Transforming Tea Catechins into Potent Anticancer Compound: Analysis of Three Boronated-PEG Delivery System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polyphenol Nanoparticles (NPs) Synthesis
2.3. Cell Culture
2.4. In Vitro Cellular Uptake
2.5. In Vitro Cell Cytotoxicity
2.6. Dynamic Light Scattering (DLS) Analysis of Polyphenol NPs
2.7. Transmission Electron Microscopy (TEM) Analysis
2.8. Tumor Retention Properties of Polyphenol NPs In Vivo
3. Results
3.1. The Nanoparticle Forming Abilities of EPI, PIC and EGCG Nanoparticles
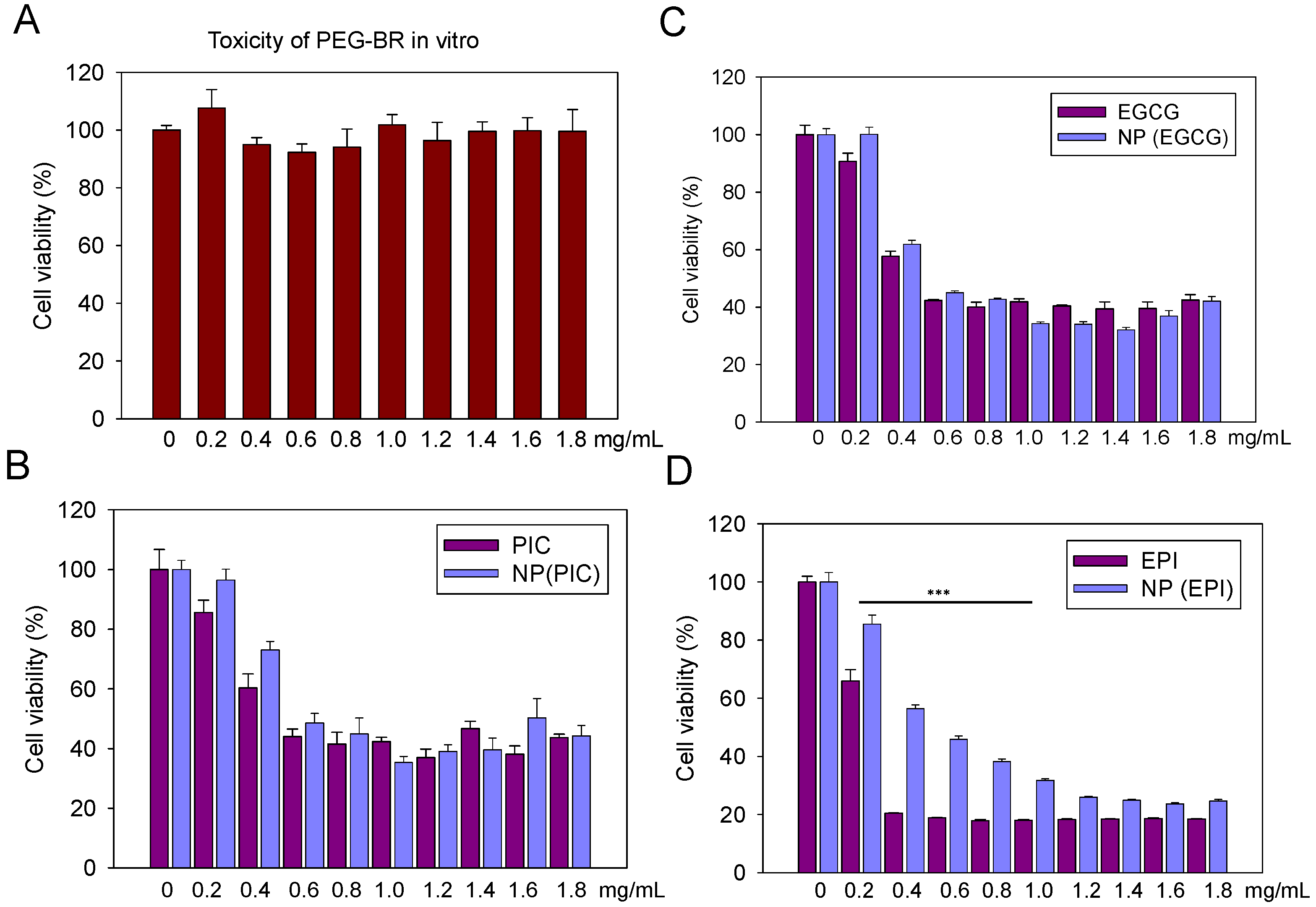
3.2. The In Vitro Cytotoxicity of PEG-Br, EPI, PIC, EGCG and Their Nanoparticles
3.3. The In Vitro Cellular Uptake of EPI, PIC and EGCG Nanoparticles
3.4. The In Vivo Tumoral Uptake of EPI, PIC and EGCG Nanoparticles
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fenske, D.B.; Chonn, A.; Cullis, P.R. Liposomal nanomedicines: An emerging field. Toxicol. Pathol. 2008, 36, 21–29. [Google Scholar] [CrossRef]
- Lee, D.E.; Koo, H.; Sun, I.C.; Ryu, J.H.; Kim, K.; Kwon, I.C. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem. Soc. Rev. 2012, 41, 2656–2672. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, N. Nanomedicine: Nanocarriers shape up for long life. Nat. Nanotechnol. 2007, 2, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saw, P.E.; Lee, S.; Jon, S. Naturally Occurring Bioactive Compound-Derived Nanoparticles for Biomedical Applications. Adv. Ther. 2019, 2, 1800146. [Google Scholar] [CrossRef]
- Saw, P.E.; Jiang, S. The significance of interdisciplinary integration in academic research and application. BIO Integr. 2020, 1, 2–5. [Google Scholar] [CrossRef]
- Shii, T.; Tanaka, T.; Watarumi, S.; Matsuo, Y.; Miyata, Y.; Tamaya, K.; Tamaru, S.; Tanaka, K.; Matsui, T.; Kouno, I. Polyphenol composition of a functional fermented tea obtained by tea-rolling processing of green tea and loquat leaves. J. Agric. Food Chem. 2011, 59, 7253–7260. [Google Scholar] [CrossRef]
- Chennasamudram, S.P.; Kudugunti, S.; Boreddy, P.R.; Moridani, M.Y.; Vasylyeva, T.L. Renoprotective effects of (+)-catechin in streptozotocin-induced diabetic rat model. Nutr. Res. 2012, 32, 347–356. [Google Scholar] [CrossRef]
- Li, X.; Liang, S.; Tan, C.H.; Cao, S.; Xu, X.; Saw, P.E.; Tao, W. Nanocarriers in the Enhancement of Therapeutic Efαcacy of Natural Drugs. BIO Integr. 2021, 2, 40–49. [Google Scholar] [CrossRef]
- Chen, D.; Chen, G.; Sun, Y.; Zeng, X.; Ye, H. Physiological genetics, chemical composition, health benefits and toxicology of tea (Camellia sinensis L.) flower: A review. Food Res. Int. 2020, 137, 109584. [Google Scholar] [CrossRef]
- Kochman, J.; Jakubczyk, K.; Antoniewicz, J.; Mruk, H.; Janda, K. Health Benefits and Chemical Composition of Matcha Green Tea: A Review. Molecules 2020, 26, 85. [Google Scholar] [CrossRef] [PubMed]
- Diniz, L.R.L.; Elshabrawy, H.A.; Souza, M.T.S.; Duarte, A.B.S.; Datta, S.; de Sousa, D.P. Catechins: Therapeutic Perspectives in COVID-19-Associated Acute Kidney Injury. Molecules 2021, 26, 5951. [Google Scholar] [CrossRef] [PubMed]
- Granja, A.; Frias, I.; Neves, A.R.; Pinheiro, M.; Reis, S. Therapeutic Potential of Epigallocatechin Gallate Nanodelivery Systems. BioMed Res. Int. 2017, 2017, 5813793. [Google Scholar] [CrossRef]
- Cheng, X.; Xu, H.D.; Ran, H.H.; Liang, G.; Wu, F.G. Glutathione-Depleting Nanomedicines for Synergistic Cancer Therapy. ACS Nano 2021, 15, 8039–8068. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wang, Z.; Yang, K.; Yue, L.; Cheng, Q.; Ma, Y.L.; Lu, S.; Chen, G.; Wang, R. Polyamine-Responsive Morphological Transformation of a Supramolecular Peptide for Specific Drug Accumulation and Retention in Cancer Cells. Small 2021, 17, e2101139. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Xu, Y.; Guo, X.; Yuan, C.; Mu, D.; Xiao, Y. Cancer cell membrane-coated biomimetic platform for targeted therapy of breast cancer in an orthotopic mouse model. J. Biomater. Sci. Polym. Ed. 2020, 31, 1538–1551. [Google Scholar] [CrossRef]
- Xie, B.; Wan, J.; Chen, X.; Han, W.; Wang, H. Preclinical Evaluation of a Cabazitaxel Prodrug Using Nanoparticle Delivery for the Treatment of Taxane-Resistant Malignancies. Mol. Cancer Ther. 2020, 19, 822–834. [Google Scholar] [CrossRef] [Green Version]
- Suvitha, A.; Venkataramanan, N.S.; Sahara, R.; Kawazoe, Y. A theoretical exploration of the intermolecular interactions between resveratrol and water: A DFT and AIM analysis. J. Mol. Model. 2019, 25, 56. [Google Scholar] [CrossRef]
- Casanova, E.; Priego, E.M.; Jimeno, M.L.; Aguado, L.; Negri, A.; Gago, F.; Camarasa, M.J.; Perez-Perez, M.J. Intramolecular cation-pi interactions as the driving force to restrict the conformation of certain nucleosides. J. Org. Chem. 2010, 75, 1974–1981. [Google Scholar] [CrossRef]
- Saw, P.E.; Yao, H.; Lin, C.; Tao, W.; Farokhzad, O.C.; Xu, X. Stimuli-Responsive Polymer-Prodrug Hybrid Nanoplatform for Multistage siRNA Delivery and Combination Cancer Therapy. Nano Lett. 2019, 19, 5967–5974. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Saw, P.E.; Tao, W.; Li, Y.; Ji, X.; Bhasin, S.; Liu, Y.; Ayyash, D.; Rasmussen, J.; Huo, M.; et al. ROS-Responsive Polyprodrug Nanoparticles for Triggered Drug Delivery and Effective Cancer Therapy. Adv. Mater. 2017, 29, 29. [Google Scholar] [CrossRef]
- Xu, X.; Saw, P.E.; Tao, W.; Li, Y.; Ji, X.; Yu, M.; Mahmoudi, M.; Rasmussen, J.; Ayyash, D.; Zhou, Y.; et al. Tumor Microenvironment-Responsive Multistaged Nanoplatform for Systemic RNAi and Cancer Therapy. Nano Lett. 2017, 17, 4427–4435. [Google Scholar] [CrossRef]
- Basini, G.; Bianco, F.; Grasselli, F. Epigallocatechin-3-gallate from green tea negatively affects swine granulosa cell function. Domest. Anim. Endocrinol. 2005, 28, 243–256. [Google Scholar] [CrossRef]
- Basini, G.; Bianco, F.; Grasselli, F. EGCG, a major component of green tea, inhibits VEGF production by swine granulosa cells. Biofactors 2005, 23, 25–33. [Google Scholar] [CrossRef]
- Taleghani, A.; Nasseri, M.A.; Iranshahi, M. Synthesis of dual-action parthenolide prodrugs as potent anticancer agents. Bioorg. Chem. 2017, 71, 128–134. [Google Scholar] [CrossRef]
- Du, G.J.; Zhang, Z.; Wen, X.D.; Yu, C.; Calway, T.; Yuan, C.S.; Wang, C.Z. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Saw, P.E.; Park, J.; Lee, E.; Ahn, S.; Lee, J.; Kim, H.; Kim, J.; Choi, M.; Farokhzad, O.C.; Jon, S. Effect of PEG pairing on the efficiency of cancer-targeting liposomes. Theranostics 2015, 5, 746–754. [Google Scholar] [CrossRef] [Green Version]
- Maeda, H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J. Control. Release 2012, 164, 138–144. [Google Scholar] [CrossRef]
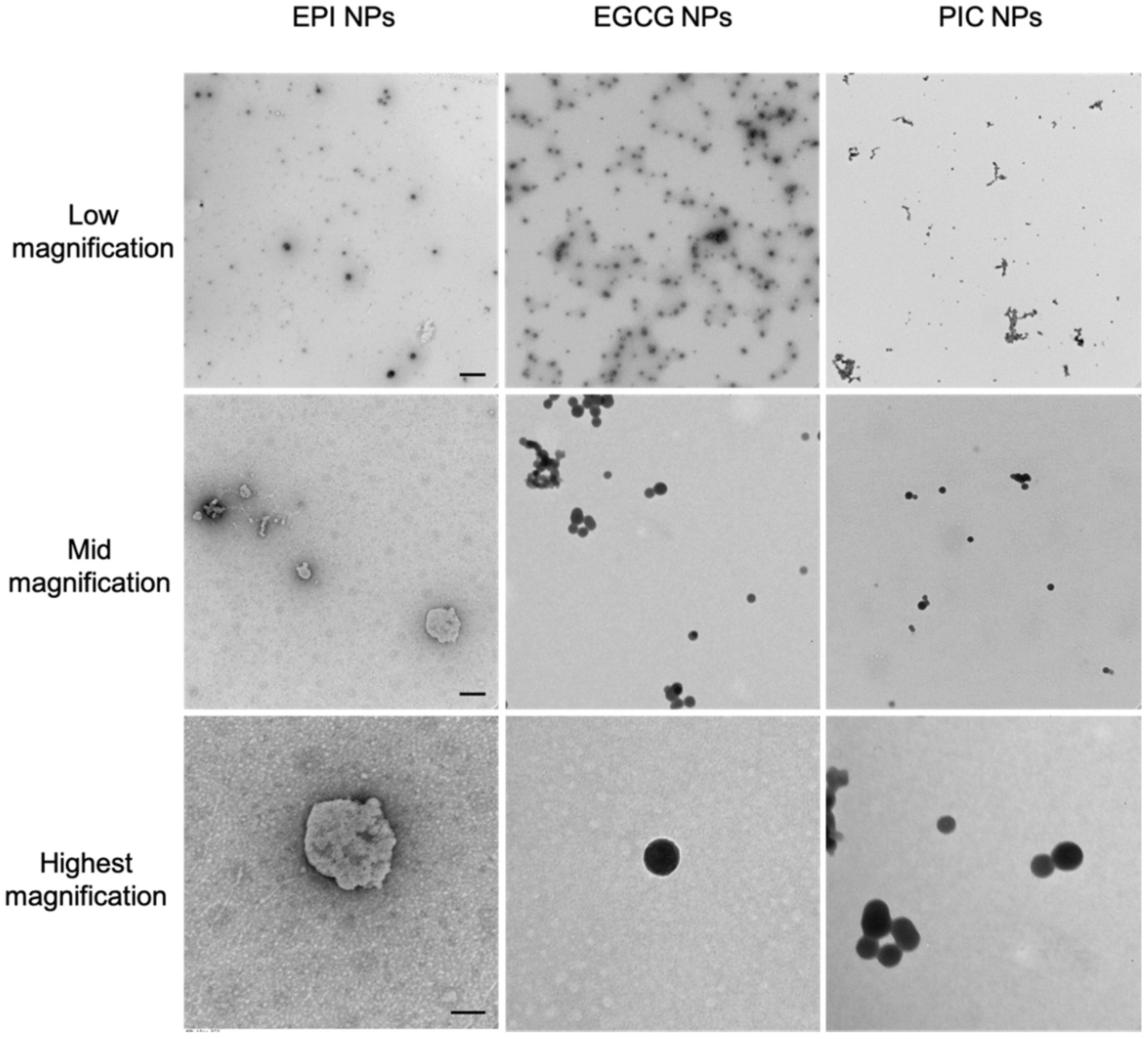



| NP | Run1 (nm) | Run2 (nm) | Run3 (nm) | Mean (nm) |
|---|---|---|---|---|
| EPI | 400 | 458 | 416.7 | 425.2 |
| EGCG | 473.5 | 464.9 | 456.5 | 465 |
| PIC | 302.5 | 306.1 | 297.1 | 301.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; Marek, L.; Liang, Y.; Saw, P.E. Transforming Tea Catechins into Potent Anticancer Compound: Analysis of Three Boronated-PEG Delivery System. Micromachines 2022, 13, 45. https://doi.org/10.3390/mi13010045
Guo M, Marek L, Liang Y, Saw PE. Transforming Tea Catechins into Potent Anticancer Compound: Analysis of Three Boronated-PEG Delivery System. Micromachines. 2022; 13(1):45. https://doi.org/10.3390/mi13010045
Chicago/Turabian StyleGuo, Mingyan, Lukas Marek, Yixia Liang, and Phei Er Saw. 2022. "Transforming Tea Catechins into Potent Anticancer Compound: Analysis of Three Boronated-PEG Delivery System" Micromachines 13, no. 1: 45. https://doi.org/10.3390/mi13010045
APA StyleGuo, M., Marek, L., Liang, Y., & Saw, P. E. (2022). Transforming Tea Catechins into Potent Anticancer Compound: Analysis of Three Boronated-PEG Delivery System. Micromachines, 13(1), 45. https://doi.org/10.3390/mi13010045






