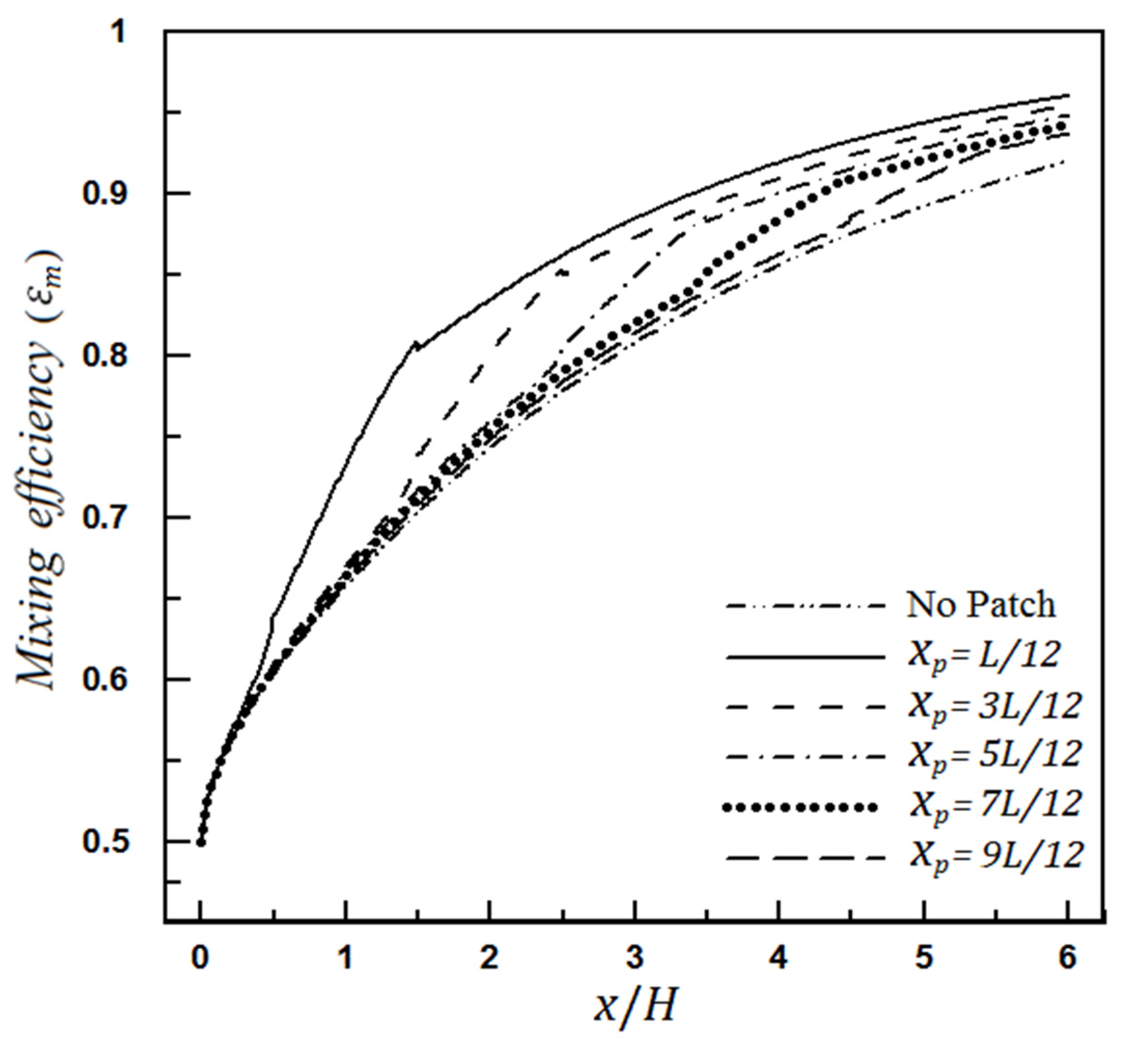
4.1.1. The Effect of Heterogeneity Position
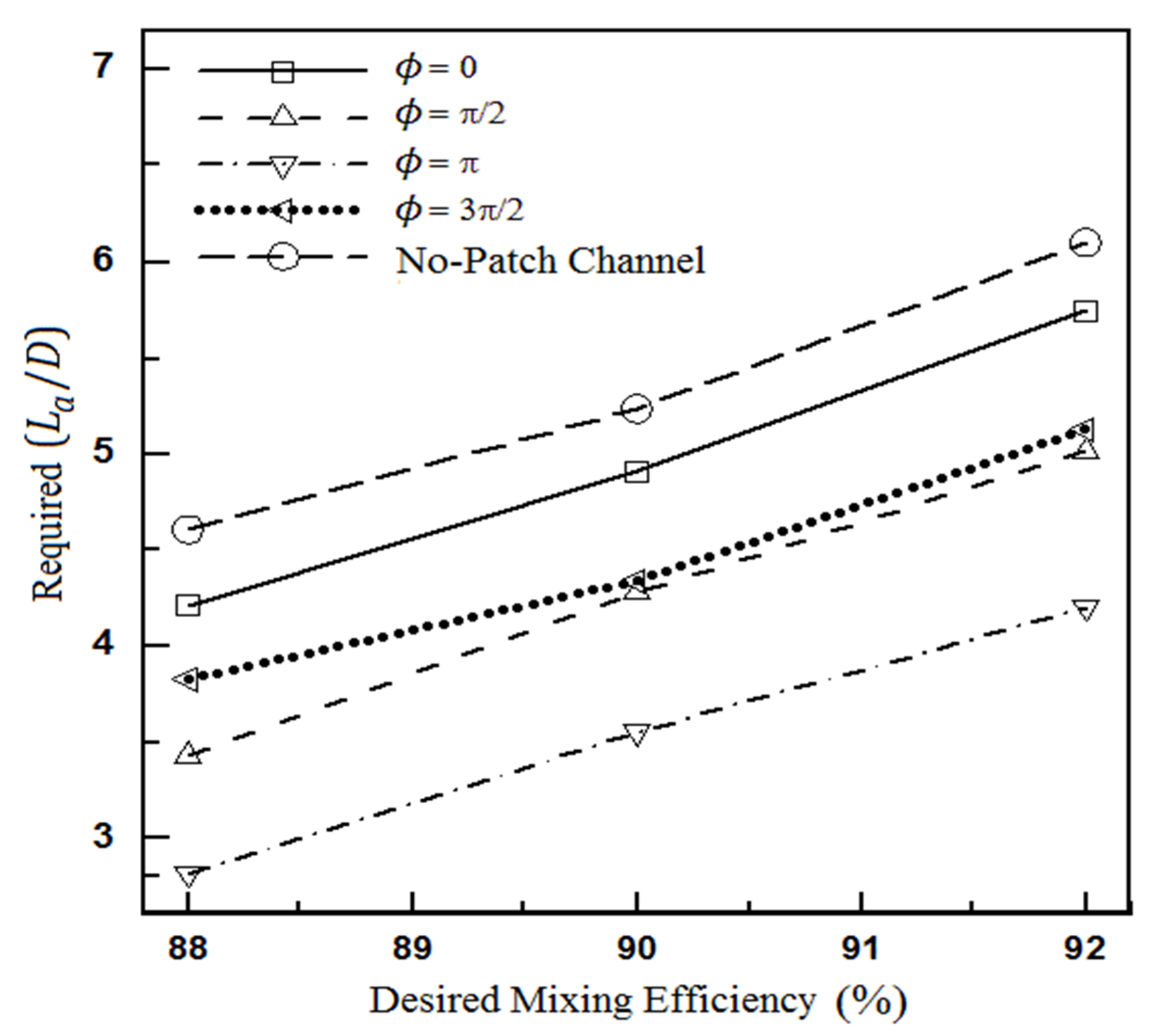
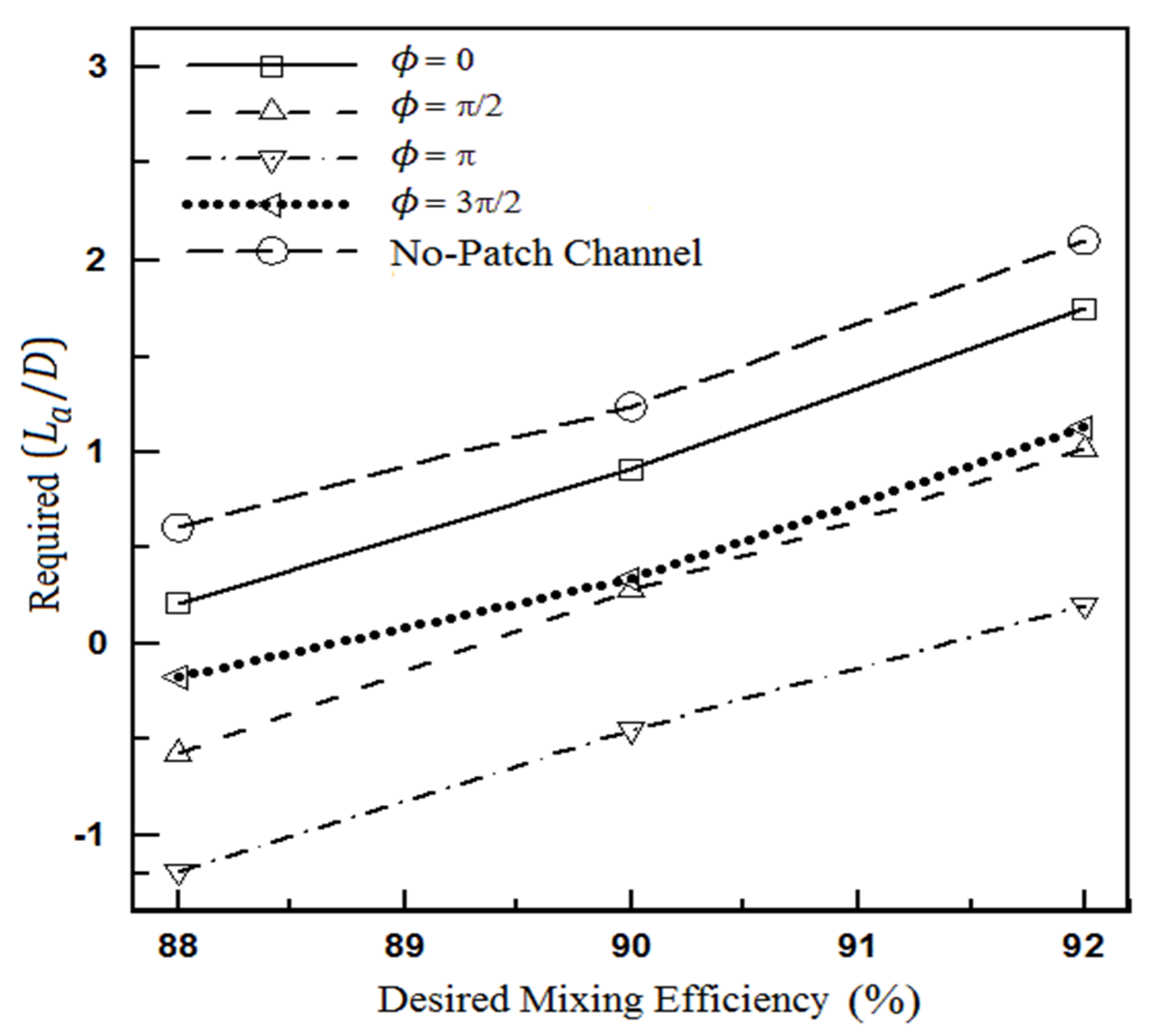
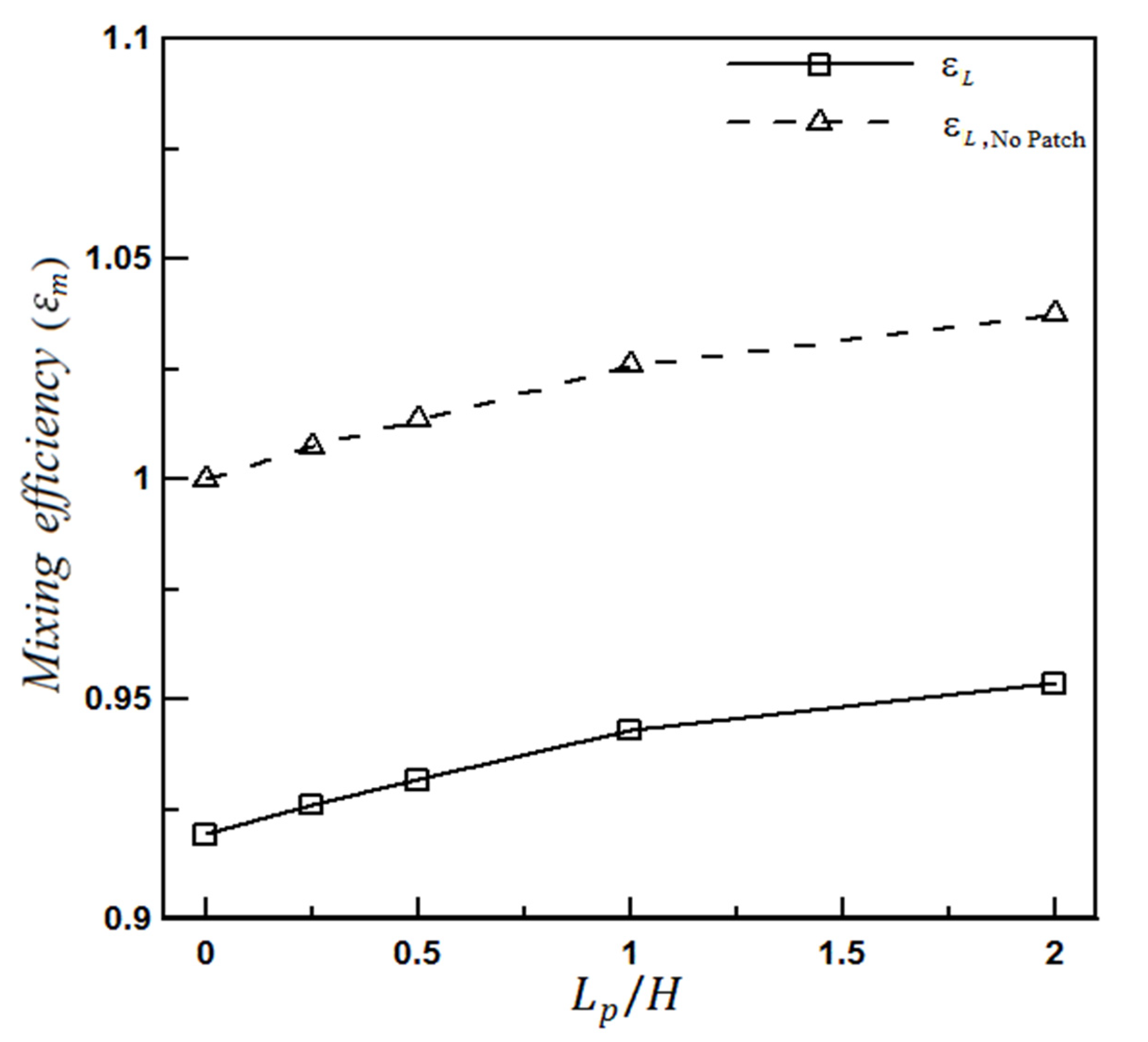
4.1.2. The Effect of Heterogeneity Size
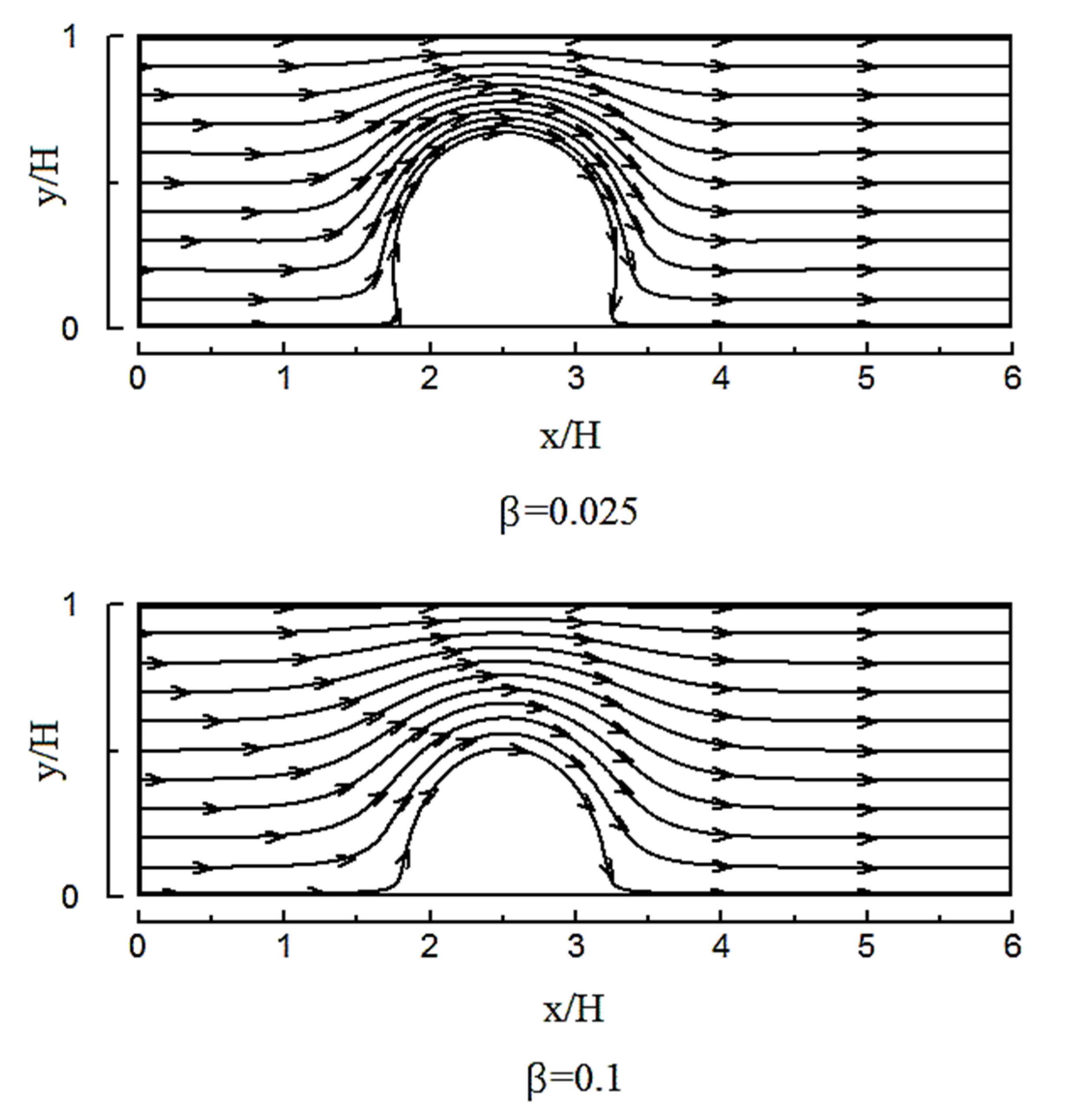
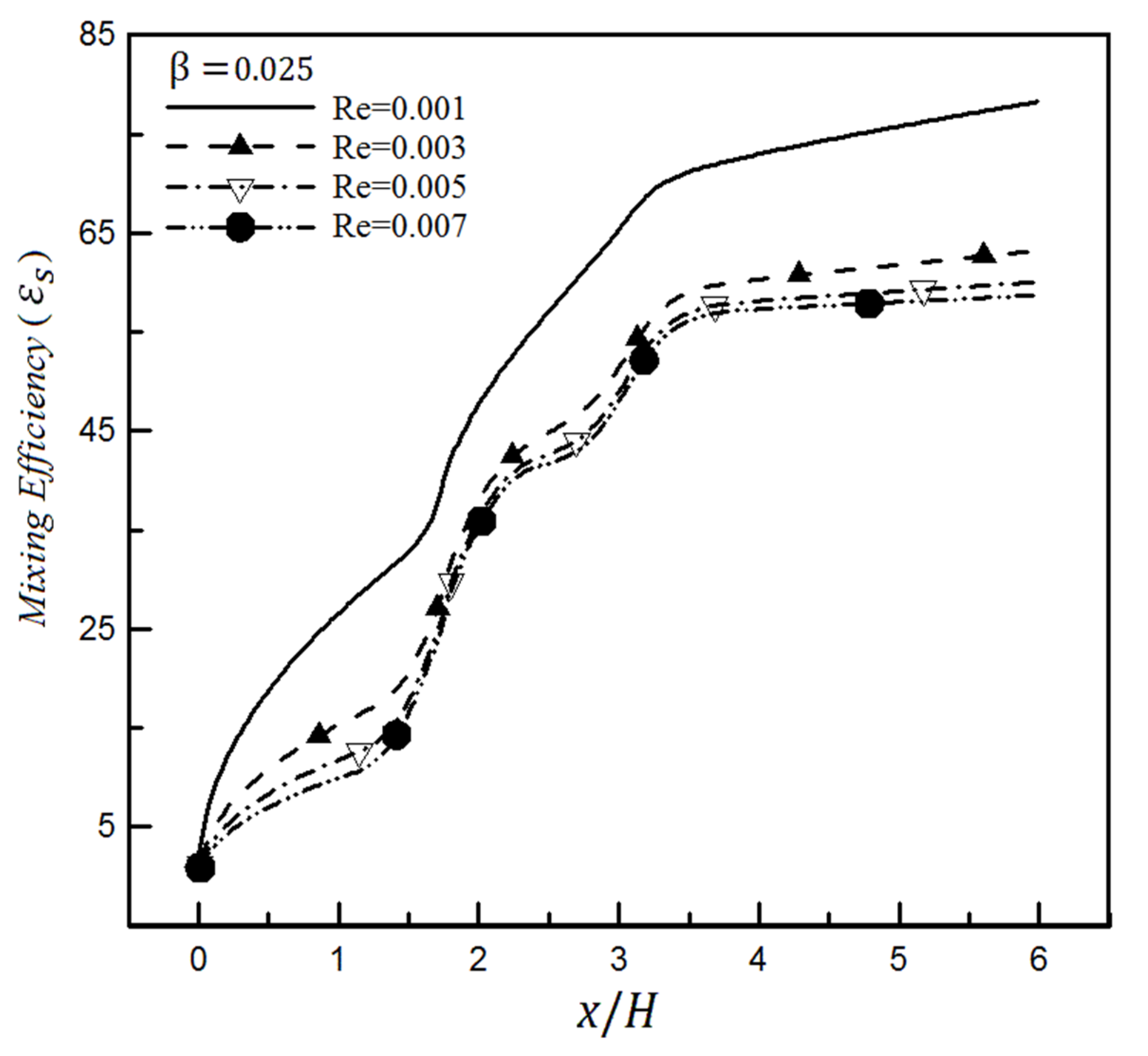
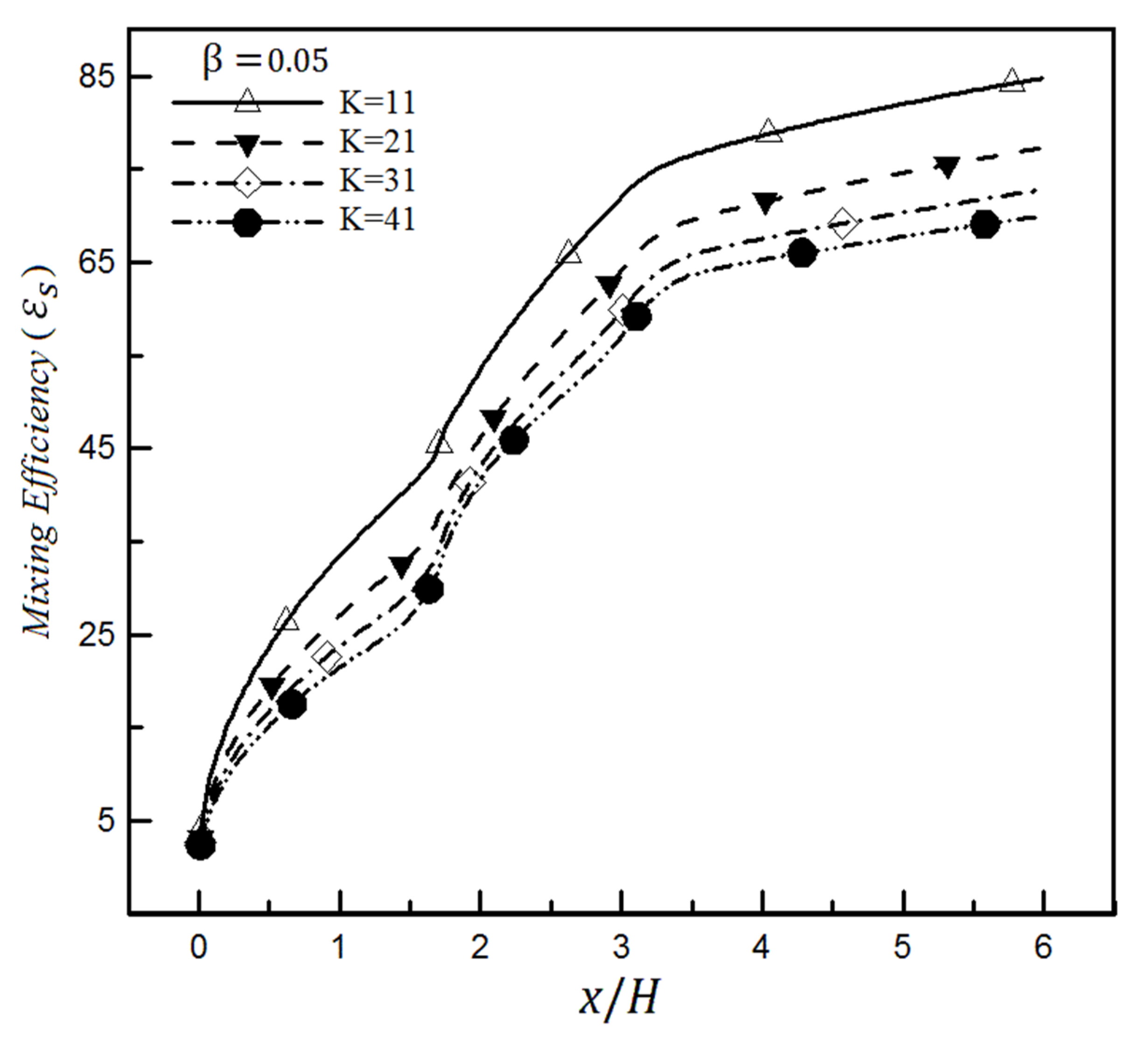
4.2. The Effect of Slip on a Heterogeneity Patch
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Parameter | Name |
| C | Concentration |
| Mean concentration at each cross-section | |
| D | Molecular diffusion coefficient |
| e | Elementary charge |
| E | Electric field strength |
| Eext | Applied electric field strength |
| H | Microchannel height |
| K | Dimensionless Debye–Huckel parameter |
| Kb | Boltzmann constant |
| L | Channel length |
| n0 | Bulk ionic concentration |
| P | Pressure |
| Re | Reynolds number |
| Smix | Mixing entropy |
| Sc | Schmidt number |
| T | Absolute temperature |
| us | Slip velocity at the wall |
| Velocity vector | |
| Z | Valance number of ions for a symmetric electrolyte |
| β | Slip coefficient |
| Mixing efficiency based on the concentration | |
| εs | Mixing efficiency based on entropy |
| ε | Electrolyte permittivity coefficient |
| λ | The thickness of the electric double layer |
| The inverse of the electric double layer (EDL) thickness | |
| μ | Dynamic viscosity |
| ζ | Zeta-potential |
| Density | |
| ρe | Net electric charge density |
| Angle | |
| External electric field | |
| ψ | Internal electric field (due to EDL) |
| Concentration deviation without weight function | |
| Concentration deviation with weight function |
References
- Kong, T.; Shum, H.C.; Weitz, D.A. The Fourth Decade of Microfluidics. Small 2020, 16, 2000070. [Google Scholar] [CrossRef]
- Lei, L.; Bergstrom, D.; Zhang, B.; Zhang, H.; Yin, R.; Song, K.-Y.; Zhang, W. Micro/nanospheres generation by fluid-fluid interaction technology: A literature review. Recent Pat. Nanotechnol. 2017, 11, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhang, H.; Yang, S.; Yin, R.; Yao, X.; Zhang, W. Comparison of the degradation behavior of PLGA scaffolds in micro-channel, shaking, and static conditions. Biomicrofluidics 2018, 12, 034106. [Google Scholar] [CrossRef]
- Farahinia, A.; Zhang, W.J.; Badea, I. Novel microfluidic approaches to circulating tumor cell separation and sorting of blood cells: A review. J. Sci. Adv. Mater. Devices 2021, 6, 303–320. [Google Scholar] [CrossRef]
- Papadopoulos, V.E.; Kefala, I.N.; Kaprou, G.; Kokkoris, G.; Moschou, D.; Papadakis, G.; Gizeli, E.; Tserepi, A. A passive micromixer for enzymatic digestion of DNA. Microelectron. Eng. 2014, 124, 42–46. [Google Scholar] [CrossRef]
- Farahinia, A.; Zhang, W. Numerical analysis of a microfluidic mixer and the effects of different cross-sections and various input angles on its mixing performance. J. Braz. Soc. Mech. Sci. Eng. 2020, 42, 1–18. [Google Scholar] [CrossRef]
- Farahinia, A.; Zhang, W.J. Numerical investigation into the mixing performance of micro T-mixers with different patterns of obstacles. J. Braz. Soc. Mech. Sci. Eng. 2019, 41, 491–504. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, X.; Wu, Z.; Zheng, Y. Numerical study on stagger Koch fractal baffles micromixer. Int. J. Heat Mass Transf. 2019, 133, 1065–1073. [Google Scholar] [CrossRef]
- Dreher, S.; Kockmann, N.; Woias, P. Characterization of Laminar Transient Flow Regimes and Mixing in T-shaped Micromixers. Heat Transf. Eng. 2009, 30, 91–100. [Google Scholar] [CrossRef]
- Jamaati, J.; Farahinia, A.; Niazmand, H. Mixing Investigation In Combined Electroosmotic/Pressure-driven Micromixers With Heterogeneous Wall Charges. Modares Mech. Eng. 2015, 15, 297–306. [Google Scholar]
- Zhang, F.; Marre, S.; Erriguible, A. Mixing intensification under turbulent conditions in a high pressure microreactor. Chem. Eng. J. 2020, 382, 122859. [Google Scholar] [CrossRef] [Green Version]
- Jamaati, J.; Farahinia, A.; Niazmand, H. Numerical Investigation of Electro-osmotic Flow in Heterogeneous Microchannels. Modares Mech. Eng. 2015, 15, 260–270. [Google Scholar]
- Bhattacharyya, S.; Bera, S. Nonlinear Electroosmosis Pressure-Driven Flow in a Wide Microchannel With Patchwise Surface Heterogeneity. J. Fluids Eng. 2013, 135, 021303. [Google Scholar] [CrossRef]
- Kunti, G.; Bhattacharya, A.; Chakraborty, S. Rapid mixing with high-throughput in a semi-active semi-passive micromixer. Electrophoresis 2017, 38, 1310–1317. [Google Scholar] [CrossRef]
- Ai, B.-Q.; Shao, Z.-G.; Zhong, W.-R. Mixing and demixing of binary mixtures of polar chiral active particles. Soft Matter 2018, 14, 4388–4395. [Google Scholar] [CrossRef] [Green Version]
- Le The, H.; Le Thanh, H.; Dong, T.; Ta, B.Q.; Tran Minh, N.; Karlsen, F. An effective passive micromixer with shifted trapezoidal blades using wide Reynolds number range. Chem. Eng. Res. Des. 2015, 93, 1–11. [Google Scholar] [CrossRef]
- Gidde, R.R.; Pawar, P.M.; Ronge, B.P.; Misal, N.D.; Kapurkar, R.B.; Parkhe, A.K. Evaluation of the mixing performance in a planar passive micromixer with circular and square mixing chambers. Microsyst. Technol. 2018, 24, 2599–2610. [Google Scholar] [CrossRef]
- Alam, A.; Afzal, A.; Kim, K.Y. Mixing performance of a planar micromixer with circular obstructions in a curved microchannel. Chem. Eng. Res. Des. 2014, 92, 423–434. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Bera, S. Combined electroosmosis-pressure driven flow and mixing in a microchannel with surface heterogeneity. Appl. Math. Model. 2015, 39, 4337–4350. [Google Scholar] [CrossRef]
- Kamali, R.; Mansoorifar, A.; Dehghan Manshadi, M.K. Effect of baffle geometry on mixing performance in the passive micromixer. Trans. Mech. Eng. 2014, 38, 351–360. [Google Scholar]
- Hossain, S.; Fuwad, A.; Kim, K.-Y.; Jeon, T.-J.; Kim, S.M. Investigation of mixing performance of two-dimensional micromixer using Tesla structures with different shapes of obstacles. Ind. Eng. Chem. Res. 2020, 59, 3636–3643. [Google Scholar] [CrossRef]
- Zhou, R.; Surendran, A.; Wang, J. Fabrication and characteristic study on mixing enhancement of a magnetofluidic mixer. Sens. Actuators A Phys. 2021, 326, 112733. [Google Scholar] [CrossRef]
- Xiong, S.; Chen, X.; Ma, Y. Simulation analysis of micromixer with three-dimensional fractal structure with electric field effect. J. Braz. Soc. Mech. Sci. Eng. 2021, 43, 1–8. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Hasanzadeh-Barforoushi, A.; Nejat, A.; Kowsary, F. Numerical study of mixing and heat transfer in mixed electroosmotic/pressure driven flow through T-shaped microchannels. Int. J. Heat Mass Transf. 2014, 75, 565–580. [Google Scholar] [CrossRef]
- Peng, R.; Li, D. Effects of ionic concentration gradient on electroosmotic flow mixing in a microchannel. J. Colloid Interface Sci. 2015, 440, 126–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, S.K.; Pati, S.; Mondal, P.K. Numerical study of the vortex induced electroosmotic mixing of non-Newtonian biofluids in a non-uniformly charged wavy microchannel: Effect of finite ion size. Electrophoresis 2021. [Google Scholar] [CrossRef] [PubMed]
- Salari, A.; Dalton, C. Simultaneous pumping and mixing of biological fluids in a double-array electrothermal microfluidic device. Micromachines 2019, 10, 92. [Google Scholar] [CrossRef] [Green Version]
- Mei, L.; Cui, D.; Shen, J.; Dutta, D.; Brown, W.; Zhang, L.; Dabipi, I.K. Electroosmotic Mixing of Non-Newtonian Fluid in a Microchannel with Obstacles and Zeta Potential Heterogeneity. Micromachines 2021, 12, 431. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Fimbres Weihs, G.A.; Wiley, D.E. Approximation for modelling electro-osmotic mixing in the boundary layer of membrane systems. J. Membr. Sci. 2014, 450, 18–27. [Google Scholar] [CrossRef]
- Ng, C.O.; Qi, C. Electroosmotic flow of a power-law fluid in a non-uniform microchannel. J. Non-Newton. Fluid Mech. 2014, 208–209, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Bera, S.; Bhattacharyya, S. On mixed electroosmotic-pressure driven flow and mass transport in microchannels. Int. J. Eng. Sci. 2013, 62, 165–176. [Google Scholar] [CrossRef]
- Nayak, A.K. Analysis of mixing for electroosmotic flow in micro/nano channels with heterogeneous surface potential. Int. J. Heat Mass Transf. 2014, 75, 135–144. [Google Scholar] [CrossRef]
- Farahinia, A.; Jamaati, J.; Niazmand, H.; Zhang, W.J. Investigation of an electro-osmotic micromixer with heterogeneous zeta-potential distribution at the wall. Eng. Res. Express 2019, 1, 015024. [Google Scholar] [CrossRef]
- Farahinia, A.; Jamaati, J.; Niazmand, H. Investigation of slip effects on electroosmotic mixing in heterogeneous microchannels based on entropy index. SN Appl. Sci. 2019, 1, 728–740. [Google Scholar] [CrossRef] [Green Version]
- Aminpour, M.; Torres, S.A.G.; Scheuermann, A.; Li, L. Slip-Flow Regimes in Nanofluidics: A Universal Superexponential Model. Phys. Rev. Appl. 2021, 15, 054051. [Google Scholar] [CrossRef]
- Kavokine, N.; Netz, R.R.; Bocquet, L. Fluids at the nanoscale: From continuum to subcontinuum transport. Annu. Rev. Fluid Mech. 2021, 53, 377–410. [Google Scholar] [CrossRef]
- Hu, H.; Wang, D.; Ren, F.; Bao, L.; Priezjev, N.V.; Wen, J. A comparative analysis of the effective and local slip lengths for liquid flows over a trapped nanobubble. Int. J. Multiph. Flow 2018, 104, 166–173. [Google Scholar] [CrossRef]
- Zuo, H.; Javadpour, F.; Deng, S.; Li, H. Liquid slippage on rough hydrophobic surfaces with and without entrapped bubbles. Phys. Fluids 2020, 32, 082003. [Google Scholar] [CrossRef]
- Joly, L.; Ybert, C.; Trizac, E.; Bocquet, L. Liquid friction on charged surfaces: From hydrodynamic slippage to electrokinetics. J. Chem. Phys. 2006, 125, 204716. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S. The EDL effect in microchannel flow: A critical review. Int. J. Adv. Comput. Res. 2013, 3, 242. [Google Scholar]
- Xie, Y.; Fu, L.; Niehaus, T.; Joly, L. Liquid-solid slip on charged walls: The dramatic impact of charge distribution. Phys. Rev. Lett. 2020, 125, 014501. [Google Scholar] [CrossRef]
- Chakraborty, S. Generalization of interfacial electrohydrodynamics in the presence of hydrophobic interactions in narrow fluidic confinements. Phys. Rev. Lett. 2008, 100, 097801. [Google Scholar] [CrossRef] [PubMed]
- Park, H.M.; Choi, Y.J. A method for simultaneous estimation of inhomogeneous zeta potential and slip coefficient in microchannels. Anal. Chim. Acta 2008, 616, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Park, H.M.; Kim, T.W. Simultaneous estimation of zeta potential and slip coefficient in hydrophobic microchannels. Anal. Chim. Acta 2007, 593, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.; Xuan, X. Electrokinetic energy conversion in slip nanochannels. J. Power Sources 2008, 179, 297–300. [Google Scholar] [CrossRef]
- Ren, Y.; Stein, D. Slip-enhanced electrokinetic energy conversion in nanofluidic channels. Nanotechnology 2008, 19, 195707. [Google Scholar] [CrossRef]
- Mikelić, A. An introduction to the homogenization modeling of non-Newtonian and electrokinetic flows in porous media. In Non-Newtonian Fluid Mechanics and Complex Flows; Springer: Berlin/Heidelberg, Germany, 2018; pp. 171–227. [Google Scholar]
- Malkin, A.Y.; Patlazhan, S. Wall slip for complex liquids–phenomenon and its causes. Adv. Colloid Interface Sci. 2018, 257, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qi, H.; Yu, B.; Xiong, Z.; Xu, H. Analytical and numerical study of electroosmotic slip flows of fractional second grade fluids. Commun. Nonlinear Sci. Numer. Simul. 2017, 50, 77–87. [Google Scholar] [CrossRef]
- Afonso, A.; Ferras, L.; Nobrega, J.; Alves, M.; Pinho, F. Pressure-driven electrokinetic slip flows of viscoelastic fluids in hydrophobic microchannels. Microfluid. Nanofluidics 2013, 16, 1131–1142. [Google Scholar] [CrossRef]
- Ferras, L.L.; Afonso, A.M.; Alves, M.A.; Nobrega, J.M.; Pinho, F.T. Analytical and numerical study of the electro-osmotic annular flow of viscoelastic fluids. J. Colloid Interface Sci. 2014, 420, 152–157. [Google Scholar] [CrossRef]
- Ahmadian Yazdi, A.; Sadeghi, A.; Saidi, M.H. Electrokinetic mixing at high zeta potentials: Ionic size effects on cross stream diffusion. J. Colloid Interface Sci. 2015, 442, 8–14. [Google Scholar] [CrossRef]
- Fodor, P.; Vyhnalek, B.; Kaufman, M. Entropic Evaluation of Dean Flow Micromixer. In Proceedings of the 2013 COMSOL Conference, Boston, MA, USA, 9−11 October 2013. [Google Scholar]
- Mastrangelo, F.M.; Pennella, F.; Consolo, F.; Rasponi, M.; Redaelli, A.; Montevecchi, F.M.; Morbiducci, U. Micromixing and Microchannel Design: Vortex Shape and Entropy. In Proceedings of the 2nd Micro and Nano Flows Conference, West London, UK, 1−2 September 2009. [Google Scholar]
- Wang, M.; Wang, J.; Chen, S.; Pan, N. Electrokinetic pumping effects of charged porous media in microchannels using the lattice Poisson-Boltzmann method. J. Colloid Interface Sci. 2006, 304, 246–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masliyah, J.H. Electrokinetik Transport. Phenomena; Alberta Oil Sands Tecnology and Research Authority: Edmonton, AB, Canada, 1994. [Google Scholar]
- Mirbozorgi, S.A.; Niazmand, H.; Renksizbulut, M. Electro-Osmotic Flow in Reservoir-Connected Flat Microchannels With Non-Uniform Zeta Potential. J. Fluids Eng. 2006, 128, 1133–1143. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloid Science; Academic Press: Cambridge, MA, USA, 1981. [Google Scholar]
- Rhie, C.; Chow, W.L. Numerical study of the turbulent flow past an airfoil with trailing edge separation. AIAA J. 1983, 21, 1525–1532. [Google Scholar] [CrossRef]
- Zhang, J.; He, G.; Liu, F. Electro-osmotic flow and mixing in heterogeneous microchannels. Phys. Rev. E 2006, 73, 056305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bockelmann, H.; Heuveline, V.; Barz, D.P. Optimization of an electrokinetic mixer for microfluidic applications. Biomicrofluidics 2012, 6, 24118–24123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farahinia, A.; Jamaati, J.; Niazmand, H. Study of Slip Effect on Electro-osmotic Micromixer Performance Based on Entropy Index. Amirkabir J. Mech. Eng. 2017, 49, 535–548. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farahinia, A.; Jamaati, J.; Niazmand, H.; Zhang, W. Numerical Analysis of the Heterogeneity Effect on Electroosmotic Micromixers Based on the Standard Deviation of Concentration and Mixing Entropy Index. Micromachines 2021, 12, 1055. https://doi.org/10.3390/mi12091055
Farahinia A, Jamaati J, Niazmand H, Zhang W. Numerical Analysis of the Heterogeneity Effect on Electroosmotic Micromixers Based on the Standard Deviation of Concentration and Mixing Entropy Index. Micromachines. 2021; 12(9):1055. https://doi.org/10.3390/mi12091055
Chicago/Turabian StyleFarahinia, Alireza, Jafar Jamaati, Hamid Niazmand, and Wenjun Zhang. 2021. "Numerical Analysis of the Heterogeneity Effect on Electroosmotic Micromixers Based on the Standard Deviation of Concentration and Mixing Entropy Index" Micromachines 12, no. 9: 1055. https://doi.org/10.3390/mi12091055
APA StyleFarahinia, A., Jamaati, J., Niazmand, H., & Zhang, W. (2021). Numerical Analysis of the Heterogeneity Effect on Electroosmotic Micromixers Based on the Standard Deviation of Concentration and Mixing Entropy Index. Micromachines, 12(9), 1055. https://doi.org/10.3390/mi12091055






