Low-Cost In Vivo Full-Range Optical Coherence Tomography Using a Voice Coil Motor
Abstract
1. Introduction
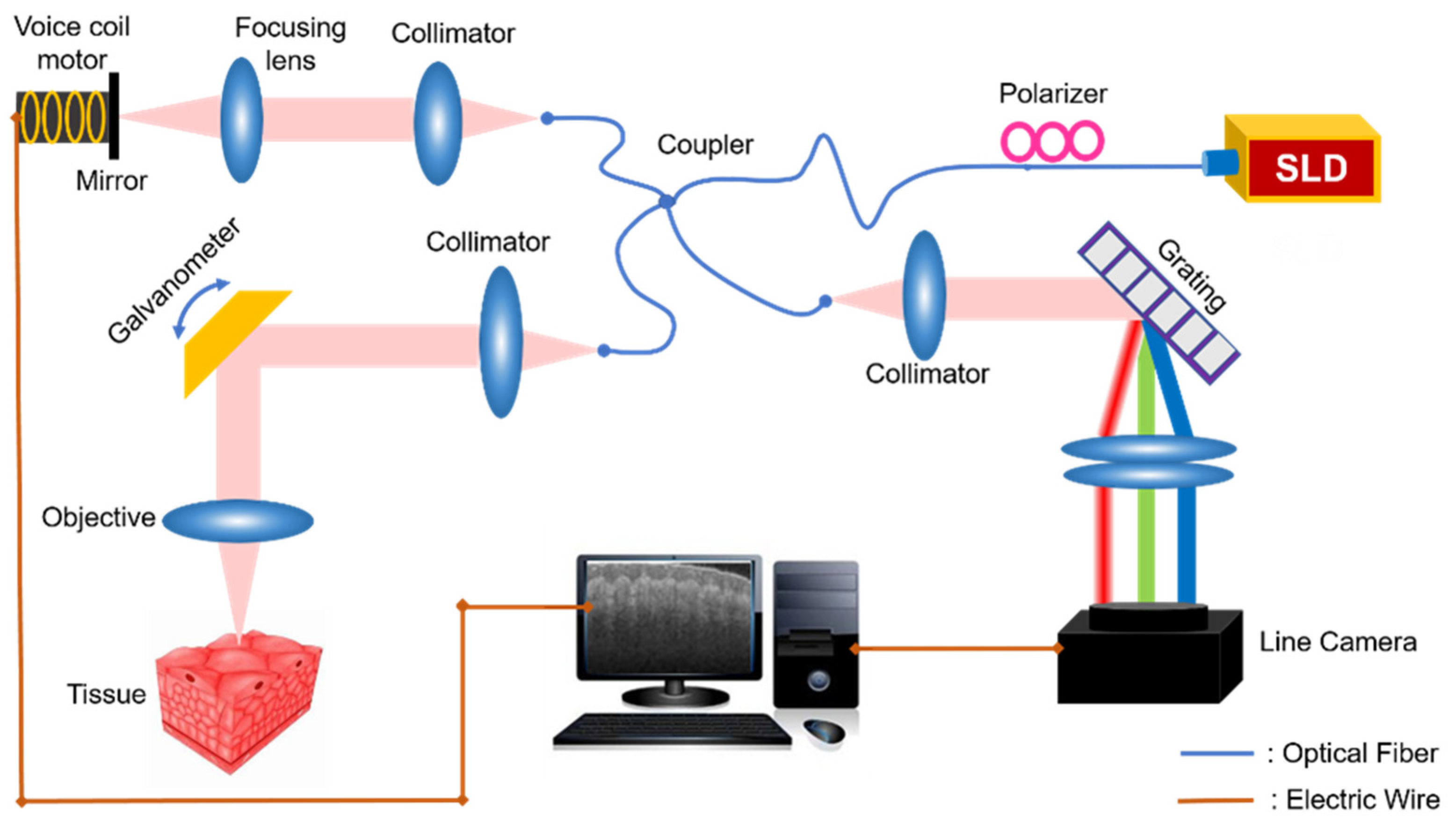
2. Experimental Setup
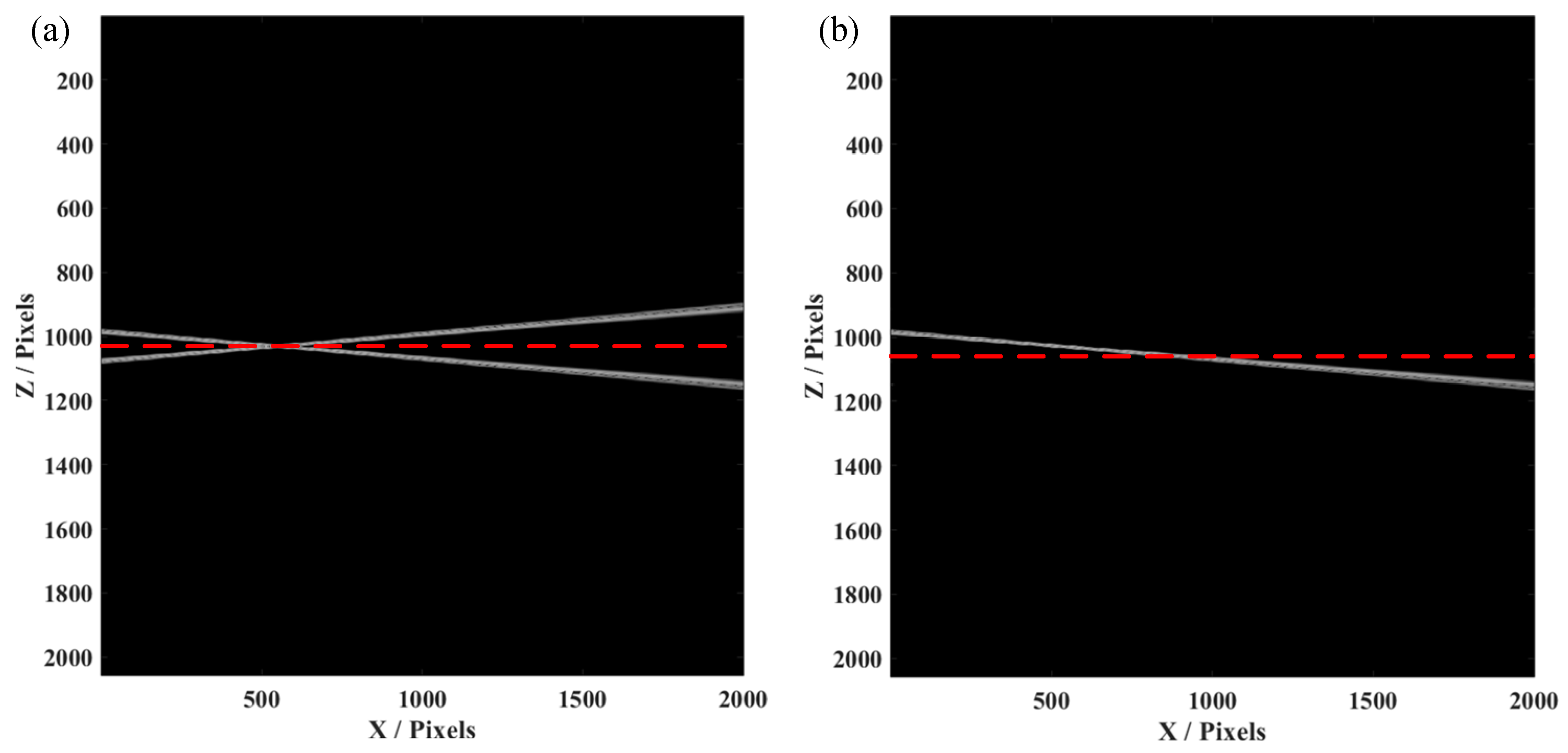
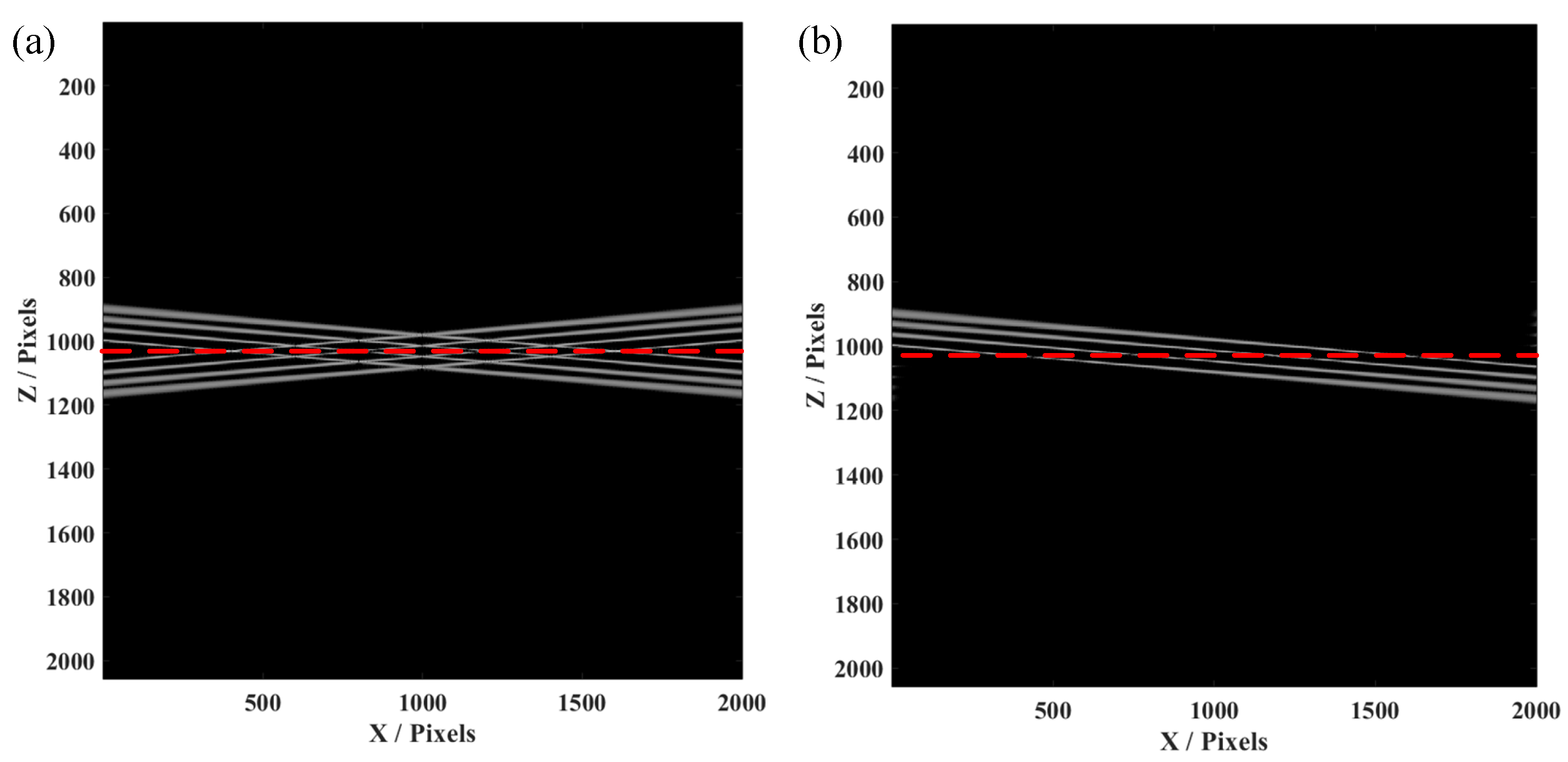
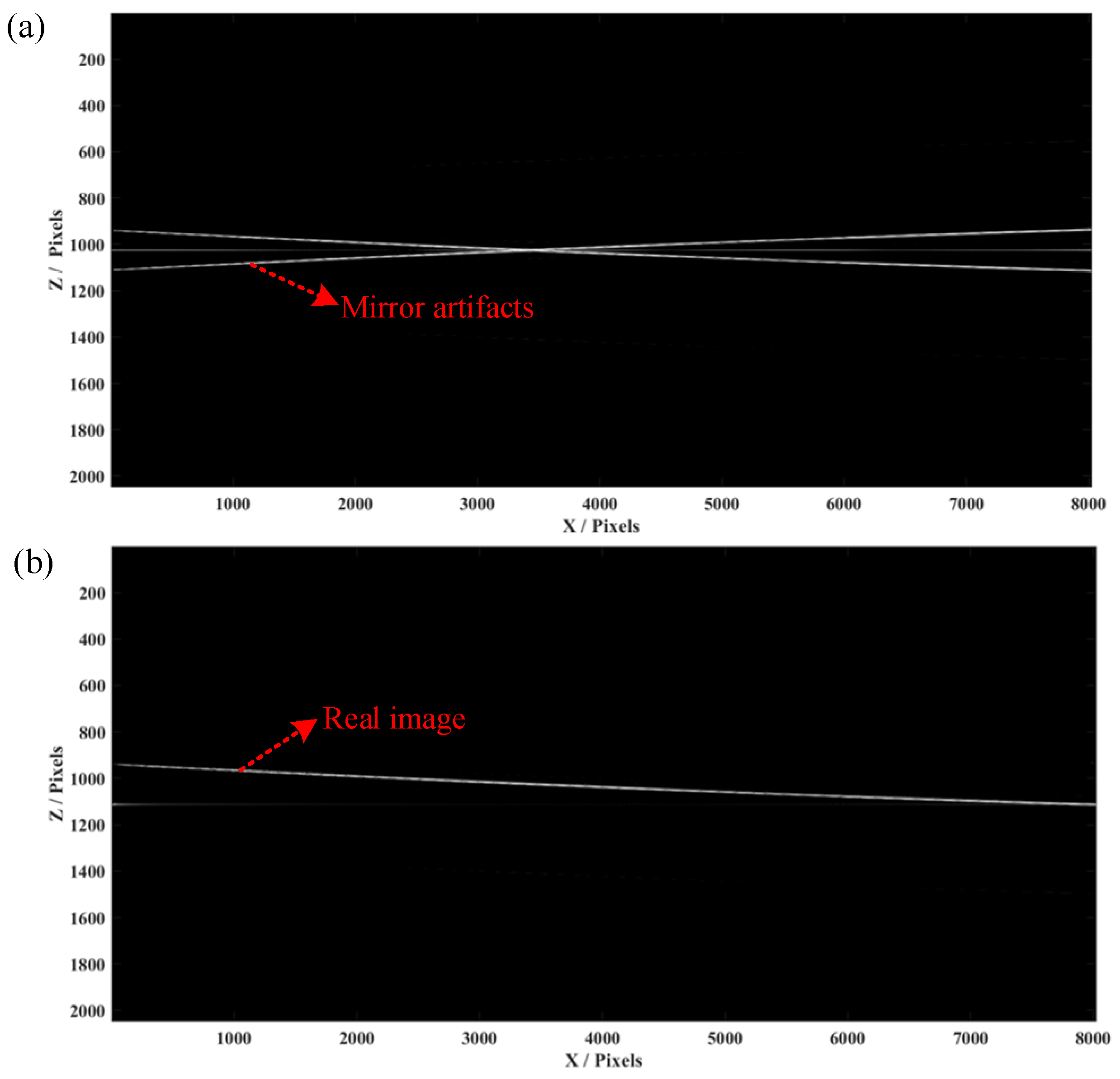
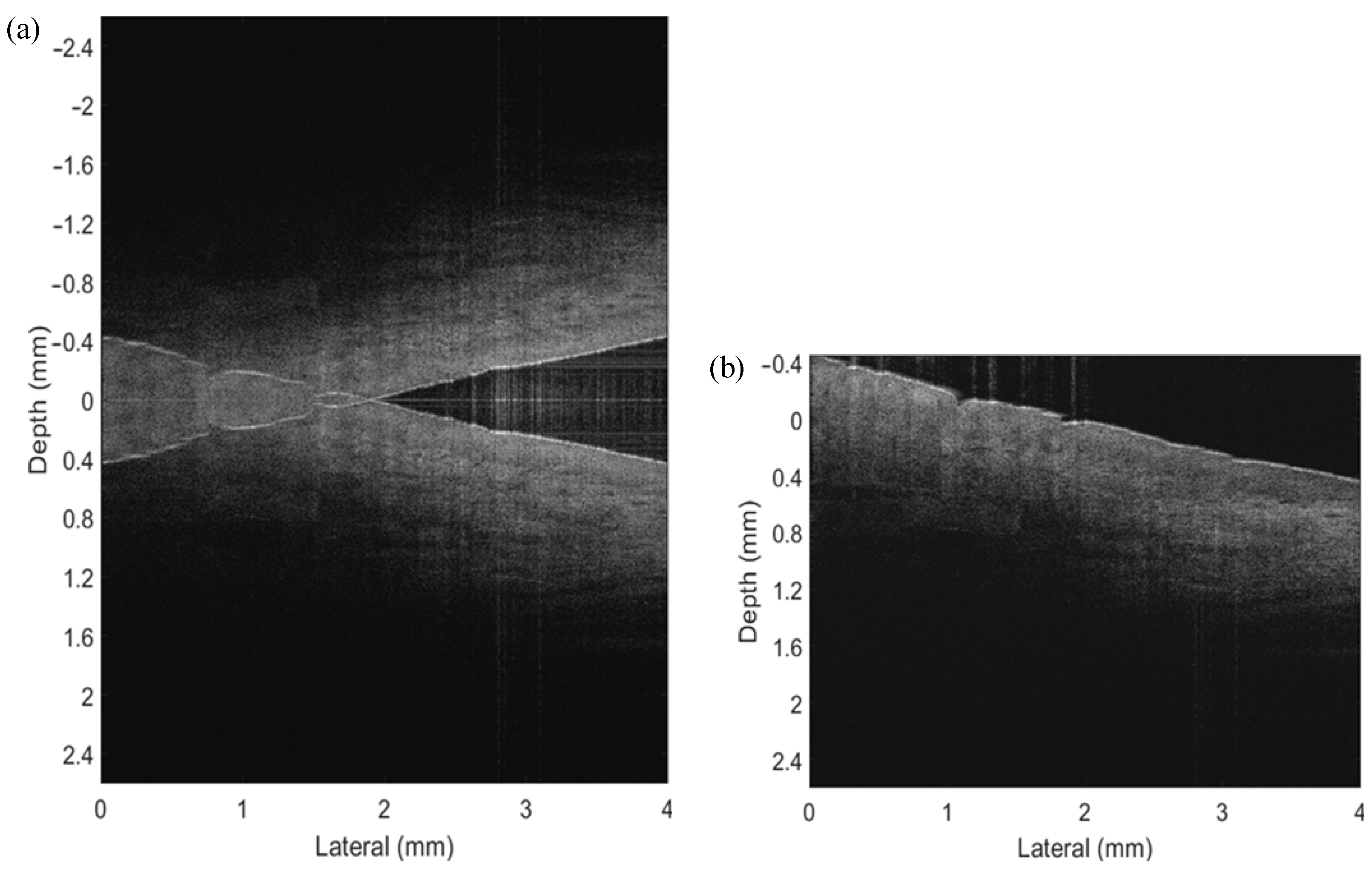
3. Simulation and Experimental Result
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical Coherence Tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- De Boer, J.F.; Cense, B.; Park, B.H.; Pierce, M.; Tearney, G.J.; Bouma, B.E. Improved signal-to-noise ratio in spectral-domain compared with time-domain optical coherence tomography. Opt. Lett. 2003, 28, 2067–2069. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-H.; Leggett, C.L.; Trindade, A.J.; Sethi, A.; Swager, A.-F.; Joshi, V.; Bergman, J.J.; Mashimo, H.; Nishioka, N.S.; Namati, E. Optical coherence tomography in gastroenterology: A review and future outlook. J. Biomed. Opt. 2017, 22, 121716. [Google Scholar] [CrossRef] [PubMed]
- Naseripour, M.; Ghasemi Falavarjani, K.; Mirshahi, R.; Sedaghat, A. Optical coherence tomography angiography (OCTA) applications in ocular oncology. Eye 2020, 34, 1535–1545. [Google Scholar] [CrossRef]
- Gil, C.J.; Tomov, M.L.; Theus, A.S.; Cetnar, A.; Mahmoudi, M.; Serpooshan, V. In Vivo Tracking of Tissue Engineered Constructs. Micromachines 2019, 10, 474. [Google Scholar] [CrossRef]
- Tsai, M.-T.; Huang, B.-H.; Yeh, C.-C.; Lei, K.F.; Tsang, N.-M. Non-Invasive Quantification of the Growth of Cancer Cell Colonies by a Portable Optical Coherence Tomography. Micromachines 2019, 10, 35. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, D.; Yoon, Y.K.; Xie, H. A Silicon Optical Bench-Based Forward-View Two-Axis Scanner for Microendoscopy Applications. Micromachines 2020, 11, 1051. [Google Scholar] [CrossRef]
- Leitgeb, R.A.; Schmetterer, L.; Hitzenberger, C.K.; Fercher, A.F.; Berisha, F.; Wojtkowski, M.; Bajraszewski, T. Real-time measurement of in vitro flow by Fourier-domain color Doppler optical coherence tomography. Opt. Lett. 2004, 29, 171–173. [Google Scholar] [CrossRef]
- Leitgeb, R.; Placzek, F.; Rank, E.; Krainz, L.; Haindl, R.; Li, Q.; Liu, M.; Andreana, M.; Unterhuber, A.; Schmoll, T.; et al. Enhanced medical diagnosis for dOCTors: A perspective of optical coherence tomography. J. Biomed. Opt. 2021, 26, 100601. [Google Scholar] [CrossRef]
- Lemmens, S.; Van Craenendonck, T.; Van Eijgen, J.; De Groef, L.; Bruffaerts, R.; De Jesus, D.A.; Charle, W.; Jayapala, M.; Sunaric-Mégevand, G.; Standaert, A.; et al. Combination of snapshot hyperspectral retinal imaging and optical coherence tomography to identify Alzheimer’s disease patients. Alzheimer’s Res. Ther. 2020, 12, 144. [Google Scholar] [CrossRef]
- Maheshwari, A.; Choma, M.A.; Izatt, J.A. Heterodyne swept-source optical coherence tomography for complete complex conjugate ambiguity removal. J. Biomed. Opt. 2005, 10, 064005. [Google Scholar] [CrossRef]
- Wojtkowski, M.; Kowalczyk, A.; Leitgeb, R.; Fercher, A.F. Full range complex spectral optical coherence tomography technique in eye imaging. Opt. Lett. 2002, 27, 1415–1417. [Google Scholar] [CrossRef] [PubMed]
- Fercher, A.F.; Drexler, W.; Hitzenberger, C.K.; Lasser, T. Optical coherence tomography—principles and applications. Rep. Prog. Phys. 2003, 66, 239–303. [Google Scholar] [CrossRef]
- Zhang, J.; Nelson, J.S.; Chen, Z. Removal of a mirror image and enhancement of the signal-to-noise ratio in Fourier-domain optical coherence tomography using an electro-optic phase modulator. Opt. Lett. 2005, 30, 147–149. [Google Scholar] [CrossRef]
- Aumann, S.; Donner, S.; Fischer, J.; Müller, F. Optical Coherence Tomography (OCT): Principle and Technical Realization. In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Springer: Cham, The Switzerland, 2019; pp. 59–85. ISBN 978-3-030-16638-0. [Google Scholar]
- Drexler, W.; Morgner, U.; Ghanta, R.K.; Kärtner, F.X.; Schuman, J.S.; Fujimoto, J.G. Ultrahigh-resolution ophthalmic optical coherence tomography. Nat. Med. 2001, 7, 502–507. [Google Scholar] [CrossRef]
- Husvogt, L.; Ploner, S.B.; Chen, S.; Stromer, D.; Schottenhamml, J.; Alibhai, A.Y.; Moult, E.; Waheed, N.K.; Fujimoto, J.G.; Maier, A. Maximum a posteriori signal recovery for optical coherence tomography angiography image generation and denoising. Biomed. Opt. Express 2020, 12, 55–68. [Google Scholar] [CrossRef]
- Choma, M.A.; Sarunic, M.V.; Yang, C.; Izatt, J.A. Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Opt. Express 2003, 11, 2183–2189. [Google Scholar] [CrossRef]
- Targowski, P.; Wojtkowski, M.; Kowalczyk, A.; Bajraszewski, T.; Szkulmowski, M.; Gorczynska, I. Complex spectral OCT in human eye imaging in vivo. Opt. Commun. 2004, 229, 79–84. [Google Scholar] [CrossRef]
- Bachmann, A.H.; Leitgeb, R.A.; Lasser, T. Heterodyne Fourier domain optical coherence tomography for full range probing with high axial resolution. Opt. Express 2006, 14, 1487–1496. [Google Scholar] [CrossRef]
- Leitgeb, R.A.; Hitzenberger, C.K.; Fercher, A.F.; Bajraszewski, T. Phase-shifting algorithm to achieve high-speed long-depth-range probing by frequency-domain optical coherence tomography. Opt. Lett. 2003, 28, 2201–2203. [Google Scholar] [CrossRef]
- Yasuno, Y.; Makita, S.; Endo, T.; Aoki, G.; Itoh, M.; Yatagai, T. Simultaneous B-M-mode scanning method for real-time full-range Fourier domain optical coherence tomography. Appl. Opt. 2006, 45, 1861–1865. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.K. In vivo full range complex Fourier domain optical coherence tomography. Appl. Phys. Lett. 2007, 90, 054103. [Google Scholar] [CrossRef]
- An, L.; Subhash, H.M.; Wang, R.K. Full range complex spectral domain optical coherence tomography for volumetric imaging at 47 000 A-scans per second. J. Opt. 2010, 12, 084003. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Wang, R.K. Use of a scanner to modulate spatial interferograms for in vivo full-range Fourier-domain optical coherence tomography. Opt. Lett. 2007, 32, 3423–3425. [Google Scholar] [CrossRef] [PubMed]
- Baumann, B.; Pircher, M.; Götzinger, E.; Hitzenberger, C.K. Full range complex spectral domain optical coherence tomography without additional phase shifters. Opt. Express 2007, 15, 13375–13387. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Maeno, S.; Aoshima, K.; Hasegawa, H.; Koseki, H. Real-time processing for full-range Fourier-domain optical-coherence tomography with zero-filling interpolation using multiple graphic processing units. Appl. Opt. 2010, 49, 4756–4762. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, X.; He, L.; Duan, Z.; Tian, P.; He, Y.; Deng, Q.; Ma, Z.; Song, R.; Wu, L. Low-Cost In Vivo Full-Range Optical Coherence Tomography Using a Voice Coil Motor. Micromachines 2022, 13, 1626. https://doi.org/10.3390/mi13101626
Liao X, He L, Duan Z, Tian P, He Y, Deng Q, Ma Z, Song R, Wu L. Low-Cost In Vivo Full-Range Optical Coherence Tomography Using a Voice Coil Motor. Micromachines. 2022; 13(10):1626. https://doi.org/10.3390/mi13101626
Chicago/Turabian StyleLiao, Xiaoqiao, Liang He, Zhao Duan, Peng Tian, Yu He, Qinyuan Deng, Zeyu Ma, Ruiqi Song, and Leixin Wu. 2022. "Low-Cost In Vivo Full-Range Optical Coherence Tomography Using a Voice Coil Motor" Micromachines 13, no. 10: 1626. https://doi.org/10.3390/mi13101626
APA StyleLiao, X., He, L., Duan, Z., Tian, P., He, Y., Deng, Q., Ma, Z., Song, R., & Wu, L. (2022). Low-Cost In Vivo Full-Range Optical Coherence Tomography Using a Voice Coil Motor. Micromachines, 13(10), 1626. https://doi.org/10.3390/mi13101626




