High-Throughput Dispensing of Viscous Solutions for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Scaffold Dispenser Components and Fabrication
2.3. Preparation of CA and CHA Scaffold Solutions
2.4. Scaffold Dispensing and Processing
2.5. Scanning Electron Microscopy
2.6. Macroscopic Imaging
2.7. Scaffold Pore Area Analysis
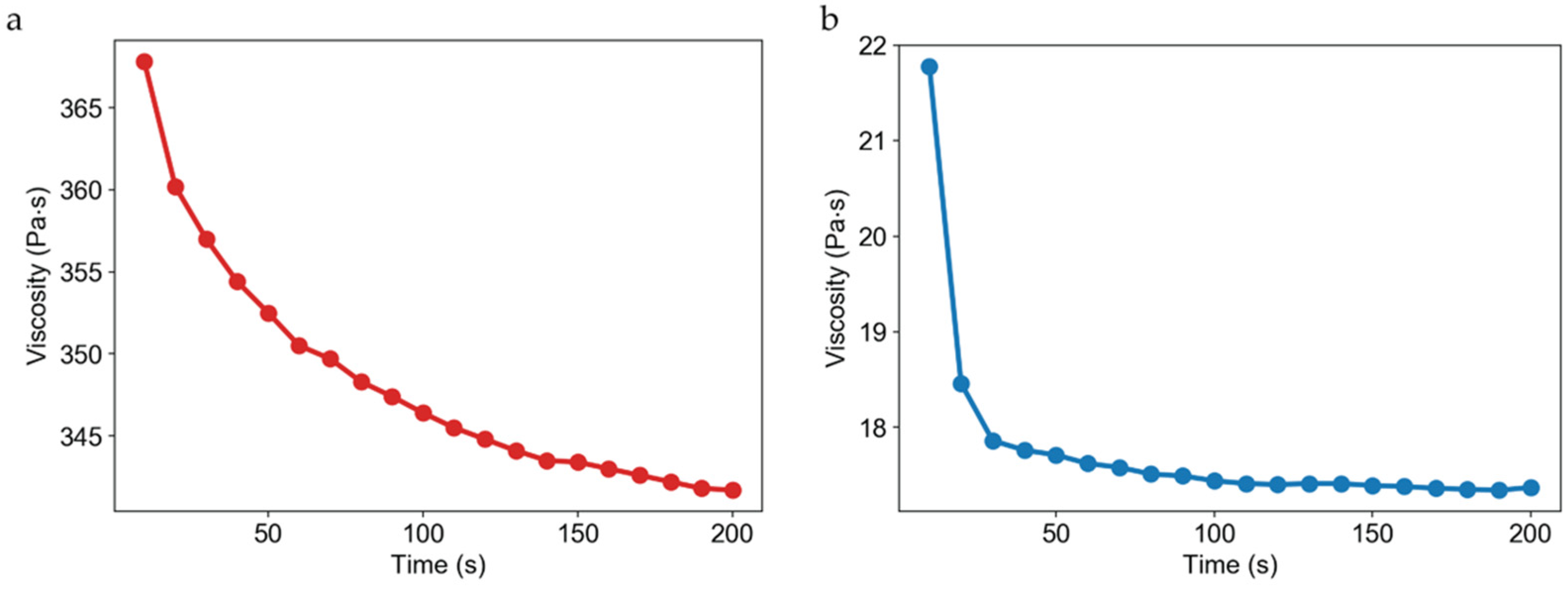
2.8. Evaluation of Scaffold Solution Viscosity
2.9. Cell Culture
2.10. In Vitro Drug Response Analysis
3. Results
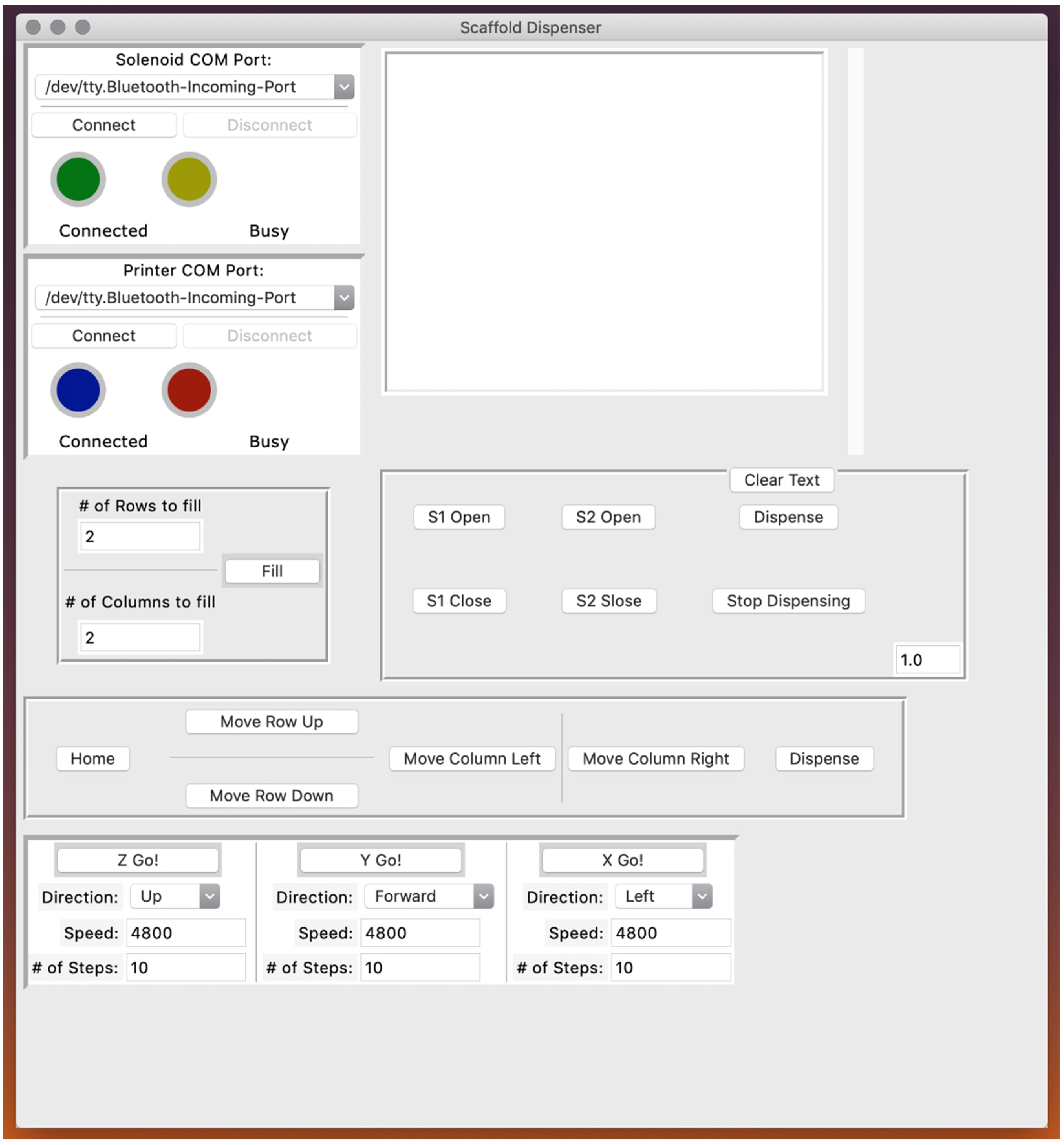
3.1. Automated Scaffold Dispenser Design
3.2. Realized Scaffold Dispensing System
3.3. High-Viscosity Scaffold Solutions
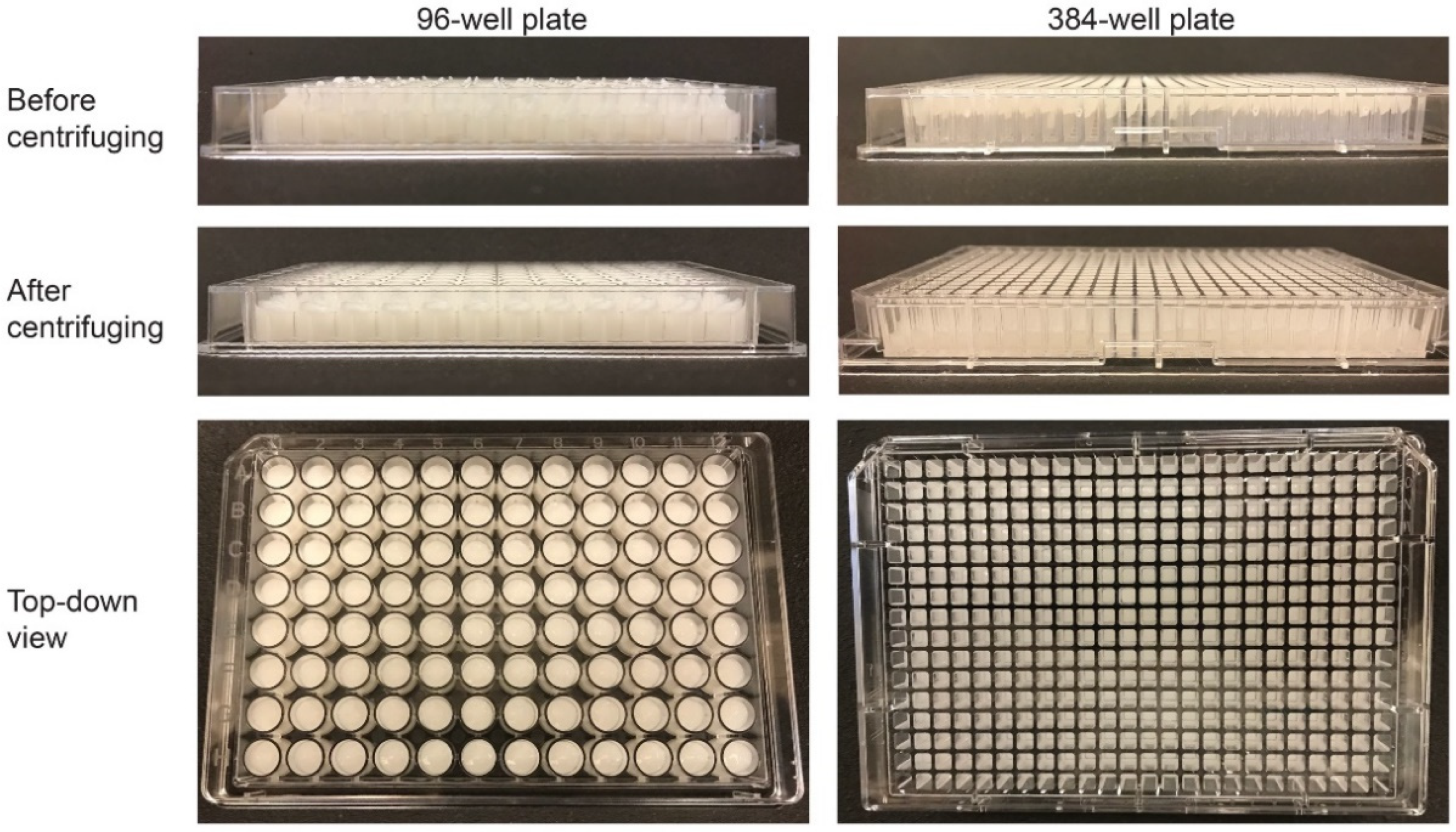
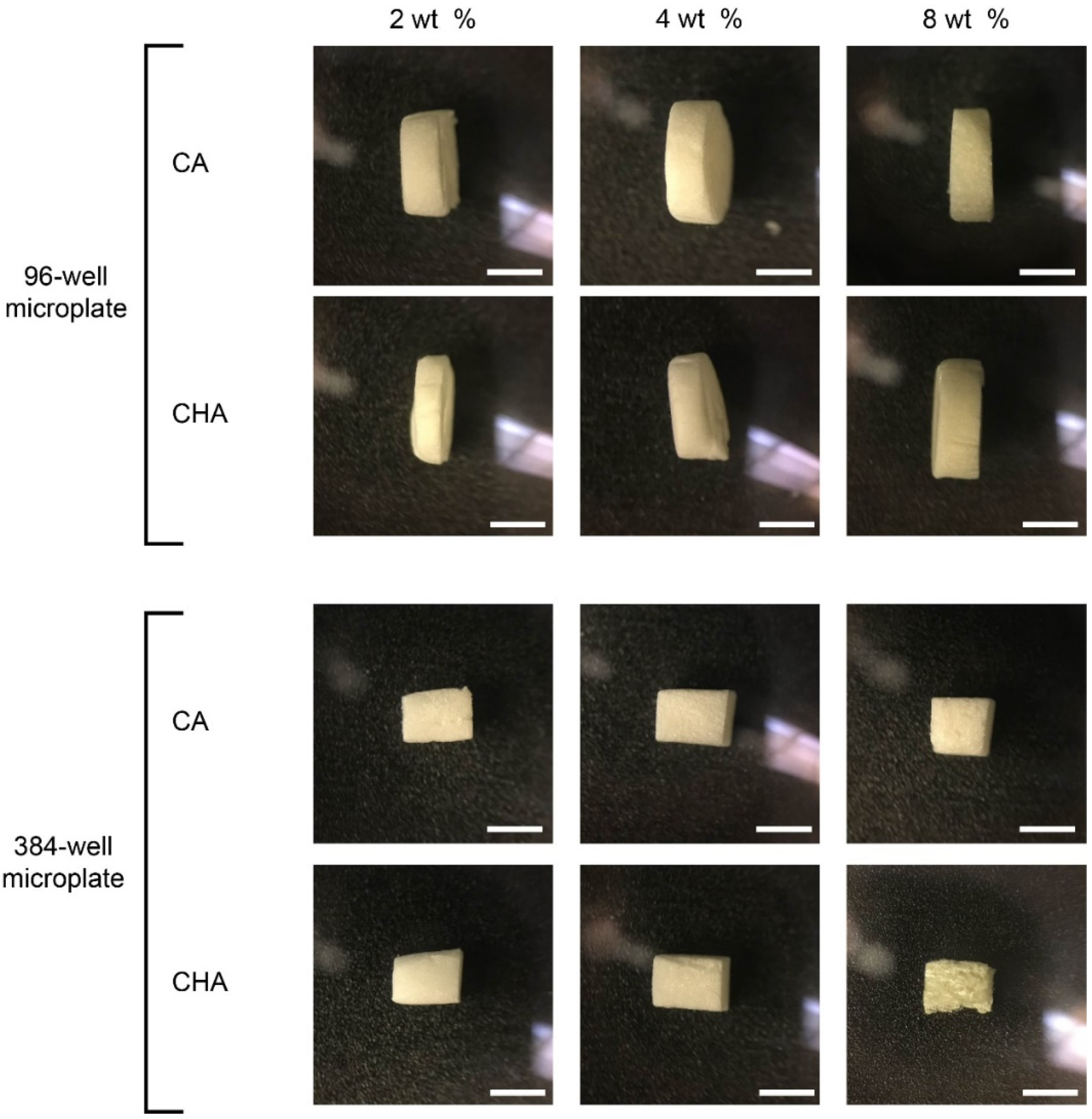
3.4. CA and CHA Scaffolds Automatically Dispensed into 96- and 384-Well Plates
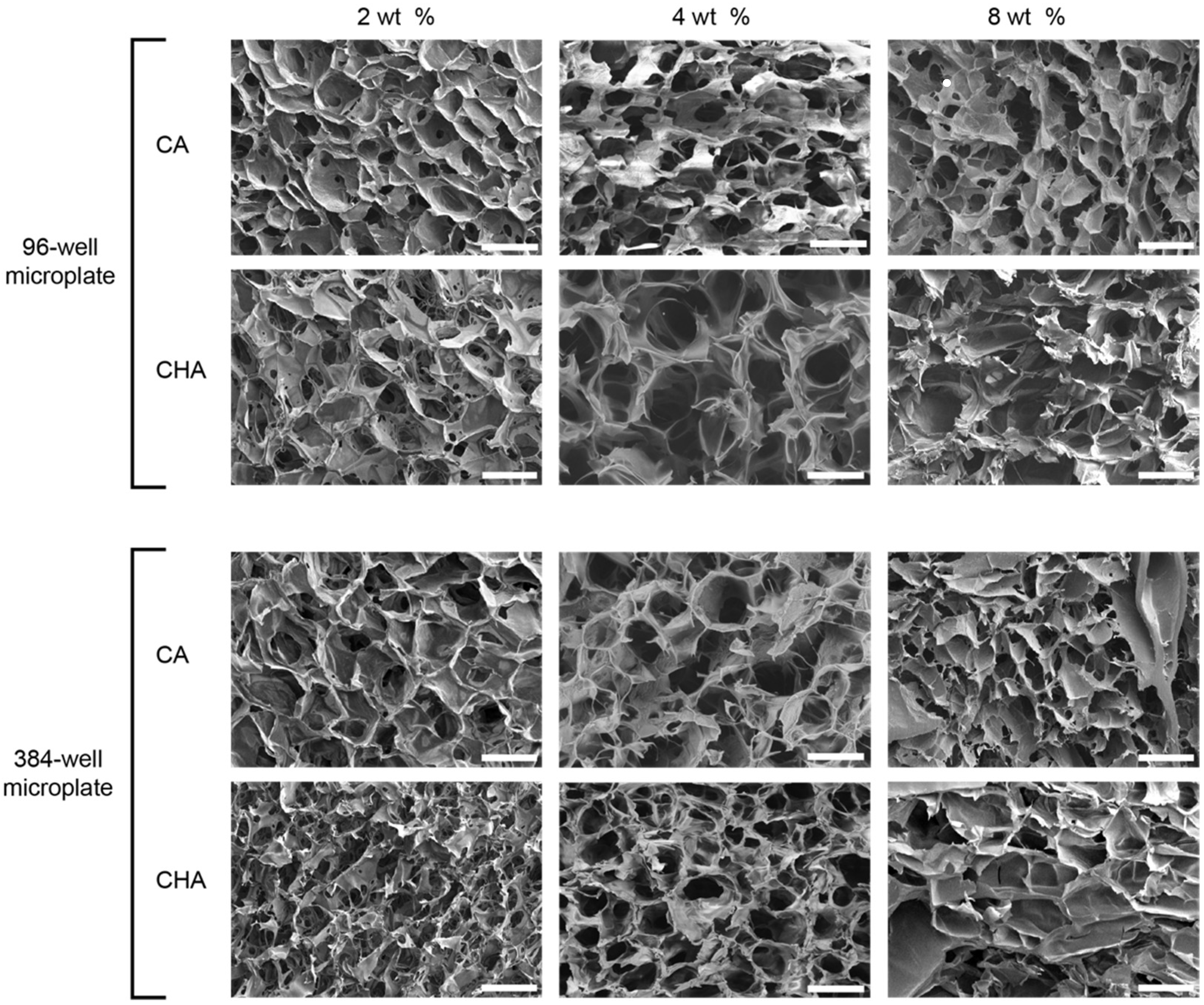
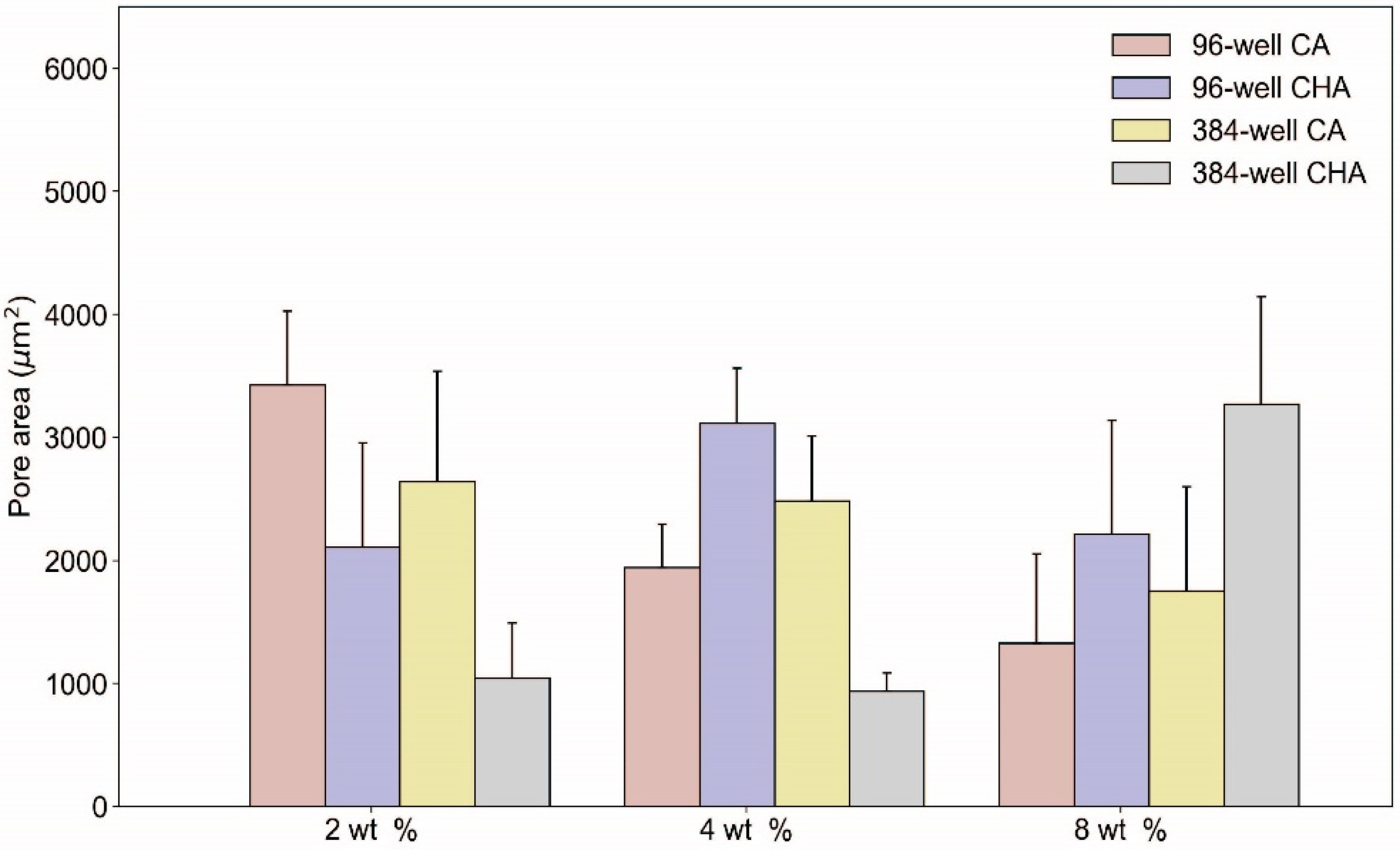
3.5. Characterization of Dispensed CA and CHA Scaffolds
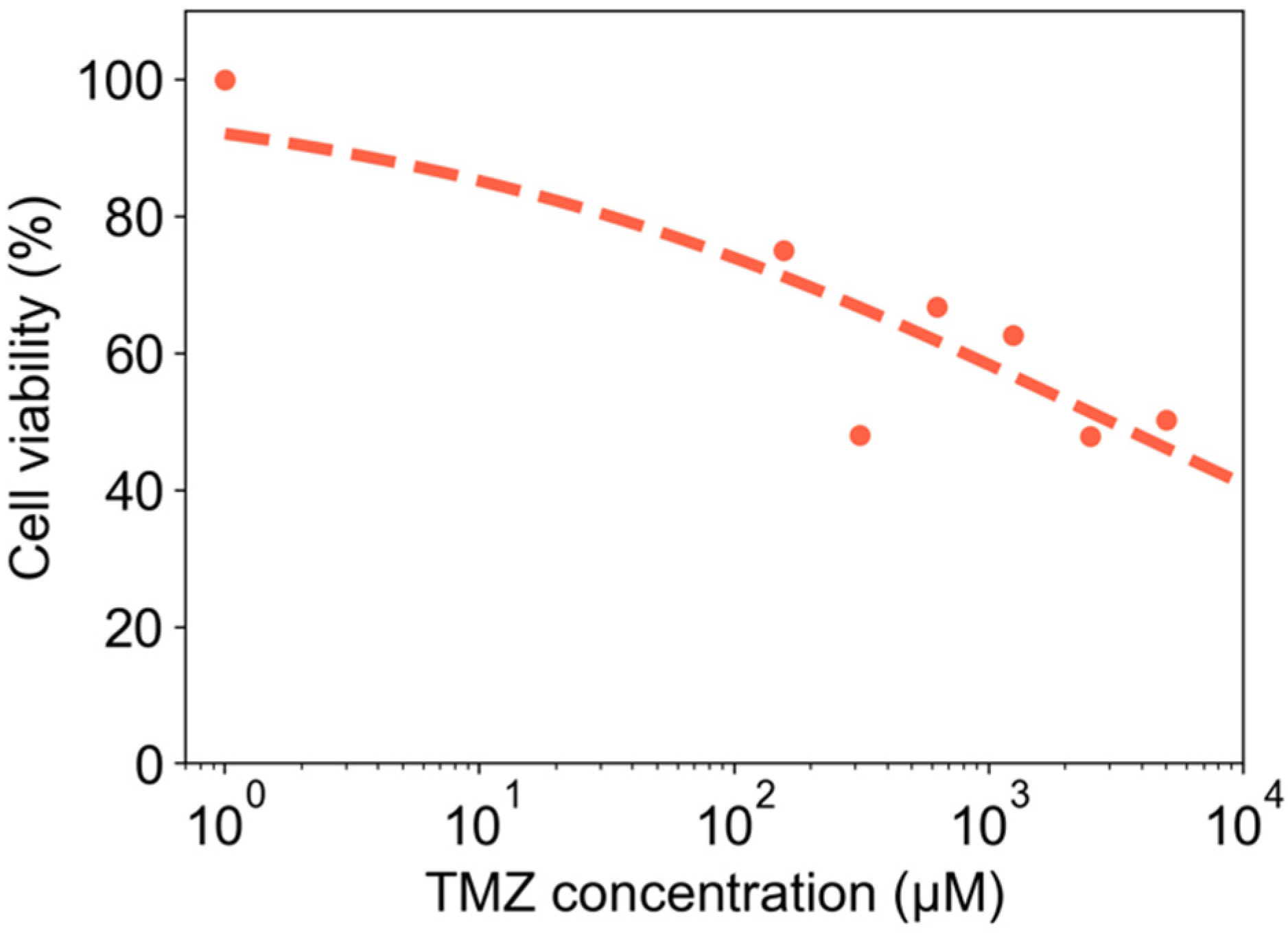
3.6. Drug Screening Proof-of-Concept
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharm. 2018, 9, 6. [Google Scholar] [CrossRef]
- Wulftange, W.J.; Rose, M.A.; Garmendia-Cedillos, M.; da Silva, D.; Poprawski, J.E.; Srinivasachar, D.; Sullivan, T.; Lim, L.; Bliskovsky, V.V.; Hall, M.D. Spatial Control of Oxygen Delivery to Three-dimensional Cultures Alters Cancer Cell Growth and Gene Expression. J. Cell Physiol. 2019, 234, 20608–20622. [Google Scholar] [CrossRef]
- Sundarakrishnan, A.; Zukas, H.; Coburn, J.; Bertini, B.T.; Liu, Z.; Georgakoudi, I.; Baugh, L.; Dasgupta, Q.; Black, L.D.; Kaplan, D.L. Bioengineered in Vitro Tissue Model of Fibroblast Activation for Modeling Pulmonary Fibrosis. ACS Biomater. Sci. Eng. 2019, 5, 2417–2429. [Google Scholar] [CrossRef]
- Badekila, A.K.; Kini, S.; Jaiswal, A.K. Fabrication Techniques of Biomimetic Scaffolds in Three-dimensional Cell Culture: A Review. J. Cell Physiol. 2021, 236, 741–762. [Google Scholar] [CrossRef]
- Baker, B.M.; Chen, C.S. Deconstructing the Third Dimension—How 3D Culture Microenvironments Alter Cellular Cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef]
- Arrowsmith, J.; Miller, P. Trial Watch: Phase II and Phase III Attrition Rates 2011–2012. Nat. Rev. Drug Discov. 2013, 12, 569. [Google Scholar] [CrossRef]
- Zhuravleva, M.; Gilazieva, Z.; Grigoriev, T.E.; Shepelev, A.D.; Tenchurin, T.K.; Kamyshinsky, R.; Krasheninnikov, S.V.; Orlov, S.; Caralogli, G.; Archipova, S. In Vitro Assessment of Electrospun Polyamide-6 Scaffolds for Esophageal Tissue Engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 253–268. [Google Scholar] [CrossRef]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-polysaccharide Composite Scaffolds for 3D Cell Culture and Tissue Engineering: Towards Natural Therapeutics. Bioeng. Transl. Med. 2019, 4, 96–115. [Google Scholar] [CrossRef]
- Ma, C.; Chang, B.; Jing, Y.; Kim, H.; Liu, X. Bio-Inspired Micropatterned Platforms Recapitulate 3D Physiological Morphologies of Bone and Dentinal Cells. Adv. Sci. 2018, 5, 1801037. [Google Scholar] [CrossRef]
- Keirouz, A.; Chung, M.; Kwon, J.; Fortunato, G.; Radacsi, N. 2D and 3D Electrospinning Technologies for the Fabrication of Nanofibrous Scaffolds for Skin Tissue Engineering: A Review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1626. [Google Scholar] [CrossRef]
- Le, M.N.; Xu, K.; Wang, Z.; Beverung, S.; Steward, R.L.; Florczyk, S.J. Evaluation of the Effect of 3D Porous Chitosan-alginate Scaffold Stiffness on Breast Cancer Proliferation and Migration. J. Biomed. Mater. Res. A 2021, 109, 1990–2000. [Google Scholar] [CrossRef]
- Kievit, F.M.; Florczyk, S.J.; Leung, M.C.; Wang, K.; Wu, J.D.; Silber, J.R.; Ellenbogen, R.G.; Lee, J.S.H.; Zhang, M. Proliferation and Enrichment of CD133+ Glioblastoma Cancer Stem Cells on 3D Chitosan-Alginate Scaffolds. Biomaterials 2014, 35, 9137–9143. [Google Scholar] [CrossRef]
- Rouhollahi, A.; Ilegbusi, O.; Florczyk, S.; Xu, K.; Foroosh, H. Effect of Mold Geometry on Pore Size in Freeze-Cast Chitosan-Alginate Scaffolds for Tissue Engineering. Ann. Biomed. Eng. 2020, 48, 1090–1102. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-Based Biomaterials for Tissue Engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Tamimi, M.; Rajabi, S.; Pezeshki-Modaress, M. Cardiac ECM/Chitosan/Alginate Ternary Scaffolds for Cardiac Tissue Engineering Application. Int. J. Biol. Macromol. 2020, 164, 389–402. [Google Scholar] [CrossRef]
- Erickson, A.E.; Sun, J.; Lan Levengood, S.K.; Swanson, S.; Chang, F.-C.; Tsao, C.T.; Zhang, M. Chitosan-Based Composite Bilayer Scaffold as an in Vitro Osteochondral Defect Regeneration Model. Biomed. Microdev. 2019, 21, 1–16. [Google Scholar] [CrossRef]
- Wang, K.; Kievit, F.M.; Erickson, A.E.; Silber, J.R.; Ellenbogen, R.G.; Zhang, M. Culture on 3D Chitosan-hyaluronic Acid Scaffolds Enhances Stem Cell Marker Expression and Drug Resistance in Human Glioblastoma Cancer Stem Cells. Adv. Healthc. Mater. 2016, 5, 3173–3181. [Google Scholar] [CrossRef]
- Lau, L.W.; Cua, R.; Keough, M.B.; Haylock-Jacobs, S.; Yong, V.W. Pathophysiology of the Brain Extracellular Matrix: A New Target for Remyelination. Nat. Rev. Neurosci. 2013, 14, 722–729. [Google Scholar] [CrossRef]
- Entekhabi, E.; Haghbin Nazarpak, M.; Shafieian, M.; Mohammadi, H.; Firouzi, M.; Hassannejad, Z. Fabrication and in Vitro Evaluation of 3D Composite Scaffold Based on Collagen/Hyaluronic Acid Sponge and Electrospun Polycaprolactone Nanofibers for Peripheral Nerve Regeneration. J. Biomed. Mater. Res. A 2021, 109, 300–312. [Google Scholar] [CrossRef]
- Erickson, A.E.; Lan Levengood, S.K.; Sun, J.; Chang, F.; Zhang, M. Fabrication and Characterization of Chitosan–Hyaluronic Acid Scaffolds with Varying Stiffness for Glioblastoma Cell Culture. Adv. Healthc. Mater. 2018, 7, 1800295. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffold Design and Fabrication Technologies for Engineering Tissues—State of the Art and Future Perspectives. J. Biomater. Sci. Polym. Ed. 2001, 12, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shi, X.; Wang, Z.; Guo, M.; Wang, Y.; Jiao, Z.; Zhang, P. Porous Scaffolds of Poly (Lactic-Co-Glycolic Acid) and Mesoporous Hydroxyapatite Surface Modified by Poly (γ-Benzyl-l-Glutamate)(PBLG) for in Vivo Bone Repair. ACS Biomater. Sci. Eng. 2019, 5, 2466–2481. [Google Scholar] [CrossRef] [PubMed]
- Sola, A.; Bertacchini, J.; D’Avella, D.; Anselmi, L.; Maraldi, T.; Marmiroli, S.; Messori, M. Development of Solvent-Casting Particulate Leaching (SCPL) Polymer Scaffolds as Improved Three-Dimensional Supports to Mimic the Bone Marrow Niche. Mater. Sci. Eng. C 2019, 96, 153–165. [Google Scholar] [CrossRef]
- Murugan, S.; Parcha, S.R. Fabrication Techniques Involved in Developing the Composite Scaffolds PCL/HA Nanoparticles for Bone Tissue Engineering Applications. J. Mater. Sci. Mater. Med. 2021, 32, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rao, F.; Yuan, Z.; Li, M.; Yu, F.; Fang, X.; Jiang, B.; Wen, Y.; Zhang, P. Expanded 3D Nanofibre Sponge Scaffolds by Gas-Foaming Technique Enhance Peripheral Nerve Regeneration. Artif. Cells Nanomed. Biotechnol. 2019, 47, 491–500. [Google Scholar] [CrossRef]
- Dattola, E.; Parrotta, E.I.; Scalise, S.; Perozziello, G.; Limongi, T.; Candeloro, P.; Coluccio, M.L.; Maletta, C.; Bruno, L.; de Angelis, M.T. Development of 3D PVA Scaffolds for Cardiac Tissue Engineering and Cell Screening Applications. RSC Adv. 2019, 9, 4246–4257. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, Y.; Zhao, D.; Zhang, K.; Wang, F.; Wang, C.; Wen, Y. High Efficiency 3D Nanofiber Sponge for Bilirubin Removal Used in Hemoperfusion. Colloids Surf. B Biointerfaces 2018, 172, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Conoscenti, G.; Carfì Pavia, F.; Ongaro, A.; Brucato, V.; Goegele, C.; Schwarz, S.; Boccaccini, A.R.; Stoelzel, K.; la Carrubba, V.; Schulze-Tanzil, G. Human Nasoseptal Chondrocytes Maintain Their Differentiated Phenotype on PLLA Scaffolds Produced by Thermally Induced Phase Separation and Supplemented with Bioactive Glass 1393. Connect. Tissue Res. 2019, 60, 344–357. [Google Scholar] [CrossRef]
- Rad, R.M.; Atila, D.; Akgün, E.E.; Evis, Z.; Keskin, D.; Tezcaner, A. Evaluation of Human Dental Pulp Stem Cells Behavior on a Novel Nanobiocomposite Scaffold Prepared for Regenerative Endodontics. Mater. Sci. Eng. C 2019, 100, 928–948. [Google Scholar]
- Grenier, J.; Duval, H.; Barou, F.; Lv, P.; David, B.; Letourneur, D. Mechanisms of Pore Formation in Hydrogel Scaffolds Textured by Freeze-Drying. Acta Biomater. 2019, 94, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Reyna-Urrutia, V.A.; Mata-Haro, V.; Cauich-Rodriguez, J.V.; Herrera-Kao, W.A.; Cervantes-Uc, J.M. Effect of Two Crosslinking Methods on the Physicochemical and Biological Properties of the Collagen-Chitosan Scaffolds. Eur. Polym. J. 2019, 117, 424–433. [Google Scholar] [CrossRef]
- Baldino, L.; Concilio, S.; Cardea, S.; Reverchon, E. Interpenetration of Natural Polymer Aerogels by Supercritical Drying. Polymers 2016, 8, 106. [Google Scholar] [CrossRef]
- Yahya, E.B.; Amirul, A.A.; HPS, A.K.; Olaiya, N.G.; Iqbal, M.O.; Jummaat, F.; AK, A.S.; Adnan, A.S. Insights into the Role of Biopolymer Aerogel Scaffolds in Tissue Engineering and Regenerative Medicine. Polymers 2021, 13, 1612. [Google Scholar] [CrossRef] [PubMed]
- Worthington, P.; Drake, K.M.; Li, Z.; Napper, A.D.; Pochan, D.J.; Langhans, S.A. Implementation of a High-Throughput Pilot Screen in Peptide Hydrogel-Based Three-Dimensional Cell Cultures. SLAS DISCOVERY Adv. Life Sci. R D 2019, 24, 714–723. [Google Scholar] [CrossRef]
- Li, Z.; Ramay, H.R.; Hauch, K.D.; Xiao, D.; Zhang, M. Chitosan-Alginate Hybrid Scaffolds for Bone Tissue Engineering. Biomaterials 2005, 26, 3919–3928. [Google Scholar] [CrossRef]
- Schulz, A.; Rickmann, A.; Wahl, S.; Germann, A.; Stanzel, B.V.; Januschowski, K.; Szurman, P. Alginate-and Hyaluronic Acid–Based Hydrogels as Vitreous Substitutes: An in Vitro Evaluation. Transl. Vis. Sci. Technol. 2020, 9, 34. [Google Scholar] [CrossRef]
- Chang, P.-H.; Chao, H.-M.; Chern, E.; Hsu, S. Chitosan 3D Cell Culture System Promotes Naïve-like Features of Human Induced Pluripotent Stem Cells: A Novel Tool to Sustain Pluripotency and Facilitate Differentiation. Biomaterials 2021, 268, 120575. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Chen, L.; Han, S.; Niu, G.; Ren, J.; Ke, C. Preparation and Characterization of a Novel Triple Composite Scaffold Containing Silk Fiborin, Chitosan, and Alginate for 3D Culture of Colonic Carcinoma Cells In Vitro. Med. Sci. Monit. 2020, 26, e922935-1. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, D.A.; Wu, D.; He, F.; Wang, H.; Huang, L.; Shi, D.; Liu, Q.; Ni, N.; Pakvasa, M. Applications of Biocompatible Scaffold Materials in Stem Cell-Based Cartilage Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 603444. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Goh, J.C.H.; Teoh, S.H. An Introduction to Biodegradable Materials for Tissue Engineering Applications. Ann. Acad. Med. Singap. 2001, 30, 183–191. [Google Scholar] [PubMed]
- Suh, J.-K.F.; Matthew, H.W.T. Application of Chitosan-Based Polysaccharide Biomaterials in Cartilage Tissue Engineering: A Review. Biomaterials 2000, 21, 2589–2598. [Google Scholar] [PubMed]
- Eslahi, M.; Dana, P.M.; Asemi, Z.; Hallajzadeh, J.; Mansournia, M.A.; Yousefi, B. The Effects of Chitosan-Based Materials on Glioma: Recent Advances in Its Applications for Diagnosis and Treatment. Int. J. Biol. Macromol. 2021, 168, 124–129. [Google Scholar] [CrossRef]
- Reig-Vano, B.; Tylkowski, B.; Montané, X.; Giamberini, M. Alginate-Based Hydrogels for Cancer Therapy and Research. Int. J. Biol. Macromol. 2021, 170, 424–436. [Google Scholar] [CrossRef]
- Levengood, S.K.L.; Zhang, M. Chitosan-Based Scaffolds for Bone Tissue Engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef] [PubMed]
- Florczyk, S.J.; Kievit, F.M.; Wang, K.; Erickson, A.E.; Ellenbogen, R.G.; Zhang, M. 3D Porous Chitosan-Alginate Scaffolds Promote Proliferation and Enrichment of Cancer Stem-like Cells. J. Mater. Chem. B 2016, 4, 6326–6334. [Google Scholar] [CrossRef]
- Kievit, F.M.; Florczyk, S.J.; Leung, M.C.; Veiseh, O.; Park, J.O.; Disis, M.L.; Zhang, M. Chitosan–Alginate 3D Scaffolds as a Mimic of the Glioma Tumor Microenvironment. Biomaterials 2010, 31, 5903–5910. [Google Scholar] [CrossRef]
- Li, Z.; Leung, M.; Hopper, R.; Ellenbogen, R.; Zhang, M. Feeder-Free Self-Renewal of Human Embryonic Stem Cells in 3D Porous Natural Polymer Scaffolds. Biomaterials 2010, 31, 404–412. [Google Scholar] [CrossRef]
- Rahmati, M.; Pennisi, C.P.; Mobasheri, A.; Mozafari, M. Bioengineered Scaffolds for Stem Cell Applications in Tissue Engineering and Regenerative Medicine. Cell Biol. Transl. Med. 2018, 3, 73–89. [Google Scholar]
- Wang, X.; He, J.; Wang, Y.; Cui, F.-Z. Hyaluronic Acid-Based Scaffold for Central Neural Tissue Engineering. Interface Focus 2012, 2, 278–291. [Google Scholar] [PubMed]
- Correia, C.R.; Moreira-Teixeira, L.S.; Moroni, L.; Reis, R.L.; van Blitterswijk, C.A.; Karperien, M.; Mano, J.F. Chitosan Scaffolds Containing Hyaluronic Acid for Cartilage Tissue Engineering. Tissue Eng. Part C Methods 2011, 17, 717–730. [Google Scholar] [CrossRef]
- Yoo, H.S.; Lee, E.A.; Yoon, J.J.; Park, T.G. Hyaluronic Acid Modified Biodegradable Scaffolds for Cartilage Tissue Engineering. Biomaterials 2005, 26, 1925–1933. [Google Scholar] [CrossRef]
- Florczyk, S.J.; Kim, D.; Wood, D.L.; Zhang, M. Influence of Processing Parameters on Pore Structure of 3D Porous Chitosan-Alginate Polyelectrolyte Complex Scaffolds. J. Biomed. Mater. Res. A 2011, 98, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Tetik, H.; Yang, G.; Tan, W.; Fong, A.; Lei, S.; Weker, J.N.; Lin, D. High Speed In-Situ X-Ray Imaging of 3D Freeze Printing of Aerogels. Addit. Manuf. 2020, 36, 101513. [Google Scholar] [CrossRef]
- Tetik, H.; Wang, Y.; Sun, X.; Cao, D.; Shah, N.; Zhu, H.; Qian, F.; Lin, D. Additive Manufacturing of 3D Aerogels and Porous Scaffolds: A Review. Adv. Funct. Mater. 2021, 31, 2103410. [Google Scholar] [CrossRef]
- Iglesias-Mejuto, A.; García-González, C.A. 3D-Printed Alginate-Hydroxyapatite Aerogel Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C 2021, 131, 112525. [Google Scholar] [CrossRef]
- Lee, H.; Kim, G. Cryogenically Fabricated Three-Dimensional Chitosan Scaffolds with Pore Size-Controlled Structures for Biomedical Applications. Carbohydr. Polym. 2011, 85, 817–823. [Google Scholar] [CrossRef]
- Kim, G.; Ahn, S.; Yoon, H.; Kim, Y.; Chun, W. A Cryogenic Direct-Plotting System for Fabrication of 3D Collagen Scaffolds for Tissue Engineering. J. Mater. Chem. 2009, 19, 8817–8823. [Google Scholar] [CrossRef]
- Morris, V.B.; Nimbalkar, S.; Younesi, M.; McClellan, P.; Akkus, O. Mechanical Properties, Cytocompatibility and Manufacturability of Chitosan: PEGDA Hybrid-Gel Scaffolds by Stereolithography. Ann. Biomed. Eng. 2017, 45, 286–296. [Google Scholar] [CrossRef]
- Yan, Y.; Xiong, Z.; Hu, Y.; Wang, S.; Zhang, R.; Zhang, C. Layered Manufacturing of Tissue Engineering Scaffolds via Multi-Nozzle Deposition. Mater. Lett. 2003, 57, 2623–2628. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Chen, W.; Yuan, Z.; You, B.; Liu, Y.; Yang, S.; Li, F.; Qu, C.; Zhang, X. A Novel 3D in Vitro Tumor Model Based on Silk Fibroin/Chitosan Scaffolds to Mimic the Tumor Microenvironment. ACS Appl. Mater. Interfaces 2018, 10, 36641–36651. [Google Scholar] [CrossRef]
- Rijal, G.; Bathula, C.; Li, W. Application of Synthetic Polymeric Scaffolds in Breast Cancer 3D Tissue Cultures and Animal Tumor Models. Int. J. Biomater. 2017, 2017, 8074890. [Google Scholar] [CrossRef]
- Naciri, M.; Kuystermans, D.; Al-Rubeai, M. Monitoring PH and Dissolved Oxygen in Mammalian Cell Culture Using Optical Sensors. Cytotechnology 2008, 57, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Han, N.; Lee, R.; Choi, I.-H.; Park, Y.-B.; Shin, J.-S.; Yoo, K.-H. Real-Time Monitoring of 3D Cell Culture Using a 3D Capacitance Biosensor. Biosens. Bioelectron. 2016, 77, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Ham, J.; Lee, H.; Lee, K.; Koh, W.-G. Integration of a Fiber-Based Cell Culture and Biosensing System for Monitoring of Multiple Protein Markers Secreted from Stem Cells. Biosens. Bioelectron. 2021, 193, 113531. [Google Scholar] [CrossRef]


| CA | CHA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Well Plate | 96 | 384 | 96 | 384 | ||||||||
| Weight Percent (%) | 2 | 4 | 8 | 2 | 4 | 8 | 2 | 4 | 8 | 2 | 4 | 8 |
| Pressure (kPa) | 15 | 30 | 60 | 15 | 30 | 60 | 10 | 20 | 40 | 10 | 20 | 40 |
| Dwell Time (ms) | 120 | 120 | 120 | 120 | 120 | 120 | 100 | 100 | 100 | 100 | 100 | 100 |
| Total Time (min) | 4 | 4 | 4 | 15 | 15 | 15 | 4 | 4 | 4 | 15 | 15 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Revia, R.A.; Wagner, B.; James, M.; Zhang, M. High-Throughput Dispensing of Viscous Solutions for Biomedical Applications. Micromachines 2022, 13, 1730. https://doi.org/10.3390/mi13101730
Revia RA, Wagner B, James M, Zhang M. High-Throughput Dispensing of Viscous Solutions for Biomedical Applications. Micromachines. 2022; 13(10):1730. https://doi.org/10.3390/mi13101730
Chicago/Turabian StyleRevia, Richard A., Brandon Wagner, Matthew James, and Miqin Zhang. 2022. "High-Throughput Dispensing of Viscous Solutions for Biomedical Applications" Micromachines 13, no. 10: 1730. https://doi.org/10.3390/mi13101730
APA StyleRevia, R. A., Wagner, B., James, M., & Zhang, M. (2022). High-Throughput Dispensing of Viscous Solutions for Biomedical Applications. Micromachines, 13(10), 1730. https://doi.org/10.3390/mi13101730






