A Digital Microfluidic RT-qPCR Platform for Multiple Detections of Respiratory Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. RNA Extraction
2.3. Primer and Probe Sequences
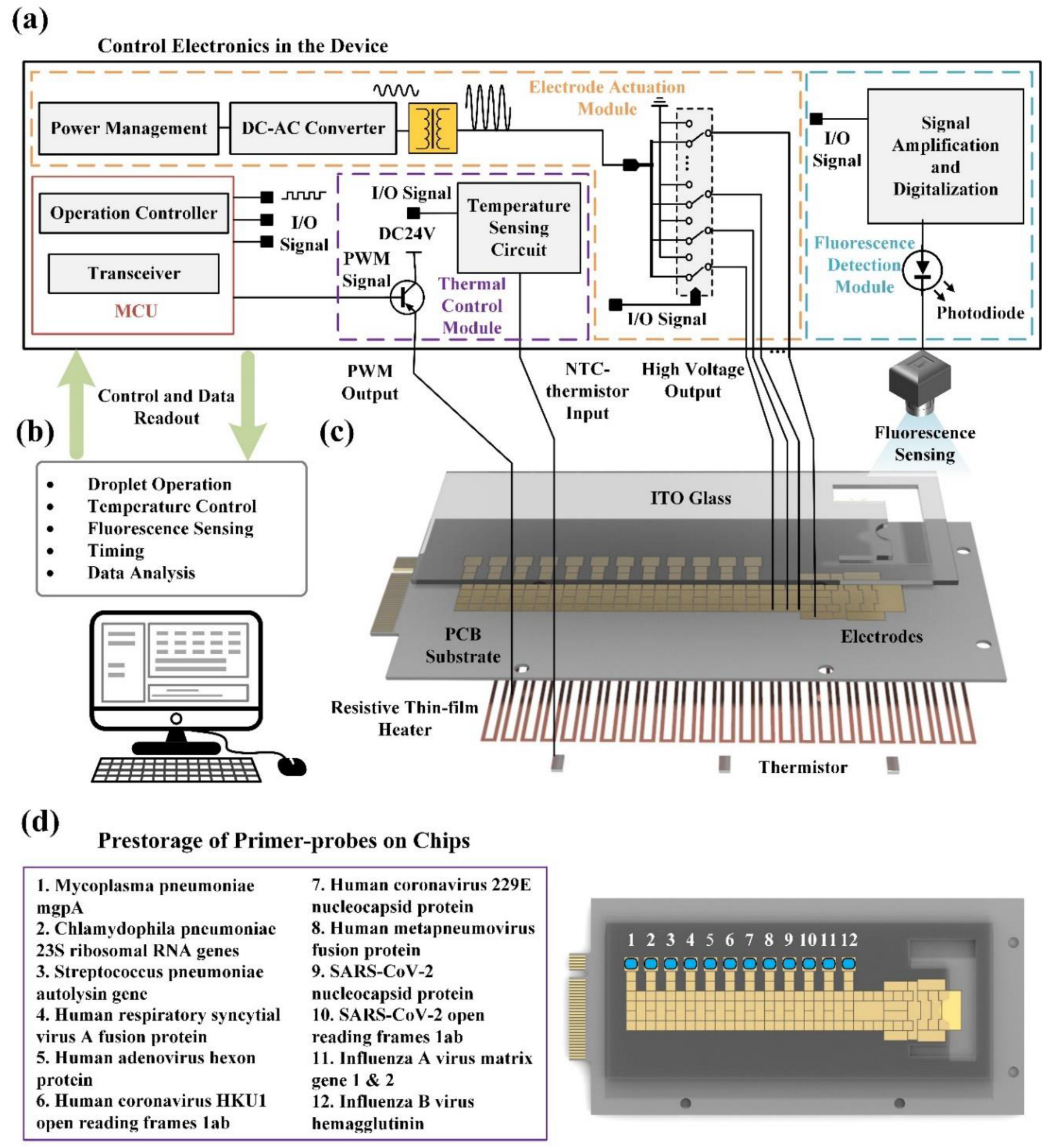
2.4. System Configuration
2.5. RT-qPCR Off-Chip
2.6. Prestorage of Primer-Probes On-Chips
2.7. RT-qPCR On-Chip
2.8. Data Analysis
3. Results
3.1. Optimization of RT-qPCR Reaction
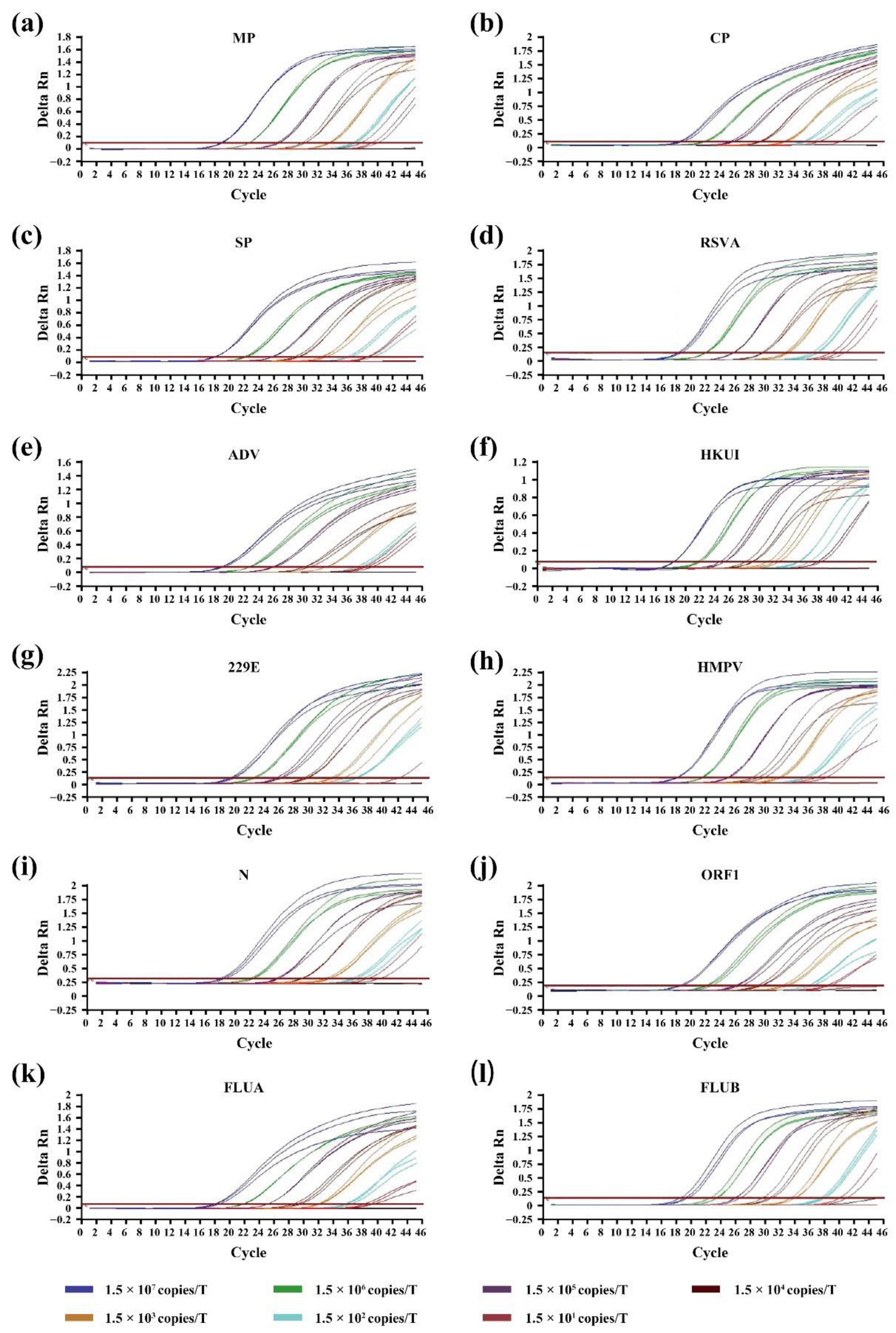
3.2. Sensitivity of RT-qPCR On-Chip and Off-Chip
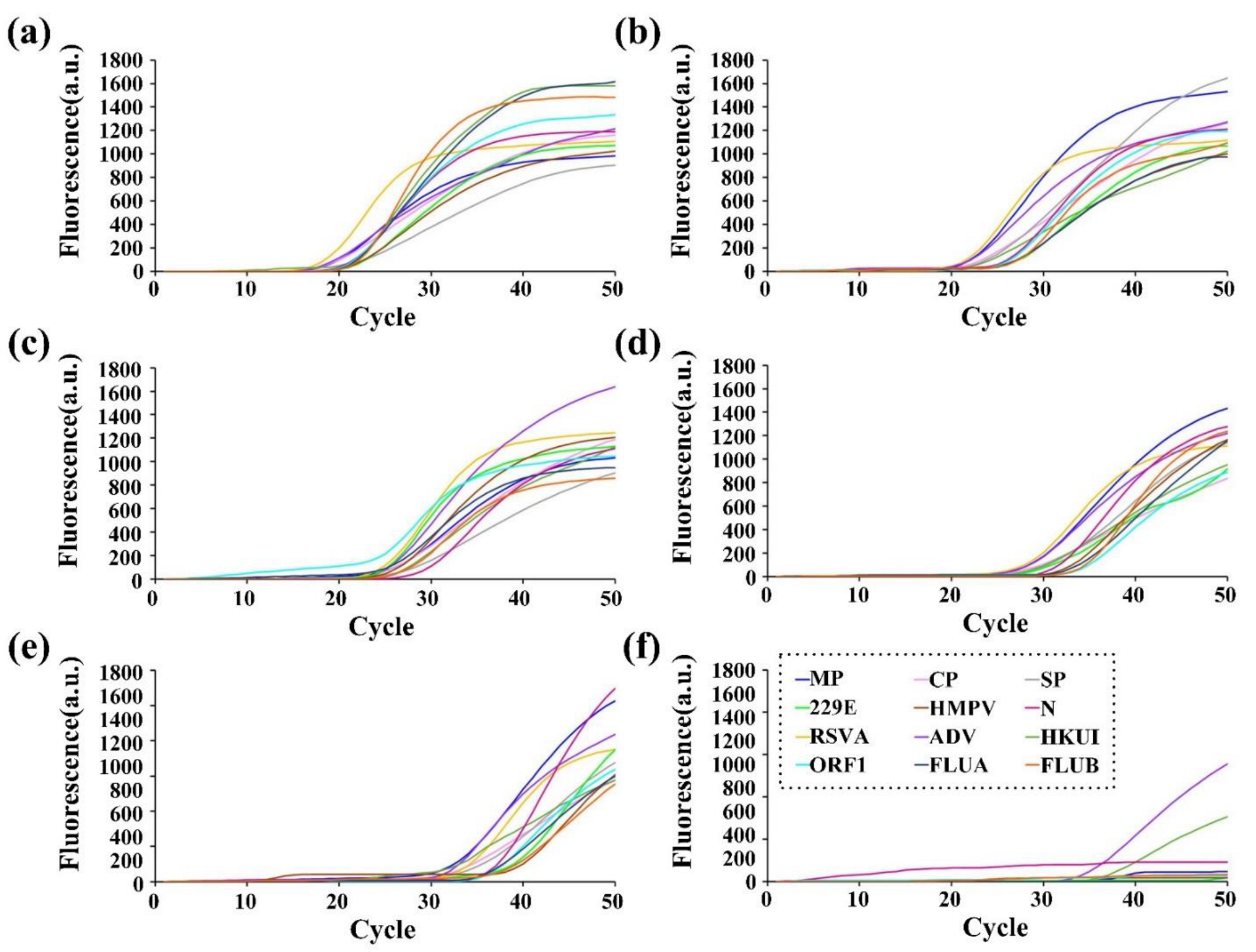
3.3. Specificity of RT-qPCR On-Chip
3.4. Evaluation of On-Chip RT-qPCR Assays Using Clinical Samples
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beck, E.T.; Henrickson, K.J. Molecular diagnosis of respiratory viruses. Future Microbiol. 2010, 5, 901–916. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.J.; Loddenkemper, R.; Lundbäck, B.; Sibille, Y. Respiratory health and disease in Europe: The new European Lung White Book. Eur. Respir. J. 2013, 42, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Kumar, B.K.; Deekshit, V.K.; Karunasagar, I.; Karunasagar, I. Detection technologies and recent developments in the diagnosis of COVID-19 infection. Appl. Microbiol. Biotechnol. 2021, 105, 441–455. [Google Scholar] [CrossRef]
- Babady, N.E.; Mead, P.; Stiles, J.; Brennan, C.; Li, H.; Shuptar, S.; Stratton, C.W.; Tang, Y.W.; Kamboj, M. Comparison of the Luminex xTAG RVP Fast assay and the Idaho Technology FilmArray RP assay for detection of respiratory viruses in pediatric patients at a cancer hospital. J. Clin. Microbiol. 2012, 50, 2282–2288. [Google Scholar] [CrossRef] [PubMed]
- Salez, N.; Vabret, A.; Leruez-Ville, M.; Andreoletti, L.; Carrat, F.; Renois, F.; de Lamballerie, X. Evaluation of Four Commercial Multiplex Molecular Tests for the Diagnosis of Acute Respiratory Infections. PLoS ONE 2015, 10, e0130378. [Google Scholar] [CrossRef]
- Pérez-Ruiz, M.; Pedrosa-Corral, I.; Sanbonmatsu-Gámez, S.; Navarro-Marí, M. Laboratory detection of respiratory viruses by automated techniques. Open Virol. J. 2012, 6, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Ginocchio, C.C. Detection of respiratory viruses using non-molecular based methods. J. Clin. Virol. Off. Publ. Pan. Am. Soc. Clin. Virol. 2007, 40, S11–S14. [Google Scholar] [CrossRef]
- Kehl, S.C.; Kumar, S. Utilization of nucleic acid amplification assays for the detection of respiratory viruses. Clin. Lab. Med. 2009, 29, 661–671. [Google Scholar] [CrossRef]
- Hymas, W.C.; Mills, A.; Ferguson, S.; Langer, J.; She, R.C.; Mahoney, W.; Hillyard, D.R. Development of a multiplex real-time RT-PCR assay for detection of influenza A, influenza B, RSV and typing of the 2009-H1N1 influenza virus. J. Virol. Methods 2010, 167, 113–118. [Google Scholar] [CrossRef]
- Rogan, D.T.; Kochar, M.S.; Yang, S.; Quinn, J.V. Impact of Rapid Molecular Respiratory Virus Testing on Real-Time Decision Making in a Pediatric Emergency Department. J. Mol. Diagn. JMD 2017, 19, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Rappo, U.; Schuetz, A.N.; Jenkins, S.G.; Calfee, D.P.; Walsh, T.J.; Wells, M.T.; Hollenberg, J.P.; Glesby, M.J. Impact of Early Detection of Respiratory Viruses by Multiplex PCR Assay on Clinical Outcomes in Adult Patients. J. Clin. Microbiol. 2016, 54, 2096–2103. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Kaplan, S.E.; Lopez, J.C.; Stiles, J.; Lu, X.; Tang, Y.W. Parallel Validation of Three Molecular Devices for Simultaneous Detection and Identification of Influenza A and B and Respiratory Syncytial Viruses. J. Clin. Microbiol. 2018, 56, e01691-17. [Google Scholar] [CrossRef]
- Park, B.H.; Oh, S.J.; Jung, J.H.; Choi, G.; Seo, J.H.; Kim, D.H.; Lee, E.Y.; Seo, T.S. An integrated rotary microfluidic system with DNA extraction, loop-mediated isothermal amplification, and lateral flow strip based detection for point-of-care pathogen diagnostics. Biosens. Bioelectron. 2017, 91, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Sayad, A.; Ibrahim, F.; Mukim Uddin, S.; Cho, J.; Madou, M.; Thong, K.L. A microdevice for rapid, monoplex and colorimetric detection of foodborne pathogens using a centrifugal microfluidic platform. Biosens. Bioelectron. 2018, 100, 96–104. [Google Scholar] [CrossRef]
- Choi, K.; Ng, A.H.; Fobel, R.; Wheeler, A.R. Digital microfluidics. Annu. Rev. Anal. Chem. 2012, 5, 413–440. [Google Scholar] [CrossRef] [PubMed]
- Janasek, D.; Franzke, J.; Manz, A. Scaling and the design of miniaturized chemical-analysis systems. Nature 2006, 442, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Kirby, A.E.; Wheeler, A.R. Digital microfluidics: An emerging sample preparation platform for mass spectrometry. Anal. Chem. 2013, 85, 6178–6184. [Google Scholar] [CrossRef]
- Bruijns, B.; van Asten, A.; Tiggelaar, R.; Gardeniers, H. Microfluidic Devices for Forensic DNA Analysis: A Review. Biosensors 2016, 6, 41. [Google Scholar] [CrossRef]
- Kanagal-Shamanna, R. Digital PCR: Principles and Applications. Methods Mol. Biol. 2016, 1392, 43–50. [Google Scholar] [CrossRef]
- Sista, R.S.; Eckhardt, A.E.; Wang, T.; Graham, C.; Rouse, J.L.; Norton, S.M.; Srinivasan, V.; Pollack, M.G.; Tolun, A.A.; Bali, D.; et al. Digital microfluidic platform for multiplexing enzyme assays: Implications for lysosomal storage disease screening in newborns. Clin. Chem. 2011, 57, 1444–1451. [Google Scholar] [CrossRef]
- Ng, A.H.; Lee, M.; Choi, K.; Fischer, A.T.; Robinson, J.M.; Wheeler, A.R. Digital microfluidic platform for the detection of rubella infection and immunity: A proof of concept. Clin. Chem. 2015, 61, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Wulff-Burchfield, E.; Schell, W.A.; Eckhardt, A.E.; Pollack, M.G.; Hua, Z.; Rouse, J.L.; Pamula, V.K.; Srinivasan, V.; Benton, J.L.; Alexander, B.D.; et al. Microfluidic platform versus conventional real-time polymerase chain reaction for the detection of Mycoplasma pneumoniae in respiratory specimens. Diagn. Microbiol. Infect. Dis. 2010, 67, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Center, C.N.l. Technical Guidelines for National Influenza Surveillance. Available online: http://ivdc.chinacdc.cn/cnic/zyzx/jcfa/201709/P020170930331067634607.pdf (accessed on 30 March 2017). (In Chinese).
- China, N.o.P.R. Technical guidance for laboratory testing of 2019-nCoV infection (Third Edition). Biosaf. Health 2020, 2, 3–5. [Google Scholar] [CrossRef]
- Liu, J.; Kabir, F.; Manneh, J.; Lertsethtakarn, P.; Begum, S.; Gratz, J.; Becker, S.M.; Operario, D.J.; Taniuchi, M.; Janaki, L.; et al. Development and assessment of molecular diagnostic tests for 15 enteropathogens causing childhood diarrhoea: A multicentre study. Lancet. Infect. Dis. 2014, 14, 716–724. [Google Scholar] [CrossRef]
- Tarim, E.A.; Karakuzu, B.; Oksuz, C.; Sarigil, O.; Kizilkaya, M.; Al-Ruweidi, M.; Yalcin, H.C.; Ozcivici, E.; Tekin, H.C. Microfluidic-based virus detection methods for respiratory diseases. Emergent. Mater. 2021, 4, 143–168. [Google Scholar] [CrossRef] [PubMed]
- Gradisteanu Pircalabioru, G.; Iliescu, F.S.; Mihaescu, G.; Cucu, A.I.; Ionescu, O.N.; Popescu, M.; Simion, M.; Burlibasa, L.; Tica, M.; Chifiriuc, M.C.; et al. Advances in the Rapid Diagnostic of Viral Respiratory Tract Infections. Front. Cell. Infect. Microbiol. 2022, 12, 807253. [Google Scholar] [CrossRef]
- Basiri, A.; Heidari, A.; Nadi, M.F.; Fallahy, M.T.P.; Nezamabadi, S.S.; Sedighi, M.; Saghazadeh, A.; Rezaei, N. Microfluidic devices for detection of RNA viruses. Rev. Med. Virol. 2021, 31, 1–11. [Google Scholar] [CrossRef]

| Spot | Target Region | Primer Source | Label | Sequence |
|---|---|---|---|---|
| 1 | Mycoplasma pneumoniae mgpA | designed in Primer Premier 5 | MP-FP | AGAATCTACCCTTTAACAATAACCG |
| MP-RP | CCGCTAAAACGAGTTCCCTA | |||
| MP-P | FAM-TACCACGGATGGCAGTTGCTGG-BHQ1 | |||
| 2 | Chlamydophila pneumoniae 23S ribosomal RNA genes | designed in Primer Premier 5 | CP-FP | TATGACCCGGAGGTATCCG |
| CP-RP | CGGGTGTCGCCTTATATGC | |||
| CP-P | HRF550-TGGGGCAACCCGATAGACTAATAG-BHQ1 | |||
| 3 | Streptococcus pneumoniae autolysin gene | designed in Primer Premier 5 | SP-FP | CGAACTCTTACGCAATCTAGCA |
| SP-RP | TTCGTGCAATACTCGTGCG | |||
| SP-P | HRF550-CCAGCTAAACTCCCTGTATCAAGCG-BHQ1 | |||
| 4 | Human respiratory syncytial virus A fusion protein | designed in Primer Premier 5 | RSVA-FP | AAACAGATGTAAGCAGCTCCGT |
| RSVA-RP | CGATTTTTATTGGATGCTGTACAT | |||
| RSVA-P | FAM-ACATCTCTAGGAGCCATTGTGTCATG-BHQ1 | |||
| 5 | Human adenovirus hexon protein | designed in Primer Premier 5 | ADV-FP | CTCGGAGTACCTGAGTCCGG |
| ADV-RP | CGTGGGATTTCTAAACTCATTTC | |||
| ADV-P | HRF550-CGCCACAGACACCTACTTCAATCTG-BHQ1 | |||
| 6 | Human coronavirus HKU1 open reading frames 1ab | designed in Primer Premier 5 | HKU1-FP | CACATGGTGATAGATTTTATCGC |
| HKU1-RP | ATAATAGCAACCGCCACACAT | |||
| HKU1-P | HRF550-CTTGCGAATGAATGTGCTCAAGTTT-BHQ1 | |||
| 7 | Human coronavirus 229E nucleocapsid protein | designed in Primer Premier 5 | 229E-FP | CAGAAAACGAAAGATTGCTTCA |
| 229E-RP | CAAGCAAAGGGCTATAAAGAGA | |||
| 229E-P | HRF550-ATGGCTACAGTCAAATGGGCTGATGC-BHQ1 | |||
| 8 | Human metapneumovirus fusion protein | designed in Primer Premier 5 | HMPV-FP | CTGTCAGCTTCAGTCARTTCAAC |
| HMPV-RP | CAATGATATTGCYGGTGTTAT | |||
| HMPV-P | FAM-TGTTGTGCGGCAGTTTTCAGACAAT-BHQ1 | |||
| 9 | SARS-CoV-2 nucleocapsid protein | NHC of P.R. China, 2020 | N-FP | GGGGAACTTCTCCTGCTAGAAT |
| N-RP | CAGACATTTTGCTCTCAAGCTG | |||
| N-P | FAM-TTGCTGCTGCTTGACAGATT-TAMRA | |||
| 10 | SARS-CoV-2 open reading frames 1ab | NHC of P.R. China, 2020 | ORF1-FP | CCCTGTGGGTTTTACACTTAA |
| ORF1-RP | ACGATTGTGCATCAGCTGA | |||
| ORF1-P | FAM-CCGTCTGCGGTATGTGGAAAGGTTATGG-BHQ1 | |||
| 11 | Influenza A virus matrix gene 1 & 2 | Chinese National lnfluenza Center, 2017 | FLUA-FP | GACCRATCCTGTCACCTCTGAC |
| FLUA-RP | GGGCATTYTGGACAAAKCGTCTACG | |||
| FLUA-P | FAM-TGCAGTCCTCGCTCACTGGGCACG-BHQ1 | |||
| 12 | Influenza B virus hemagglutinin | Chinese National lnfluenza Center, 2017 | FLUB-FP | AGACCAGAGGGAAACTATGCCC |
| FLUB-RP | TCCGGATGTAACAGGTCTGACTT | |||
| FLUB-P1 | FAM-CAGACCAAAATGCACGGGGAAHATACC-BHQ1 | |||
| FLUB-P2 | FAM-CAGRCCAATGTGTGTGGGGAYCACACC-BHQ1 |
| Target | Primer F Final Concentration | Primer R Final Concentration | Primer P Final Concentration | Maximum RFI |
|---|---|---|---|---|
| MP | 0.4 μM | 0.4 μM | 133 nM | 1.6 |
| CP | 0.4 μM | 0.4 μM | 200 nM | 1.6 |
| SP | 0.4 μM | 0.4 μM | 200 nM | 1.5 |
| RSVA | 0.4 μM | 0.4 μM | 240 nM | 1.8 |
| ADV | 0.4 μM | 0.4 μM | 400 nM | 1.5 |
| HKUI | 0.4 μM | 0.4 μM | 200 nM | 1.3 |
| 229E | 0.4 μM | 0.4 μM | 160 nM | 1.8 |
| HMPV | 0.4 μM | 0.4 μM | 100 nM | 1.8 |
| N | 0.4 μM | 0.4 μM | 200 nM | 1.6 |
| ORF1ab | 0.4 μM | 0.4 μM | 260 nM | 1.8 |
| FLUA | 0.4 μM | 0.4 μM | 160 nM | 1.7 |
| FLUB | 0.4 μM | 0.4 μM | 200 nM | 1.5 |
| Type of Infection | Dynamic Range | Standard Curve | Rsq | Mean Ct | Detection Limit (Copies/T) |
|---|---|---|---|---|---|
| MP | 1.5 × 101 to 1.5 × 107 | y = −3.4256x + 41.514 | 0.995 | 22.50 | 15 |
| CP | 1.5 × 102 to 1.5 × 107 | y = −3.4841x + 41.053 | 0.998 | 21.75 | 150 |
| SP | 1.5 × 102 to 1.5 × 107 | y = −3.7385x + 42.311 | 0.998 | 22.06 | 150 |
| RSV | 1.5 × 101 to 1.5 × 107 | y = −3.6142x + 41.853 | 0.999 | 21.95 | 15 |
| ADV | 1.5 × 101 to 1.5 × 107 | y = −3.4086x + 41.313 | 0.989 | 22.51 | 15 |
| HKUI | 1.5 × 102 to 1.5 × 107 | y = −3.4600x + 39.304 | 0.999 | 21.99 | 150 |
| 229E | 1.5 × 102 to 1.5 × 107 | y = −3.4672x + 39.896 | 0.998 | 22.36 | 150 |
| HMPV | 1.5 × 102 to 1.5 × 107 | y = −3.4859x + 38.982 | 0.998 | 21.29 | 150 |
| N | 1.5 × 102 to 1.5 × 107 | y = −3.5771x + 41.425 | 0.999 | 22.01 | 150 |
| ORF1ab | 1.5 × 102 to 1.5 × 107 | y = −3.4684x + 41.344 | 0.998 | 22.30 | 150 |
| FLUA | 1.5 × 101 to 1.5 × 107 | y = −3.4674x + 40.598 | 0.999 | 21.59 | 15 |
| FLUB | 1.5 × 102 to 1.5 × 107 | y = −3.7135x + 43.109 | 0.999 | 23.04 | 150 |
| Type of Infection | Dynamic Range | Standard Curve | Rsq | Mean Ct | Detection Limit (Copies/T) |
|---|---|---|---|---|---|
| MP | 1.2 × 102 to 1.2 × 106 | y = −3.5667x + 38.085 | 0.986 | 20.76 | 120 |
| CP | 1.2 × 102 to 1.2 × 106 | y = −3.9103x + 40.054 | 0.984 | 21.13 | 120 |
| SP | 1.2 × 102 to 1.2 × 106 | y = −3.5293x + 40.611 | 0.939 | 23.36 | 120 |
| RSV | 1.2 × 102 to 1.2 × 106 | y = −3.7060x + 37.213 | 0.996 | 18.91 | 120 |
| ADV | 1.2 × 101 to 1.2 × 106 | y = −3.6477x + 38.470 | 0.981 | 21.26 | 12 |
| HKUI | 1.2 × 101 to 1.2 × 106 | y = −3.4883x + 38.630 | 0.971 | 21.63 | 12 |
| 229E | 1.2 × 102 to 1.2 × 106 | y = −3.7153x + 41.180 | 0.933 | 22.80 | 120 |
| HMPV | 1.2 × 102 to 1.2 × 106 | y = −4.2580x + 44.229 | 0.977 | 23.32 | 120 |
| N | 1.2 × 102 to 1.2 × 106 | y = −4.3620x + 42.910 | 0.994 | 21.15 | 120 |
| ORF1ab | 1.2 × 102 to 1.2 × 106 | y = −3.9967x + 42.816 | 0.947 | 24.22 | 120 |
| FLUA | 1.2 × 102 to 1.2 × 106 | y = −3.0850x + 40.021 | 0.958 | 25.34 | 120 |
| FLUB | 1.2 × 102 to 1.2 × 106 | y = −3.6880x + 42.363 | 0.982 | 24.69 | 120 |
| RT-qPCR Off-Chip | ||||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| RT-qPCR On-Chip | Positive | 28 | 0 | 28 |
| Negative | 2 | 450 | 452 | |
| Total | 30 | 450 | 480 | |
| Value | Asymp. Std. Error 1 | Approx. T 2 | Approx. Siq. | |
|---|---|---|---|---|
| Measure of Agreement Kappa | 0.963 | 0.026 | 21.119 | 0.000 |
| N of Valid Cases | 480 |
| Method | Multiple Detection | Within 2 h | High Sensitivity | High Specificity | |
|---|---|---|---|---|---|
| Conventional diagnosis [26,27,28] | Bacterial culture | × | × | √ | √ |
| Immunofluorescence | × | √ | × | × | |
| ELISA | × | √ | × | × | |
| Molecular diagnostics [26,27,28] | DMF with LAMP | × | √ | √ | √ |
| DMF with RT-qPCR | √ | √ | √ | √ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Huang, K.; Sun, Y.; Luo, D.; Wang, M.; Chen, T.; Li, M.; Duan, J.; Huang, L.; Dong, C. A Digital Microfluidic RT-qPCR Platform for Multiple Detections of Respiratory Pathogens. Micromachines 2022, 13, 1650. https://doi.org/10.3390/mi13101650
Huang H, Huang K, Sun Y, Luo D, Wang M, Chen T, Li M, Duan J, Huang L, Dong C. A Digital Microfluidic RT-qPCR Platform for Multiple Detections of Respiratory Pathogens. Micromachines. 2022; 13(10):1650. https://doi.org/10.3390/mi13101650
Chicago/Turabian StyleHuang, Huitao, Kaisong Huang, Yun Sun, Dasheng Luo, Min Wang, Tianlan Chen, Mingzhong Li, Junwei Duan, Liqun Huang, and Cheng Dong. 2022. "A Digital Microfluidic RT-qPCR Platform for Multiple Detections of Respiratory Pathogens" Micromachines 13, no. 10: 1650. https://doi.org/10.3390/mi13101650






