Design of a Hand-Held and Battery-Operated Digital Microfluidic Device Using EWOD for Lab-on-a-Chip Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. System Architecture
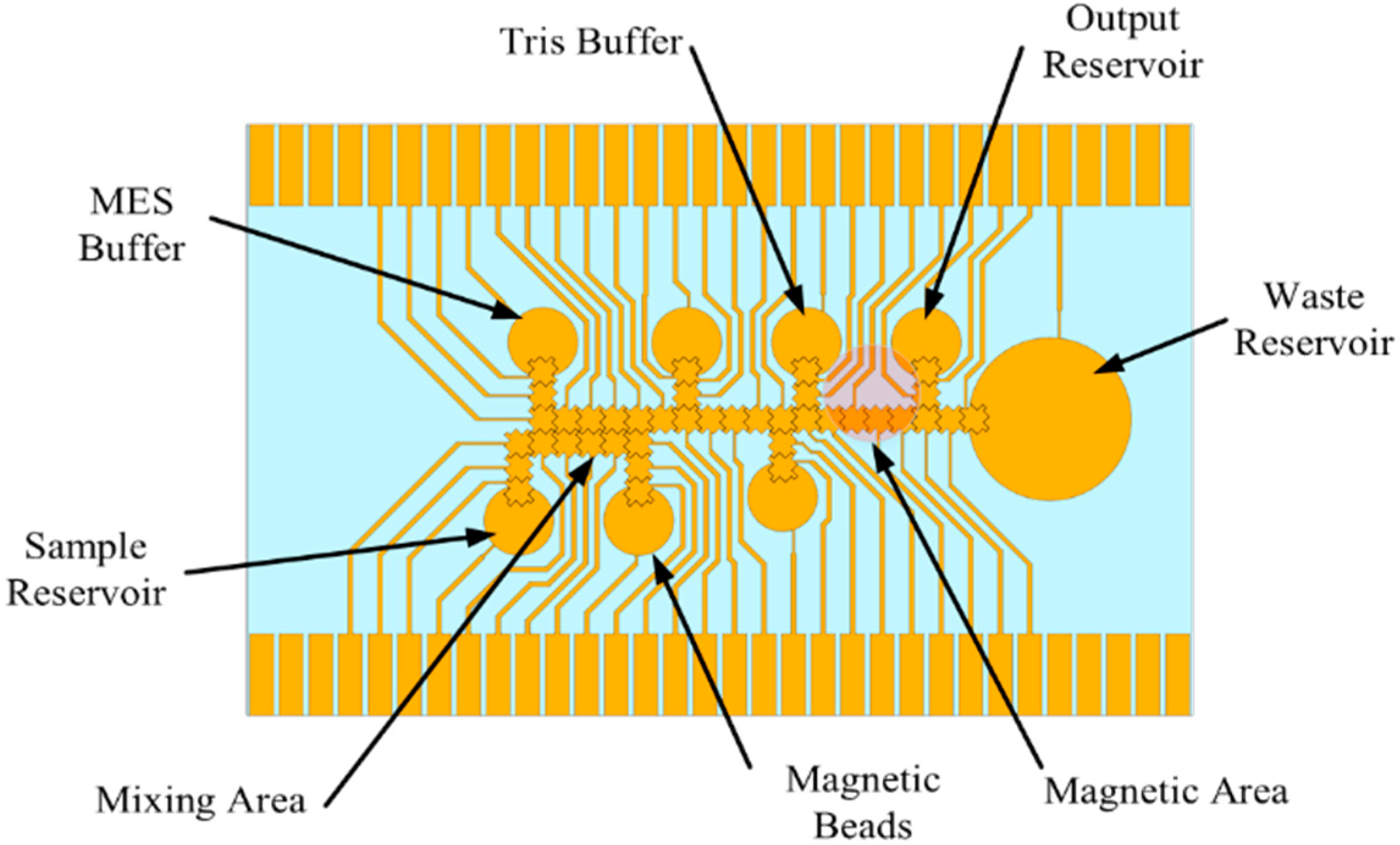
2.2. EWOD Electrode Chip for DNA Isolation and Extraction Electrode
2.3. Position Feedback
2.4. Supporting Circuits
2.5. User Control and Software Interface
2.6. EWOD System Preparation
2.7. DNA Isolation
3. Results and Discussion
3.1. High Voltage Power Supply
3.2. Adaptive Activation Control from Droplet Position Feedback
3.3. DNA Isolation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ren, H.; Fair, R.B.; Pollack, M.G. Automated on-chip droplet dispensing with volume control by electro-wetting actuation and capacitance metering. Sens. Actuators B Chem. 2004, 98, 319–327. [Google Scholar] [CrossRef]
- Karuwan, C.; Sukthang, K.; Wisitsoraat, A.; Phokharatkul, D.; Patthanasettakul, V.; Wechsatol, W.; Tuantranont, A. Electrochemical detection on electrowetting-on-dielectric digital microfluidic chip. Talanta 2011, 84, 1384–1389. [Google Scholar] [CrossRef] [PubMed]
- Mousa, N.A.; Jebrail, M.J.; Yang, H.; Abdelgawad, M.; Metalnikov, P.; Chen, J.; Wheeler, A.R.; Casper, R.F. Droplet-Scale Estrogen Assays in Breast Tissue, Blood, and Serum Movie S1 (wmv format). Sci. Transl. Med. 2009, 1, 1ra2. [Google Scholar] [CrossRef] [PubMed]
- Aijian, A.P.; Garrell, R.L. Digital Microfluidics for Automated Hanging Drop Cell Spheroid Culture. J. Lab. Autom. 2015, 20, 283–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, J.; Li, H.; Wong, A.H.H.; Dong, C.; Yi, S.; Jia, Y.; Mak, P.I.; Deng, C.X.; Martins, R.P. A digital microfluidic system with 3D microstructures for single-cell culture. Microsyst. Nanoeng. 2020, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Vergauwe, N.; Witters, D.; Ceyssens, F.; Vermeir, S.; Verbruggen, B.; Puers, R.; Lammertyn, J. A versatile electrowetting-based digital microfluidic platform for quantitative homogeneous and heterogeneous bio-assays. J. Micromech. Microeng. 2011, 21, 054026. [Google Scholar] [CrossRef]
- Srinivasan, V.; Pamula, V.K.; Fair, R.B. An integrated digital microfluidic lab-on-a-chip for clinical diagnostics on human physiological fluids. Lab Chip 2004, 4, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.H.; Fan, S.K.; Kim, C.J.; Yao, D.J. EWOD microfluidic systems for biomedical applications. Microfluid. Nanofluidics 2014, 16, 965–987. [Google Scholar] [CrossRef]
- Gong, J.; Fan, S.-K.; Kim, C.J. Portable digital microfluidics platform with active but disposable Lab-On-Chip. In Proceedings of the 17th IEEE Interitional Conference on Micro Electro Mechanical Systems, Maastricht, The Netherlands, 25–29 January 2004; Volume 3, pp. 355–358. [Google Scholar] [CrossRef]
- Joshi, K.; Velasco, V.; Esfandyarpour, R. A low-cost, disposable and portable inkjet-printed biochip for the developing world. Sensors 2020, 20, 3593. [Google Scholar] [CrossRef] [PubMed]
- Jang, L.S.; Hsu, C.Y.; Chen, C.H. Effect of electrode geometry on performance of EWOD device driven by battery-based system. Biomed. Microdevices 2009, 11, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Sista, R.; Hua, Z.; Thwar, P.; Sudarsan, A.; Srinivasan, V.; Eckhardt, A.; Pollack, M.; Pamula, V. Development of a digital microfluidic platform for point of care testing. Lab Chip 2008, 8, 2091–2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fobel, R.; Fobel, C.; Wheeler, A.R. DropBot: An open-source digital microfluidic control system with precise control of electrostatic driving force and instantaneous drop velocity measurement. Appl. Phys. Lett. 2013, 102, 193513. [Google Scholar] [CrossRef] [Green Version]
- Shih, S.C.C.; Fobel, R.; Kumar, P.; Wheeler, A.R. A feedback control system for high-fidelity digital microfluidics. Lab Chip 2011, 11, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Abdulwahab, S.; Ng, A.H.C.; Chamberlain, M.D.; Casper, R.F.; Wheeler, A.R. Lab on a Chip Towards a personalized approach to aromatase inhibitor therapy: A digital microfluidic platform for rapid analysis of estradiol in core-needle- biopsies. Lab Chip 2017, 17, 1594–1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, P.-Y.; Jiang, P.-S.; Lee, E.-F.; Fan, S.-K.; Lu, Y.-W. Genomic DNA extraction from whole blood using a digital microfluidic (DMF) platform with magnetic beads. Microsyst. Technol. 2017, 23, 313–320. [Google Scholar] [CrossRef]


















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grant, N.; Geiss, B.; Field, S.; Demann, A.; Chen, T.W. Design of a Hand-Held and Battery-Operated Digital Microfluidic Device Using EWOD for Lab-on-a-Chip Applications. Micromachines 2021, 12, 1065. https://doi.org/10.3390/mi12091065
Grant N, Geiss B, Field S, Demann A, Chen TW. Design of a Hand-Held and Battery-Operated Digital Microfluidic Device Using EWOD for Lab-on-a-Chip Applications. Micromachines. 2021; 12(9):1065. https://doi.org/10.3390/mi12091065
Chicago/Turabian StyleGrant, Nicholas, Brian Geiss, Stuart Field, August Demann, and Thomas W. Chen. 2021. "Design of a Hand-Held and Battery-Operated Digital Microfluidic Device Using EWOD for Lab-on-a-Chip Applications" Micromachines 12, no. 9: 1065. https://doi.org/10.3390/mi12091065




