A High-Throughput and Uniform Amplification Method for Cell Spheroids
Abstract
:1. Introduction
2. Materials and Methods
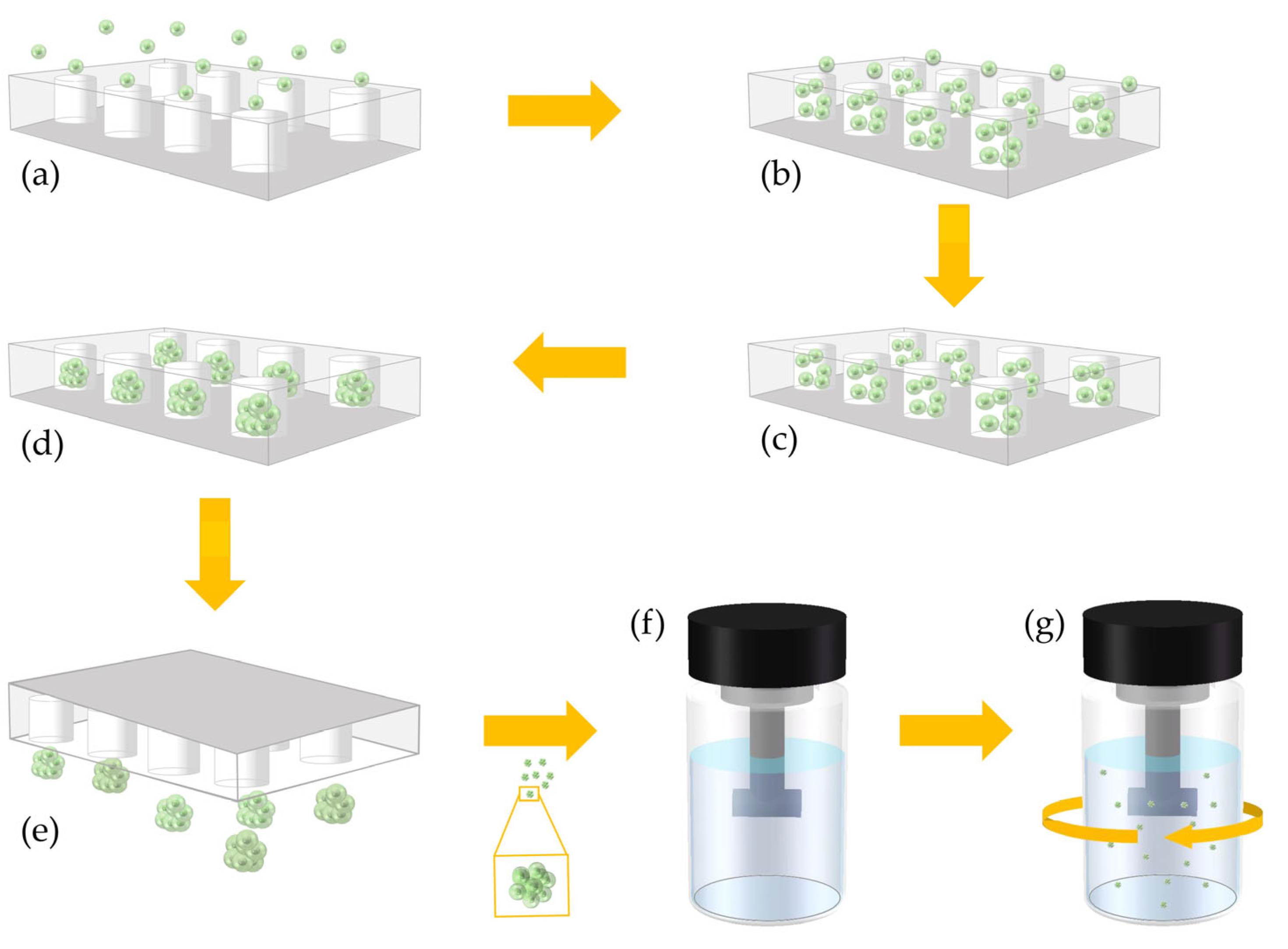
2.1. Preparation of Microwell Chip and Stirring Culture Flask
2.1.1. Materials
2.1.2. Preparation of Microwell Chips
2.1.3. Preparation of Stirring Culture Flask
2.2. Cell Culture
2.3. Cell Spheroids Formation
2.4. Cell Viability Test
3. Results
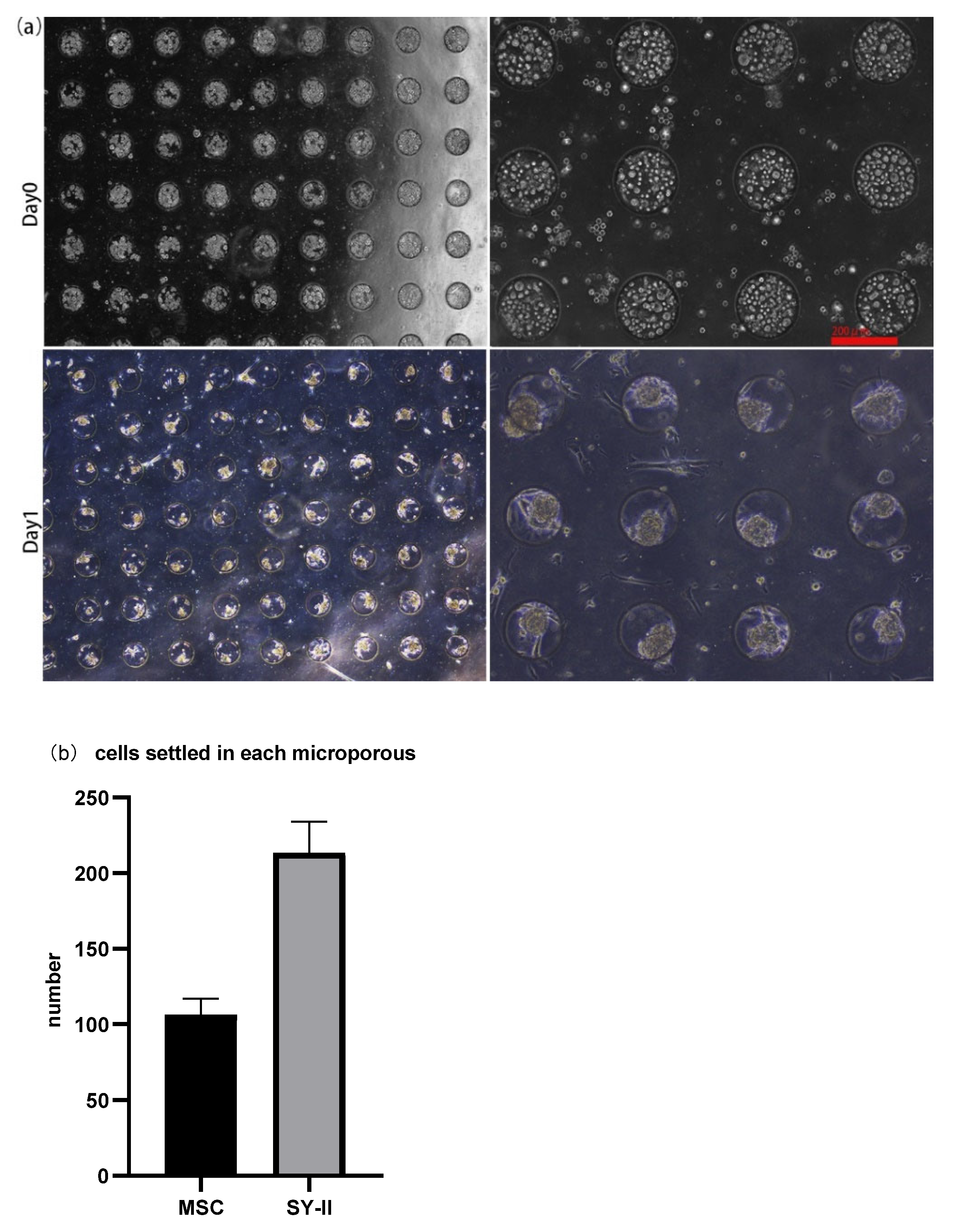
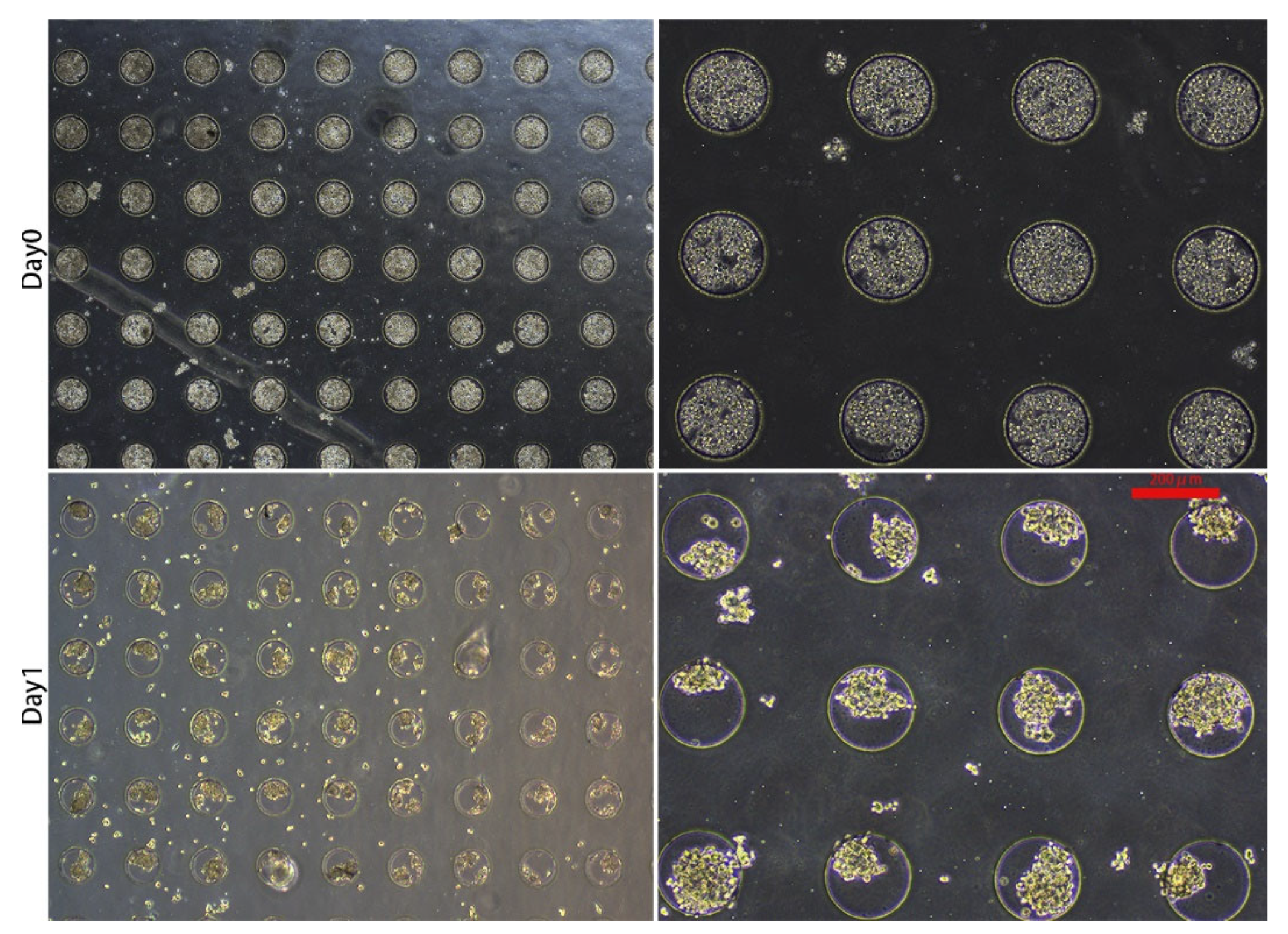
3.1. 3D Spheroid Formation and Maintenance
3.2. Cell Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neftel, C.; Laffy, J.; Filbin, M.G.; Hara, T.; Shore, M.E.; Rahme, G.J.; Richman, A.R.; Silverbush, D.; Shaw, M.L.; Hebert, C.M.; et al. An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell 2019, 178, 835–849.e21. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Komai, K.; Mise-Omata, S.; Iizuka-Koga, M.; Noguchi, Y.; Kondo, T.; Sakai, R.; Matsuo, K.; Nakayama, T.; Yoshie, O.; et al. Brain Regulatory T Cells Suppress Astrogliosis and Potentiate Neurological Recovery. Nature 2019, 565, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Muri, J.; Heer, S.; Matsushita, M.; Pohlmeier, L.; Tortola, L.; Fuhrer, T.; Conrad, M.; Zamboni, N.; Kisielow, J.; Kopf, M. The Thioredoxin-1 System Is Essential for Fueling DNA Synthesis during T-Cell Metabolic Reprogramming and Proliferation. Nat. Commun. 2018, 9, 1851. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, Y.; Li, Q.; Liu, K.; Hou, J.; Shao, C.; Wang, Y. Immunoregulatory Mechanisms of Mesenchymal Stem and Stromal Cells in Inflammatory Diseases. Nat. Rev. Nephrol. 2018, 14, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [PubMed]
- Mirbagheri, M.; Adibnia, V.; Hughes, B.R.; Waldman, S.D.; Banquy, X.; Hwang, D.K. Advanced Cell Culture Platforms: A Growing Quest for Emulating Natural Tissues. Mater. Horiz. 2019, 6, 45–71. [Google Scholar] [CrossRef]
- Breslin, S.; O’Driscoll, L. Three-Dimensional Cell Culture: The Missing Link in Drug Discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Xu, Z.; Yu, Y.; Hou, S.; Geng, J.; Xiao, T.; Liang, Y.; Dong, Q.; Mei, Y.; Wang, B.; et al. Single Cell Derived Spheres of Umbilical Cord Mesenchymal Stem Cells Enhance Cell Stemness Properties, Survival Ability and Therapeutic Potential on Liver Failure. Biomaterials 2020, 227, 119573. [Google Scholar] [CrossRef]
- Von Der Mark, K.; Gauss, V.; Von Der Mark, H.; Müller, P. Relationship between Cell Shape and Type of Collagen Synthesised as Chondrocytes Lose Their Cartilage Phenotype in Culture. Nature 1977, 267, 531–532. [Google Scholar] [CrossRef]
- Li, S.; Yang, K.; Chen, X.; Zhu, X.; Zhou, H.; Li, P.; Chen, Y.; Jiang, Y.; Li, T.; Qin, X.; et al. Simultaneous 2D and 3D Cell Culture Array for Multicellular Geometry, Drug Discovery and Tumor Microenvironment Reconstruction. Biofabrication 2021, 13, 045013. [Google Scholar] [CrossRef]
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-Dimensional Cell Culture Systems as an in Vitro Platform for Cancer and Stem Cell Modeling. World J. Stem Cells 2019, 11, 1065–1083. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Yin, Y.; Zheng, Y.; Ma, Y.; Li, Y.; Zhao, Z.; Guo, J.; Ai, Z.; Niu, Y.; Duan, K.; et al. A Developmental Landscape of 3D-Cultured Human Pre-Gastrulation Embryos. Nature 2019, 577, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Courau, T.; Bonnereau, J.; Chicoteau, J.; Bottois, H.; Remark, R.; Assante Miranda, L.; Toubert, A.; Blery, M.; Aparicio, T.; Allez, M.; et al. Cocultures of Human Colorectal Tumor Spheroids with Immune Cells Reveal the Therapeutic Potential of MICA/B and NKG2A Targeting for Cancer Treatment. J. Immunother. Cancer 2019, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Gehart, H.; Artegiani, B.; LÖpez-Iglesias, C.; Dekkers, F.; Basak, O.; van Es, J.; Chuva de Sousa Lopes, S.M.; Begthel, H.; Korving, J.; et al. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell 2018, 175, 1591–1606.e19. [Google Scholar] [CrossRef]
- Fontoura, J.C.; Viezzer, C.; dos Santos, F.G.; Ligabue, R.A.; Weinlich, R.; Puga, R.D.; Antonow, D.; Severino, P.; Bonorino, C. Comparison of 2D and 3D Cell Culture Models for Cell Growth, Gene Expression and Drug Resistance. Mater. Sci. Eng. C 2020, 107, 110264. [Google Scholar] [CrossRef]
- Alicia Boos, J.; Mark Misun, P.; Michlmayr, A.; Hierlemann, A.; Frey, O.; Boos, J.A.; Misun, P.M.; Michlmayr, A.; Hierlemann, A.; Frey, O. Microfluidic Multitissue Platform for Advanced Embryotoxicity Testing In Vitro. Adv. Sci. 2019, 6, 1900294. [Google Scholar] [CrossRef]
- Frey, O.; Misun, P.M.; Fluri, D.A.; Hengstler, J.G.; Hierlemann, A. Reconfigurable Microfluidic Hanging Drop Network for Multi-Tissue Interaction and Analysis. Nat. Commun. 2014, 5, 4250. [Google Scholar] [CrossRef]
- Wang, B.; Liu, W.; Li, J.J.; Chai, S.; Xing, D.; Yu, H.; Zhang, Y.; Yan, W.; Xu, Z.; Zhao, B.; et al. A Low Dose Cell Therapy System for Treating Osteoarthritis: In Vivo Study and In Vitro Mechanistic Investigations. Bioact. Mater. 2022, 7, 478–490. [Google Scholar] [CrossRef]
- Gava, F.; Faria, C.; Gravelle, P.; Valero, J.G.; Dobaño-López, C.; Morin, R.; Norlund, M.; Gomes, A.; Lagarde, J.M.; Rossi, C.; et al. 3D Model Characterization by 2D and 3D Imaging in t(14;18)-Positive B-NHL: Perspectives for In Vitro Drug Screens in Follicular Lymphoma. Cancers 2021, 13, 1490. [Google Scholar] [CrossRef]
- Lück, S.; Schubel, R.; Rüb, J.; Hahn, D.; Mathieu, E.; Zimmermann, H.; Scharnweber, D.; Werner, C.; Pautot, S.; Jordan, R. Tailored and Biodegradable Poly(2-Oxazoline) Microbeads as 3D Matrices for Stem Cell Culture in Regenerative Therapies. Biomaterials 2016, 79, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gill, E.L.; Willis, S.; Gerigk, M.; Cohen, P.; Zhang, D.; Li, X.; Huang, Y.Y.S. Fabrication of Designable and Suspended Microfibers via Low-Voltage 3D Micropatterning. ACS Appl. Mater. Interfaces 2019, 11, 19679–19690. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, H.; Song, J.; Zhang, T.; Wang, X.; Huang, X.; Zheng, C. A Novel Simpledrop Chip for 3d Spheroid Formation and Anti-Cancer Drug Assay. Micromachines 2021, 12, 681. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Z.; Dong, D.L.; Jang, T.S.; Knowles, J.C.; Kim, H.W.; Jin, G.Z.; Xuan, Y. 3D Culture Technologies of Cancer Stem Cells: Promising Ex Vivo Tumor Models. J. Tissue Eng. 2020, 11, 2041731420933407. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.W.; Alhasan, L.; Rezk, A.R.; Al-Abboodi, A.; Doran, P.M.; Yeo, L.Y.; Chan, P.P.Y. Fast Three-Dimensional Micropatterning of PC12 Cells in Rapidly Crosslinked Hydrogel Scaffolds Using Ultrasonic Standing Waves. Biofabrication 2019, 12, 015013. [Google Scholar] [CrossRef] [PubMed]
- Mary, G.; Van De Walle, A.; Perez, J.E.; Ukai, T.; Maekawa, T.; Luciani, N.; Wilhelm, C.; Mary, G.; Van De Walle, A.; Perez, J.E.; et al. High-Throughput Differentiation of Embryonic Stem Cells into Cardiomyocytes with a Microfabricated Magnetic Pattern and Cyclic Stimulation. Adv. Funct. Mater. 2020, 30, 2002541. [Google Scholar] [CrossRef]
- Zhao, L.; Xiu, J.; Liu, Y.; Zhang, T.; Pan, W.; Zheng, X.; Zhang, X. A 3D Printed Hanging Drop Dripper for Tumor Spheroids Analysis Without Recovery. Sci. Rep. 2019, 9, 19717. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Chuah, Y.J.; Ong, H.B.; Tay, H.M.; Dalan, R.; Hou, H.W. A Facile and Scalable Hydrogel Patterning Method for Microfluidic 3D Cell Culture and Spheroid-in-Gel Culture Array. Biosensors 2021, 11, 509. [Google Scholar] [CrossRef]
- Shao, C.; Liu, Y.; Chi, J.; Ye, F.; Zhao, Y. Hierarchically Inverse Opal Porous Scaffolds from Droplet Microfluidics for Biomimetic 3D Cell Co-Culture. Engineering 2021, 7, 1778–1785. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lou, X.; Zhang, Z.; Ingram, P.; Yoon, E. High-Throughput Cancer Cell Sphere Formation for Characterizing the Efficacy of Photo Dynamic Therapy in 3D Cell Cultures. Sci. Rep. 2015, 5, 12175. [Google Scholar] [CrossRef] [Green Version]
- Patra, B.; Peng, C.C.; Liao, W.H.; Lee, C.H.; Tung, Y.C. Drug Testing and Flow Cytometry Analysis on a Large Number of Uniform Sized Tumor Spheroids Using a Microfluidic Device. Sci. Rep. 2016, 6, 21061. [Google Scholar] [CrossRef] [PubMed]
- Grimes, D.R.; Currell, F.J. Oxygen Diffusion in Ellipsoidal Tumour Spheroids. J. R. Soc. Interface 2018, 15, 20180256. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Liu, H.; Huang, X.; Liu, X.; Zheng, C. A High-Throughput and Uniform Amplification Method for Cell Spheroids. Micromachines 2022, 13, 1645. https://doi.org/10.3390/mi13101645
Liu L, Liu H, Huang X, Liu X, Zheng C. A High-Throughput and Uniform Amplification Method for Cell Spheroids. Micromachines. 2022; 13(10):1645. https://doi.org/10.3390/mi13101645
Chicago/Turabian StyleLiu, Liyuan, Haixia Liu, Xiaowen Huang, Xiaoli Liu, and Chengyun Zheng. 2022. "A High-Throughput and Uniform Amplification Method for Cell Spheroids" Micromachines 13, no. 10: 1645. https://doi.org/10.3390/mi13101645





