Development of High-Cell-Density Tissue Method for Compressed Modular Bioactuator
Abstract
:1. Introduction
2. Materials and Methods
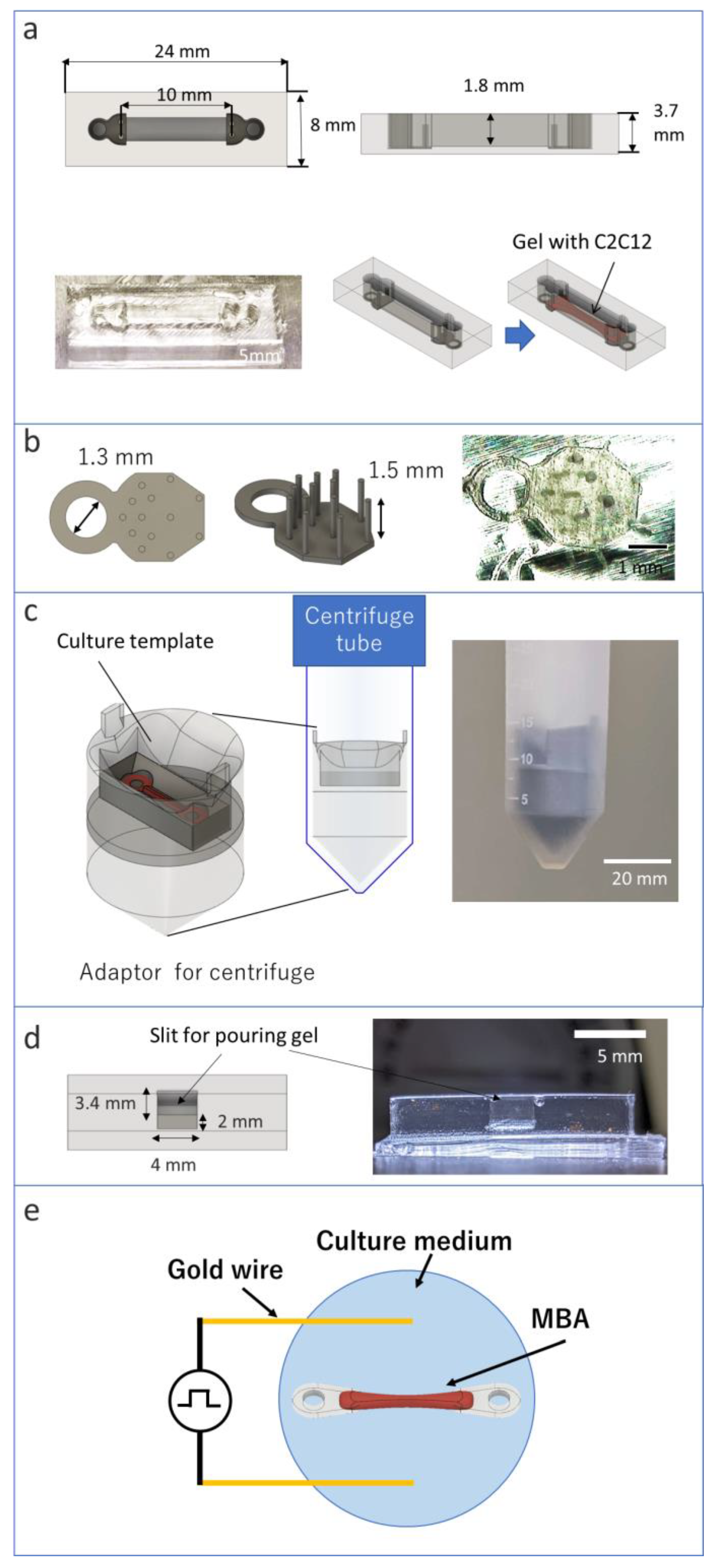
2.1. Materials of C-MBA
2.2. Preparation of Tendon Structures and Culture Template
2.3. Preparation of Myoblast Cells
2.4. Attaching Template for Centrifuge
2.5. Measuring the Effect of Centrifugal Force on Cells
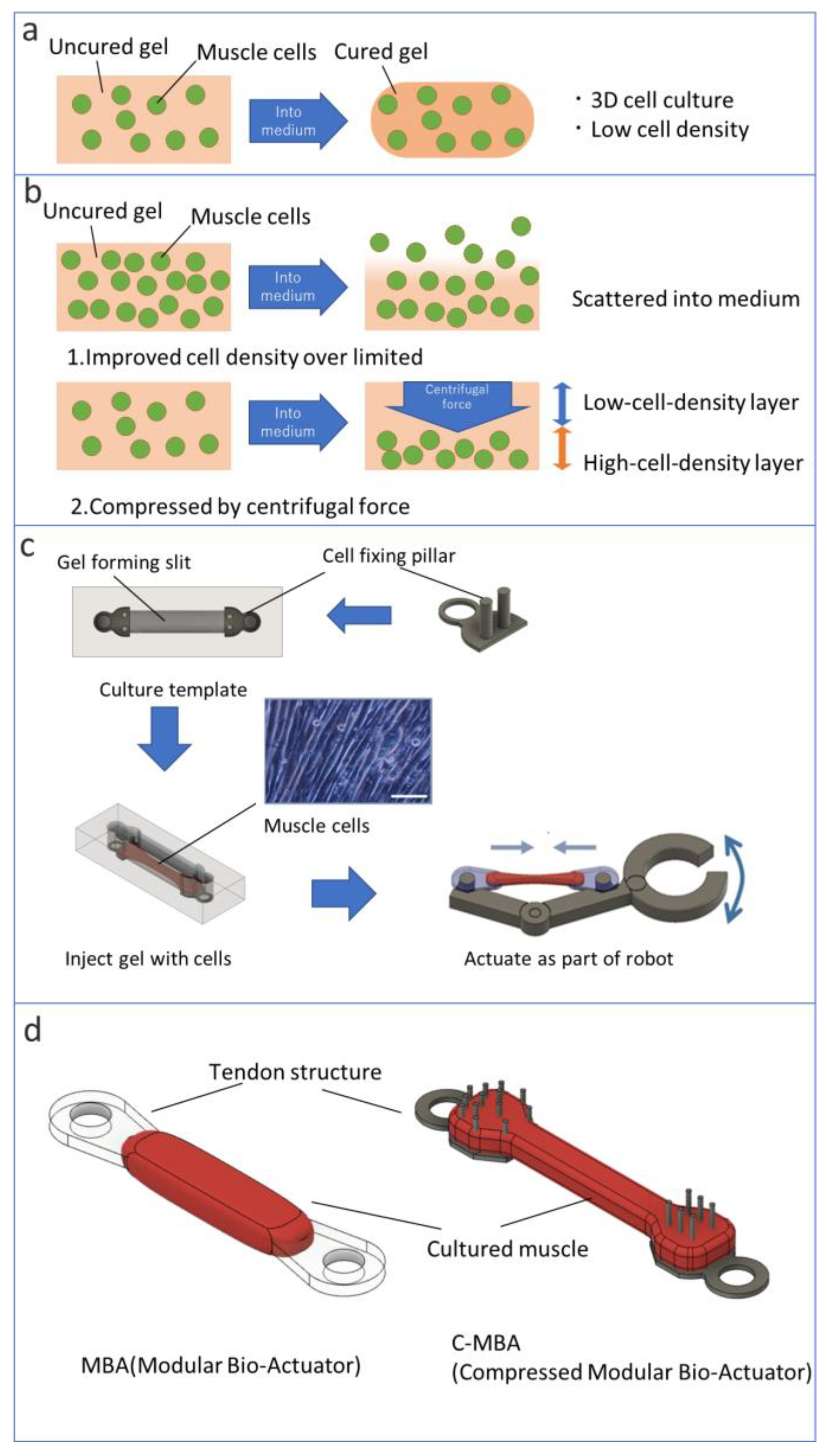
2.6. Fabrication of MBA and C-MBA
2.7. Stimulation for Bioactuator
3. Results
3.1. Effect of Centrifuge
3.2. Fabrication of C-MBA
3.3. Comparison of the Mass Density of C-MBA and MBA
3.4. Contract Force per Unit Cross-Sectional Area
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feinberg, A.W. Biological Soft Robotics. Annu. Rev. Biomed. Eng. 2015, 17, 243–265. [Google Scholar] [CrossRef]
- Park, S.-J.; Gazzola, M.; Park, K.S.; Park, S.; Santo, V.D.; Blevins, E.L.; Lind, J.U.; Campbell, P.H.; Dauth, S.; Capulli, A.K.; et al. Phototactic guidance of a tissue-engineered soft-robotic ray. Science 2016, 353, 158–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.H.; Kwon, C.H.; Lee, C.; An, J.; Phuong, T.T.T.; Park, S.H.; Lima, M.D.; Baughman, R.H.; Kang, T.M.; Kim, S.J. Bio-inspired Hybrid Carbon Nanotube Muscles. Sci. Rep. 2016, 6, 26687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holley, M.T.; Nagarajan, N.; Danielson, C.; Zorlutuna, P.; Park, K. Development and characterization of muscle-based actuators for self-stabilizing swimming biorobots. Lab Chip 2016, 16, 3473–3484. [Google Scholar] [CrossRef]
- Nawroth, J.C.; Lee, H.; Feinberg, A.W.; Ripplinger, C.M.; McCain, M.L.; Grosberg, A.; Dabiri, J.O.; Parker, K.K. A tissue-engineered jellyfish with biomimetic propulsion. Nat. Biotechnol. 2012, 30, 792–797. [Google Scholar] [CrossRef]
- Chan, V.; Park, K.; Collens, M.B.; Kong, H.; Saif, T.A.; Bashir, R. Development of Miniaturized Walking Biological Machines. Sci. Rep. 2012, 2, 857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morimoto, Y.; Kato-Negishi, M.; Onoe, H.; Takeuchi, S. Three-dimensional neuronemuscle constructs with neuromuscular junctions. Biomaterials 2013, 34, 9413–9419. [Google Scholar] [CrossRef]
- Feinberg, A.W.; Feigel, A.; Shevkoplyas, S.S.; Sheehy, S.; Whitesides, G.M.; Parker, K.K. Muscular Thin Films for Building Actuators and Powering Devices. Science 2007, 317, 1366–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lind, J.U.; Busbee, T.A.; Valentine, A.D.; Pasqualini, F.S.; Yuan, H.; Yadid, M.; Park, S.-J.; Kotikian, A.; Nesmith, A.P.; Campbell, P.H.; et al. Instrumented cardiac microphysiological devices via muti-material 3D printing. Nat. Mater. 2017, 16, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.J.; Anand, S.V.; Rajagopalan, J.; Saif, M.T.A. A self-propelled biohybrid swimmer at low Reynolds number. Nat. Commun. 2014, 5, 3081. [Google Scholar] [CrossRef] [PubMed]
- Vannozzi, L.; Yasa, I.C.; Ceylan, H.; Menciassi, A.; Ricotti, L.; Sitti, M. Self-Folded Hydrogel Tubes for Implantable Muscular Tissue Scaffolds. Macromol. Biosci. 2018, 18, 1700377. [Google Scholar] [CrossRef] [PubMed]
- Ricotti, L.; Menciassi, A. Bio-hybrid muscle cell-based actuators. Biomed. Microdevices 2012, 14, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Ricotti, L.; Menciassi, A. Nanotechnology in biorobotics: Opportunities and challenges. J. Nanopart. Res. 2015, 17, 84. [Google Scholar] [CrossRef]
- Ricotti, L.; Trimmer, B.; Feinberg, A.W.; Raman, R.; Parker, K.K.; Bashir, R.; Sitti, M.; Martel, S.; Dario, P.; Menciassi, A. Biohybrid actuators for robotics: A review of devices actuated by living cells. Sci. Robot. 2017, 2, 0495. [Google Scholar] [CrossRef] [Green Version]
- Vannozzi, L.; Ricotti, L.; Cianchetti, M.; Bearzi, C.; Rizzi, R.; Dario, P.; Menciassi, A. Self-assembly of polydimethylsiloxane structures from 2D to 3D for bio-hybrid actuation. Bioinspir. Biomim. 2015, 10, 056001. [Google Scholar] [CrossRef]
- Morimoto, Y.; Kato-Negishi, M.; Onoe, H.; Takeuchi, S. Biohybrid robot powered by an antagonistic pair of skeletal muscle tissues. Sci. Robot. 2018, 3, 4440. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Takagi, S.; Kamon, T.; Yamasaki, K.; Fujisato, T. Development and evaluation of a removable tissue-engineered muscle with artificial tendons. J. Biosci. Bioeng. 2017, 123, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Terada, R.; Hashimoto, M.; Hoshino, T.; Furukawa, Y.; Morishima, K. Rod-shaped tissue engineered skeletal muscle with artificial anchors to utilize as a bio-actuator. J. Biomech. Sci. Eng. 2010, 5, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, G.; Aung, A.; Varghese, S. Skeletal muscle-on-a-chip: An in vitro model to evaluate tissue formation and injury. Lab Chip 2017, 17, 3447–3461. [Google Scholar] [CrossRef] [PubMed]
- Kabumoto, K.; Hoshino, T.; Akiyama, Y.; Morishima, K. Voluntary Movement Controlled by the Surface EMG Signal for Tissue-Engineered Skeletal Muscle on a Gripping Tool. Tissue Eng. Part A 2013, 19, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T.; Imagawa, K.; Akiyama, Y.; Morishima, K. Cardiomyocyte-driven gel network for bio mechano-informatic wet robotics. Biomed. Microdevices 2012, 14, 969–977. [Google Scholar] [CrossRef]
- Costantini, M.; Testa, S.; Fornetti, E.; Barbetta, A.; Trombetta, M.; Cannata, M.S.; Gargioli, C. Engineering Muscle Networks in 3D Gelatin Methacryloyl Hydrogels: Influence of Mechanical Stiffness and Geometrical Confinement. Front. Bioeng. Biotechnol. 2017, 5, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudera, V.; Smith, A.S.T.; Brady, M.A.; Lewis, M.P. The Effect of Cell Density on the Maturation and Contractile Ability of Muscle Derived Cells in a 3D Tissue-Engineered Skeletal Muscle Model and Determination of the Cellular and Mechanical Stimuli Required for the Synthesis of a Postural Phenotype. J. Cell. Physiol. 2010, 225, 646–653. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ito, A.; Kato, M.; Kawabe, Y.; Shimizu, K.; Fujita, H.; Nagamori, E.; Kamihira, M. Preparation of artificial skeletal muscle tissues by a magnetic force-based tissue engineering technique. J. Biosci. Bioeng. 2009, 108, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Yeung, E.; Lui, C.; Ong, C.S.; Pitaktong, I.; Huang, C.; Inoue, T.; Matsushita, H.; Ma, C.; Hibino, N. A Net Mold-based Method of Biomaterial-free Three-Dimensional Cardiac Tissue Creation. Tissue Eng. Part C Methods 2019, 25, 243–252. [Google Scholar]
- Nomura, T.; Takeuchi, M.; Kim, E.; Huang, Q.; Hasegawa, Y.; Fukuda, T. Development of Cultured Muscles with Tendon Structures for Modular Bio-Actuators. Micromachines 2021, 12, 379. [Google Scholar] [CrossRef]
- Yamasaki, K.; Hayashi, H.; Nishiyama, K.; Kobayashi, H.; Uto, S.; Kondo, H.; Hashimoto, S.; Fujisato, T. Control of myotube contraction using electrical pulse stimulation for bio-actuator. J. Artif. Organs 2009, 12, 131–137. [Google Scholar] [CrossRef]
- Leonard, K.C.; Worden, N.; Boettcher, M.L.; Dickinson, E.; Omstead, K.M.; Burrows, A.M.; Hartstone-Rose, A. Anatomical and ontogenetic influences on muscle density. Sci. Rep. 2021, 11, 2114. [Google Scholar] [CrossRef]
- Haraguchi, Y.; Matsuura, K.; Kagawa, Y.; Hasegawa, A.; Kubo, H.; Shimizu, T. Rapid creation system of morphologically and functionally communicative three-dimensional cell-dense tissue by centrifugation. Biotechnol. Prog. 2018, 34, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, Y.; Kagawa, Y.; Hasegawa, A.; Kubo, H.; Shimizu, T. Rapid fabrication of detachable three-dimensional tissues by layering of cell sheets with heating centrifuge. Biotechnol. Prog. 2018, 34, 692–701. [Google Scholar] [CrossRef]
- Haraguchi, Y.; Hasegawa, A.; Matsuura, K.; Kobayashi, M.; Iwana, S.; Kabetani, Y.; Shimizu, T. Three-Dimensional Human Cardiac Tissue Engineered by Centrifugation of Stacked Cell Sheets and Cross-Sectional Observation of Its Synchronous Beatings by Optical Coherence Tomography. Biomed. Res. Int. 2017, 2017, 5341702. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, U.; Tonooka, Y.; Koretake, R.; Akimoto, T.; Gonda, Y.; Saito, J.; Umemura, M.; Fujita, T.; Sakuma, S.; Arai, F.; et al. Arterial graft with elastic layer structure grown from cells. Sci. Rep. 2017, 7, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, Y.; Nakayama, A.; Nakano, S.; Amiya, R.; Hirose, J. An Electrical Stimulation Culture System for Daily Maintenance-Free Muscle Tissue Production. Cyborg Bionic Syst. 2021, 2021, 9820505. [Google Scholar] [CrossRef]
- Sarathy, A.; Wuebbles, D.R.; Fontelonga, M.T.; Tarchione, A.R.; Griner, A.M.L.; Heredia, J.D.; Nunes, M.A.; Duan, S.; Brewer, D.P.; Ry, V.T.; et al. SU9516 Increases α7β1 Integrin and Ameliorates Disease Progression in the mdx Mouse Model of Duchenne Muscular Dystrophy. Mol. Ther. 2017, 25, 1395–1407. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nomura, T.; Takeuchi, M.; Kim, E.; Huang, Q.; Hasegawa, Y.; Fukuda, T. Development of High-Cell-Density Tissue Method for Compressed Modular Bioactuator. Micromachines 2022, 13, 1725. https://doi.org/10.3390/mi13101725
Nomura T, Takeuchi M, Kim E, Huang Q, Hasegawa Y, Fukuda T. Development of High-Cell-Density Tissue Method for Compressed Modular Bioactuator. Micromachines. 2022; 13(10):1725. https://doi.org/10.3390/mi13101725
Chicago/Turabian StyleNomura, Takuto, Masaru Takeuchi, Eunhye Kim, Qiang Huang, Yasuhisa Hasegawa, and Toshio Fukuda. 2022. "Development of High-Cell-Density Tissue Method for Compressed Modular Bioactuator" Micromachines 13, no. 10: 1725. https://doi.org/10.3390/mi13101725




