Designing Sensitivity: A Comparative Analysis of Microelectrode Topologies for Electrochemical Oxygen Sensing in Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
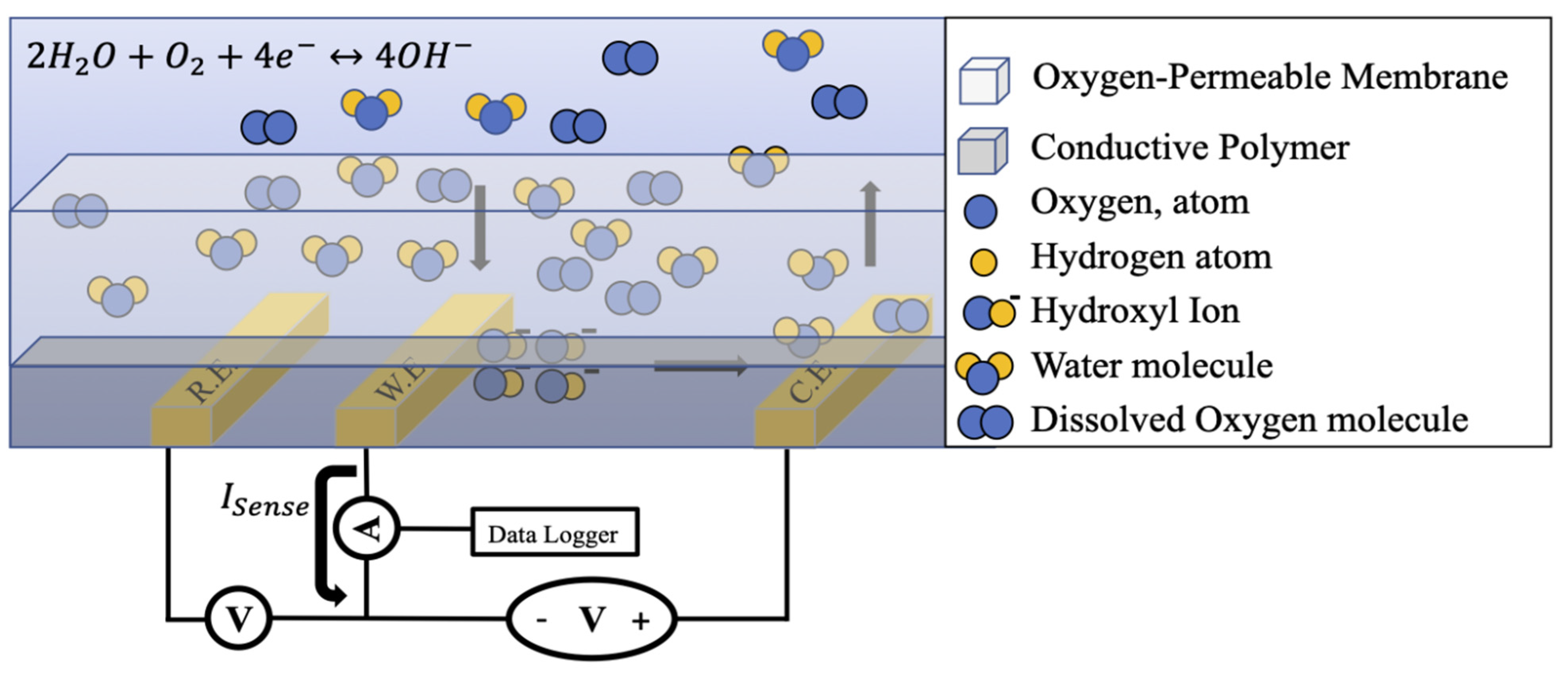
2.1. Electrochemical Sensing Principles
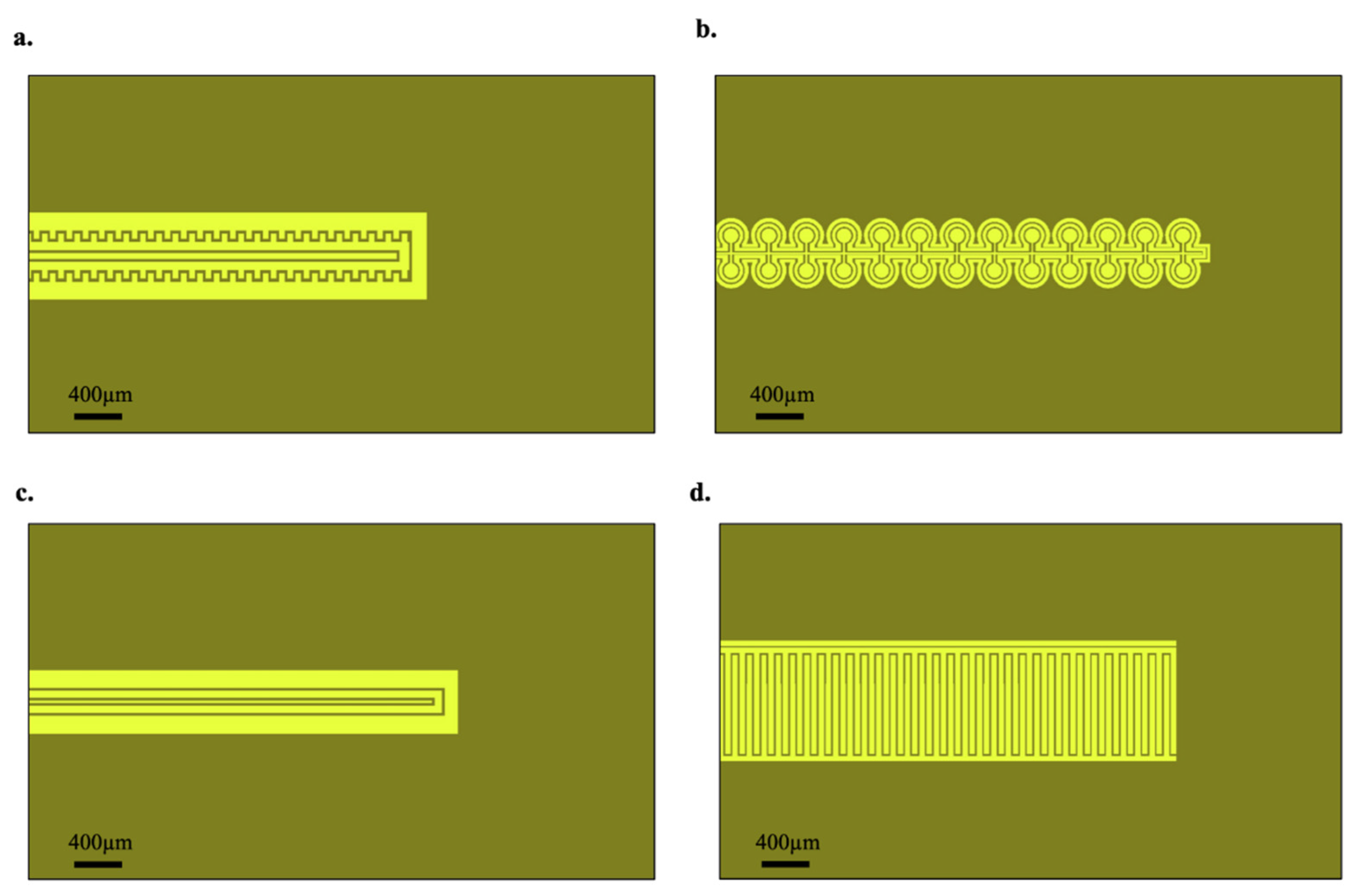
2.2. Device Fabrication
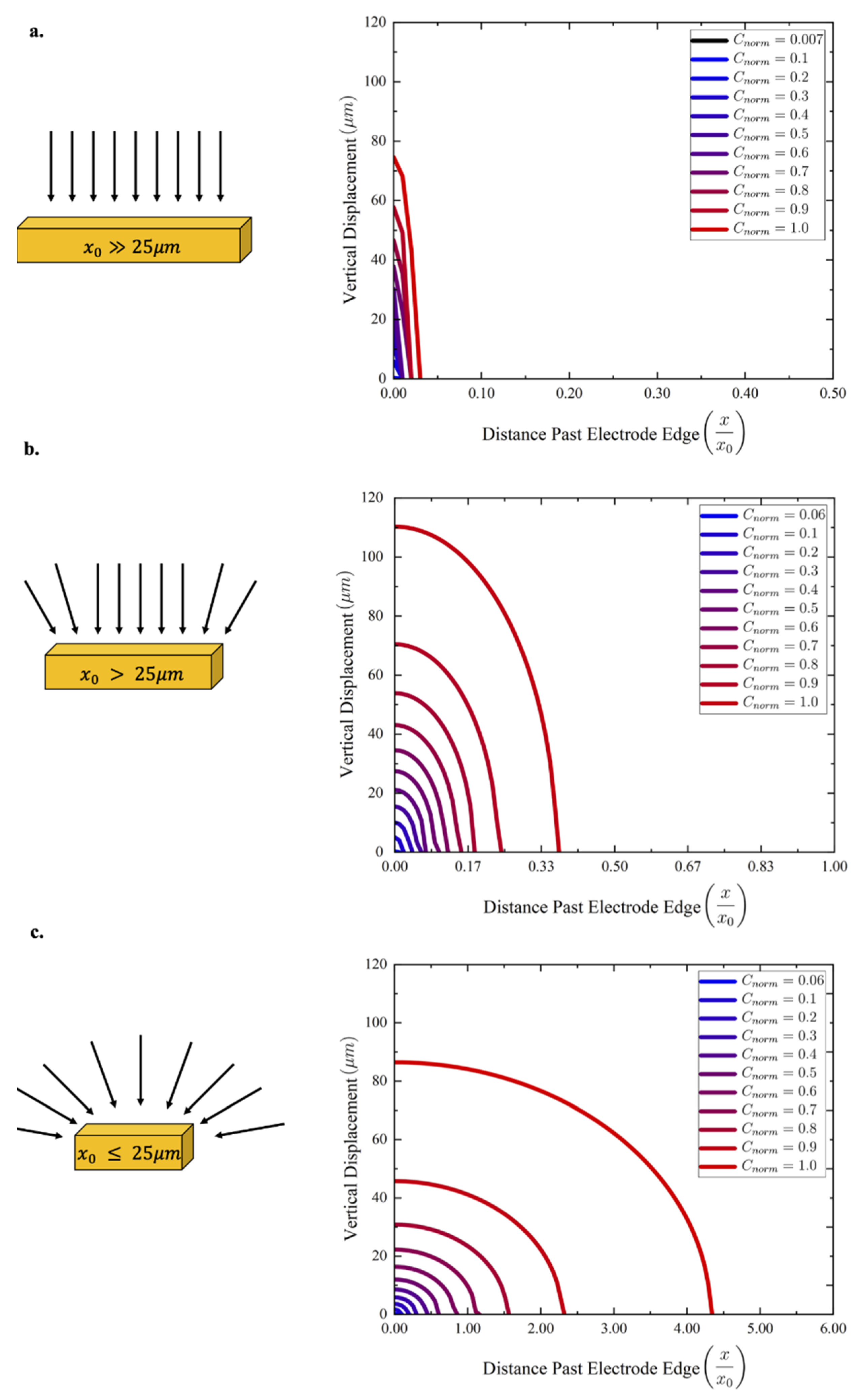
2.3. Characterization Setup and Procedure
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holenda, B.; Domokos, E.; Redey, A.; Fazakas, J. Dissolved oxygen control of the activated sludge wastewater treatment process using model predictive control. Comput. Chem. Eng. 2008, 32, 1270–1278. [Google Scholar] [CrossRef]
- Hargreaves, J.A.; Tucker, C.S. Measuring Dissolved Oxygen Concentration in Aquaculture; Southern Regional Aquaculture Center: Stoneville, MS, USA, 2002. [Google Scholar]
- Chue, C.; Leo, C.J.; Chan, W.P.; Damalerio, M.R.B.; Cheng, M.-Y.; Cheong, J.H.; Gao, Y. Characterization of CMOS Electrochemical Oxygen Sensor for Biomedicai Applications. In Proceedings of the 2015 IEEE International Conference on Electron Devices and Solid-State Circuits (EDSSC), Singapore, 1–4 June 2015; pp. 325–328. [Google Scholar]
- Xiao, Z.; Wang, J.; Yi, C.; Wang, Z.; Wang, L. The dissolved oxygen sensor design based on ultrasonic self-adaption and self-cleaning. Complexity 2020, 2020, 3297203. [Google Scholar] [CrossRef]
- Ndubuizu, O.; LaManna, J.C. Brain tissue oxygen concentration measurements. Antioxid. Redox Signal. 2007, 9, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-J.; Jurng, T.-H.; Chung, U.-H.; Hong, S.-I. Simultaneous determination of oxygen transport characteristics of six membranes by hexagonal dissolved oxygen sensor system. Sens. Actuators B Chem. 2001, 72, 11–20. [Google Scholar] [CrossRef]
- Li, C.; Ahn, C.H.; Shutter, L.A.; Narayan, R.K. Toward real-time continuous brain glucose and oxygen monitoring with a smart catheter. Biosens. Bioelectron. 2009, 25, 173–178. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, G.W.; Braden, K.; Franc, B.; Kovacs, G.T. Microfabricated solid-state dissolved oxygen sensor. Sens. Actuators B Chem. 2002, 83, 138–148. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Y.; Abruna, H.D.; Spector, J.A.; Olbricht, W.L. Micromachined dissolved oxygen sensor based on solid polymer electrolyte. Sens. Actuators B Chem. 2011, 153, 145–151. [Google Scholar] [CrossRef]
- Wiranto, G.; Widodo, S.; Hermida, I.D.P.; Manurung, R.V.; Sugandi, G.; Arifin, Z.; Wiendartun. Design and fabrication of thick film dissolved oxygen sensor based on RuO2 working electrodes for water quality monitoring. Mater. Sci. Forum 2018, 917, 59–63. [Google Scholar] [CrossRef]
- Miyata, M.; Kitazumi, Y.; Shirai, O.; Kataoka, K.; Kano, K. Diffusion-limited biosensing of dissolved oxygen by direct electron transfer-type bioelectrocatalysis of multi-copper oxidases immobilized on porous gold microelectrodes. J. Electroanal. Chem. 2020, 860, 113895. [Google Scholar] [CrossRef]
- Sosna, M.; Denuault, G.; Pascal, R.W.; Prien, R.D.; Mowlem, M. Development of a reliable microelectrode dissolved oxygen sensor. Sens. Actuators B Chem. 2007, 123, 344–351. [Google Scholar] [CrossRef]
- Carreau, A.; El Hafny-Rahbi, B.; Matejuk, A.; Grillon, C.; Kieda, C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell Mol. Med. 2011, 15, 1239–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dings, J.; Meixensberger, J.; Jager, A.; Roosen, K. Clinical experience with 118 brain tissue oxygen partial pressure catheter probes. Neurosurgery 1998, 43, 1082–1094. [Google Scholar] [CrossRef] [PubMed]
- Parrillo, J.E.; Dellinger, R.P. Critical Care Medicine E-Book: Principles of Diagnosis and Management in the Adult; Elsevier: Philadelphia, PA, USA, 2018. [Google Scholar]
- Liebisch, F.; Weltin, A.; Marzioch, J.; Urban, G.A.; Kieninger, J. Zero-consumption clark-type microsensor for oxygen monitoring in cell culture and organ-on-chip systems. Sens. Actuators B Chem. 2020, 322, 128652. [Google Scholar] [CrossRef]
- McLaughlin, G.W. Microfluidic and Biosensor Applications of Fluoropolymer Films; Stanford University: Stanford, CA, USA, 2001. [Google Scholar]
- Heinze, J. Ultramicroelectrodes in electrochemistry. Angew. Chem. Int. Ed. Engl. 1993, 32, 1268–1288. [Google Scholar] [CrossRef]
- Wang, J. Analytical Electrochemistry, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Fundamentals and applications. Electrochem. Methods 2001, 2, 580–632. [Google Scholar]
- Niwa, O. Electroanalysis with interdigitated array microelectrodes. Electroanalysis 1995, 7, 606–613. [Google Scholar] [CrossRef]
- Partel, S.; Kasemann, S.; Choleva, P.; Dincer, C.; Kieninger, J.; Urban, G.A. Novel fabrication process for sub-micron interdigitated electrode arrays for highly sensitive electrochemical detection. Sens. Actuators B Chem. 2014, 205, 193–198. [Google Scholar] [CrossRef]
- Heo, J.I.; Shim, D.S.; Teixidor, G.T.; Oh, S.; Madou, M.J.; Shin, H. Carbon interdigitated array nanoelectrodes for electrochemical applications. J. Electrochem. Soc. 2011, 158, J76–J80. [Google Scholar] [CrossRef]
- Aoki, K.; Morita, M.; Niwa, O.; Tabei, H. Quantitative analysis of reversible diffusion-controlled currents of redox soluble species at interdigitated array electrodes under steady-state conditions. J. Electroanal. Chem. Interfacial Electrochem. 1988, 256, 269–282. [Google Scholar] [CrossRef]
- Chen, I.J.; White, I.M. High-sensitivity electrochemical enzyme-linked assay on a microfluidic interdigitated microelectrode. Biosens. Bioelectron. 2011, 26, 4375–4381. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Geometry | Feature Width (µm) | Feature Separation (µm) | I (µA) | ||
|---|---|---|---|---|---|
| Microelectrode Geometries | |||||
| Serrated | 100 | 10 | 0.358 | 50.3 | 12.1 |
| IDE | 40 | 10 | 0.183 | 50.3 | 9.196 |
| IDE | 40 | 20 | 0.15 | 50.3 | 7.56 |
| Serrated | 30 | 10 | 0.04 | 50.3 | 6.1 |
| Ultramicroelectrode Geometries | |||||
| IDE | 20 | 10 | 1.27 | 50.3 | 7.13 |
| Basic | 20 | 10 | 0.5 | 50.3 | 16.2 |
| Basic | 20 | 30 | 0.171 | 50.3 | 16.3 |
| Omega | 20 | 15 | 0.1 | 50.3 | 5.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bacheschi, D.T.; Strittmatter, E.Z.; Sawtelle, S.; Nami, M. Designing Sensitivity: A Comparative Analysis of Microelectrode Topologies for Electrochemical Oxygen Sensing in Biomedical Applications. Micromachines 2022, 13, 141. https://doi.org/10.3390/mi13010141
Bacheschi DT, Strittmatter EZ, Sawtelle S, Nami M. Designing Sensitivity: A Comparative Analysis of Microelectrode Topologies for Electrochemical Oxygen Sensing in Biomedical Applications. Micromachines. 2022; 13(1):141. https://doi.org/10.3390/mi13010141
Chicago/Turabian StyleBacheschi, Daniel T., Evan Z. Strittmatter, Sonya Sawtelle, and Mohsen Nami. 2022. "Designing Sensitivity: A Comparative Analysis of Microelectrode Topologies for Electrochemical Oxygen Sensing in Biomedical Applications" Micromachines 13, no. 1: 141. https://doi.org/10.3390/mi13010141
APA StyleBacheschi, D. T., Strittmatter, E. Z., Sawtelle, S., & Nami, M. (2022). Designing Sensitivity: A Comparative Analysis of Microelectrode Topologies for Electrochemical Oxygen Sensing in Biomedical Applications. Micromachines, 13(1), 141. https://doi.org/10.3390/mi13010141






